There are millions of breast cancer survivors in the United States currently, and expectations are that the number will continue to increase in the years to come.1,2 Survivors of early-stage breast cancer are at increased risk for developing second primary breast cancers.3 Data regarding the effectiveness of surveillance mammography among survivors of early-stage breast cancer are limited,4 but suggest that mammography may reduce breast cancer mortality for this cohort5 as it does when used to screen the general population.6–8 Although national guidelines recommend annual mammographic surveillance for survivors of early-stage breast cancer,9,10 studies suggest surveillance mammography is underutilized.11,12
Mammography is not a perfect test. In fact, it may be particularly insensitive at detecting breast cancer among selected high-risk populations, such as BRCA carriers, young women, and women with dense breast tissue. Magnetic resonance imaging (MRI) has consistently demonstrated greater sensitivity versus mammography in these populations.13 However, MRI screening is more costly and time consuming, requires the injection of intravenous contrast, generates more false-positive results, and has not been shown to impact breast cancer mortality.
Debate regarding the role of MRI as a screening test led the American Cancer Society to convene an expert panel to develop guidelines in 2007. The panel recommended breast MRI screening as an adjunct to mammography for: BRCA mutation carriers and their first-degree relatives; women with a lifetime breast cancer risk ≥ 20% to 25%; women with a history of chest radiation between ages of 10 and 30 years; and women with predisposing genetic syndromes (eg, Li-Fraumeni, Cowden). The group felt there was insufficient evidence to recommend for or against MRI screening among women with a personal history of invasive breast cancer or ductal carcinoma in situ.
On one hand, MRI surveillance may seem appropriate for breast cancer survivors. Compared to women with a higher than 20% lifetime risk of primary breast cancer as defined by BRCAPRO or other family history–based models, many survivors of early-stage breast cancer could experience an even greater risk of second primary breast cancer. In addition, the sensitivity of mammography among survivors could be impaired by changes in the breast caused by previous treatments. In contrast, no studies have explored the utility of surveillance MRI among survivors. Moreover, this cohort also faces a competing risk of mortality from the primary breast cancer diagnosis. Based on personal observations, use of surveillance MRI among women with a personal history of early-stage breast cancer appears to be increasing.
These issues raise serious questions regarding the appropriate use of surveillance breast MRI among survivors of early-stage breast cancer. To explore the implications of applying the American Cancer Society risk threshold to women with a personal history of breast cancer who are not BRCA carriers, we constructed an analytic model to calculate lifetime risk of developing a second breast cancer after diagnosis of an initial breast cancer. Specifically, we sought to determine which subsets of breast cancer survivors met a 20% or 25% lifetime risk threshold of having a new cancer, taking into account the competing risk of cancer mortality from the first diagnosis and age-dependent noncancer mortality. The model was designed to provide customized values for lifetime risk of developing breast cancer for each year after diagnosis, as a function of the initial cancer (hormone receptor–positive v –negative, mortality risk), its treatment (breast-conserving surgery v mastectomy, and tamoxifen v not for hormone receptor–positive cancers), and age at initial diagnosis.
Our model assumed the risk of developing a second breast cancer was constant over time, not related to a patient's age, and the same for the ipsilateral versus contralateral breast—as demonstrated in multiple prospective and retrospective studies.14–22 Adjuvant tamoxifen therapy was associated with a 47% reduction in the risk of developing a new breast cancer over the 10-year period after the primary cancer diagnosis.21 Therefore, the risk of developing a second breast cancer was only a function of the number of intact breasts and use of tamoxifen. No assumptions were made regarding the sensitivity or specificity of MRI screening in women with a personal history of breast cancer. Mortality risk from the initial diagnosis was assumed to be constant and completed within 7 years for hormone receptor–negative cancers23 and 15 years for hormone receptor–positive cancers.22 Patients with an initial diagnosis of ductal carcinoma in situ were assumed to have no mortality risk associated with their primary breast cancer diagnosis. Nonbreast cancer mortality was determined using 2004 United States female life-tables.24 The annual likelihood of a survivor developing a second breast cancer was determined by multiplying the per year probability of developing a new breast cancer with the per year probability of being alive (ie, not dying from the primary breast cancer or a nonbreast cancer cause). The lifetime risk of developing a second breast cancer was calculated to be annual likelihood of developing a second breast cancer multiplied by the likelihood of surviving that year summed over the remaining years of life until age 99, divided by the probability of being alive at that individual age.
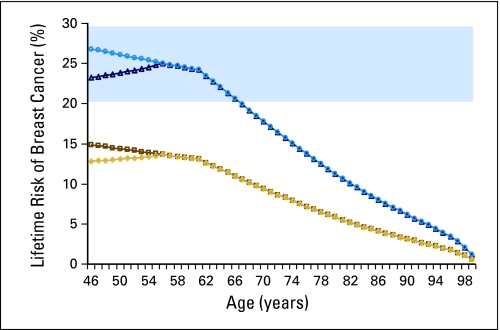
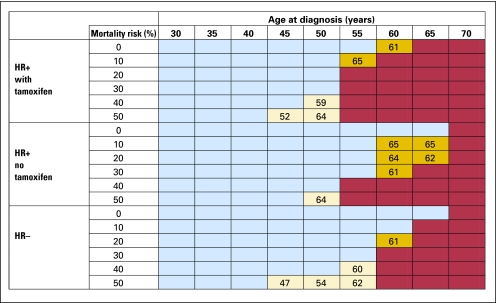
Model results for lifetime risk of breast cancer for a 45-year-old woman diagnosed with a hormone receptor–positive breast cancer carrying 30% mortality risk treated with lumpectomy or mastectomy, with or without tamoxifen, are shown in Figure 1. Tamoxifen initially lowers the risk of developing a second breast cancer. Among tamoxifen-treated patients, there is an initial increase in the lifetime risk of a second breast cancer diagnosis; this is attributable to the falling mortality risk from the primary breast cancer diagnosis as time elapses after this diagnosis. This example patient would meet the 20% lifetime-risk threshold for screening until age 66 if treated with lumpectomy, but would qualify for screening if the threshold were set at 25% only if treated with lumpectomy without tamoxifen and only until age 56. Figure 1 also shows that the same patient treated with mastectomy would never reach the 20% threshold for screening. Results after breast-conserving therapy using the 20% threshold (Fig 2) reveal that all women diagnosed at or before age 50 meet this threshold. In contrast, after mastectomy and using the 25% threshold for MRI screening, only patients between the ages of 31 and 33 would be considered screening candidates.
Fig 1.
Model results for lifetime risk of breast cancer for a 45-year-old woman diagnosed with a hormone receptor–positive breast cancer carrying 30% mortality risk treated with lumpectomy and tamoxifen (dark blue triangles), lumpectomy without tamoxifen (light blue circles), mastectomy with tamoxifen (gold diamonds), and mastectomy without tamoxifen (dark gold squares). Lifetime risk values, which lie in the blue shaded area, represent ages at which this patient would meet the 20% threshold for magnetic resonance imaging screening.
Fig 2.
Model results for screening recommendations for women who receive breast-conserving surgery using the 20% screening threshold. Recommendations are given for women as a function of age at diagnosis, type of cancer (hormone receptor [HR] positive or negative), mortality risk from primary cancer, and treatment with or without tamoxifen. Red boxes indicate women who never meet the screening threshold during their lifetime. Blue boxes indicate women who meet this threshold from the year after diagnosis to age 66. Beige boxes indicate that women meet this threshold during their lifetime but that the start date (shown in box) for screening is later than the year after diagnosis. Gold boxes indicate women who meet the threshold the year after diagnosis but fall below the screening threshold before age 66 (until age shown in box).
The model revealed screening recommendations to be very sensitive to the type of surgical treatment at diagnosis, mastectomy, or breast-conserving therapy; the lifetime risk of breast cancer was a function of the number of breasts at risk for developing new cancers. Women treated with mastectomy were less likely to meet the threshold, because they had less residual breast tissue at risk. In general, women were less likely to meet the threshold if the initial cancer was hormone receptor positive (due to the longer clinical course of hormone-positive disease), carried a high mortality risk, or were treated with tamoxifen. Women were more likely to meet the threshold if they were first diagnosed at a younger age or the lower (20%) risk threshold was used.
In summary, our model demonstrated that many women with a personal history of early-stage breast cancer who are not BRCA carriers exceed the 20% to 25% lifetime risk of developing a second breast cancer diagnosis, even when considering the competing mortality associated with primary breast cancer diagnoses. Although national guidelines recommend MRI screening for high-risk women and our analysis demonstrates that women with a personal history of breast cancer can be considered high risk, we do not believe this provides justification for the routine use of surveillance MRI testing among breast cancer survivors, for several reasons.
No clinical trials have demonstrated an improvement in outcomes associated with MRI screening among breast cancer survivors. Decision analytic models focusing on high-risk women have not found MRI screening to be cost effective.25,26 While breast cancer risk is an important factor in estimating the potential benefit of MRI screening, it is not the only important factor that must be considered. Our analysis found that the cohort of patients who demonstrated a ≥ 20% to 25% lifetime risk of developing second breast cancer was quite varied. It is not necessarily true that all women in this cohort will be more likely to survive if they have combined mammography/MRI screening versus mammographic screening alone, because the performance characteristics of MRI may not be constant across the group. MRI screening might offer incremental benefit to some women in this cohort (eg, those who are younger or have dense breast tissue, for whom mammography is less sensitive), but not others (eg, those who are postmenopausal after completing primary breast cancer therapy). Moreover, some women who experience an 18% to 19% lifetime risk of developing a second breast cancer might benefit substantially from combined mammography/MRI screening. These findings highlight the significant limitations of using lifetime risk as the primary mechanism for identifying which women should be considered eligible for MRI screening/surveillance.
While data regarding breast surveillance among survivors of early-stage breast cancer are sparse, the standard remains yearly mammography. Clinical trials are needed to evaluate the benefits and risks of surveillance MRI testing among selected subsets of breast cancer survivors. The challenge will be to determine which women, if any, experience a reduction in breast cancer mortality as a result of MRI surveillance. Studies assessing the utility of surveillance MRI testing among women who, at the time of screening, face a high risk of developing second breast cancer, a low risk of death from primary breast cancer, and a relatively greater sensitivity from MRI testing than mammography are warranted.
Acknowledgment
Supported by Grant No. 1K07 CA118629 from the National Institutes of Health (R.S.P.), American Society of Clinical Oncology Foundation Career Development Award, and Susan G. Komen for the Cure Career Catalyst in Disparities Research Grant (M.J.H.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rinaa S. Punglia, Michael J. Hassett
Collection and assembly of data: Rinaa S. Punglia, Michael J. Hassett
Data analysis and interpretation: Rinaa S. Punglia, Michael J. Hassett
Manuscript writing: Rinaa S. Punglia, Michael J. Hassett
Final approval of manuscript: Rinaa S. Punglia, Michael J. Hassett
REFERENCES
- 1.De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: Geographic variability and time trends, 2005-2015. Cancer. 2009;115:1954–1966. doi: 10.1002/cncr.24217. [DOI] [PubMed] [Google Scholar]
- 2.Sprague BL, Trentham-Dietz A. Prevalence of breast carcinoma in situ in the United States. JAMA. 2009;302:846–848. doi: 10.1001/jama.2009.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein JL, Lapinski RH, Thakore SS, et al. The descriptive epidemiology of second primary breast cancer. Epidemiology. 2003;14:552–558. doi: 10.1097/01.ede.0000072105.39021.6d. [DOI] [PubMed] [Google Scholar]
- 4.Grunfeld E, Noorani H, McGahan L, et al. Surveillance mammography after treatment of primary breast cancer: A systematic review. Breast. 2002;11:228–235. doi: 10.1054/brst.2001.0404. [DOI] [PubMed] [Google Scholar]
- 5.Lash TL, Fox MP, Buist DS, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 6.Nystrom L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet. 2002;359:909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 8.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2009;4:CD001877. doi: 10.1002/14651858.CD001877.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Carlson RW, Allred DC, Anderson BO, et al. NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 10.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17:1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 11.Keating NL, Landrum MB, Guadagnoli E, et al. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 12.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Warner E, Messersmith H, Causer P, et al. Systematic review: Using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148:671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Semenciw R, Kliewer E, et al. Incidence of second primary breast cancer among women with a first primary in Manitoba. Canada Breast Cancer Res Treat. 2001;67:35–40. doi: 10.1023/a:1010665603732. [DOI] [PubMed] [Google Scholar]
- 17.Fowble B, Hanlon A, Freedman G, et al. Second cancers after conservative surgery and radiation for stages I-II breast cancer: Identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 18.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: An analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 19.Vaittinen P, Hemminki K. Risk factors and age-incidence relationships for contralateral breast cancer. Int J Cancer. 2000;88:998–1002. doi: 10.1002/1097-0215(20001215)88:6<998::aid-ijc25>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Obedian E, Fischer DB, Haffty BG. Second malignancies after treatment of early-stage breast cancer: Lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18:2406–2412. doi: 10.1200/JCO.2000.18.12.2406. [DOI] [PubMed] [Google Scholar]
- 21.Tamoxifen for early breast cancer: An overview of the randomised trials: Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 22.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 23.Clarke M, Coates AS, Darby SC, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: Patient-level meta-analysis of randomised trials. Lancet. 2008;371:29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- 24.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 25.Moore SG, Shenoy PJ, Fanucchi L, et al. Cost-effectiveness of MRI compared to mammography for breast cancer screening in a high risk population. BMC Health Serv Res. 2009;9:9. doi: 10.1186/1472-6963-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taneja C, Edelsberg J, Weycker D, et al. Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J Am Coll Radiol. 2009;6:171–179. doi: 10.1016/j.jacr.2008.10.003. [DOI] [PubMed] [Google Scholar]