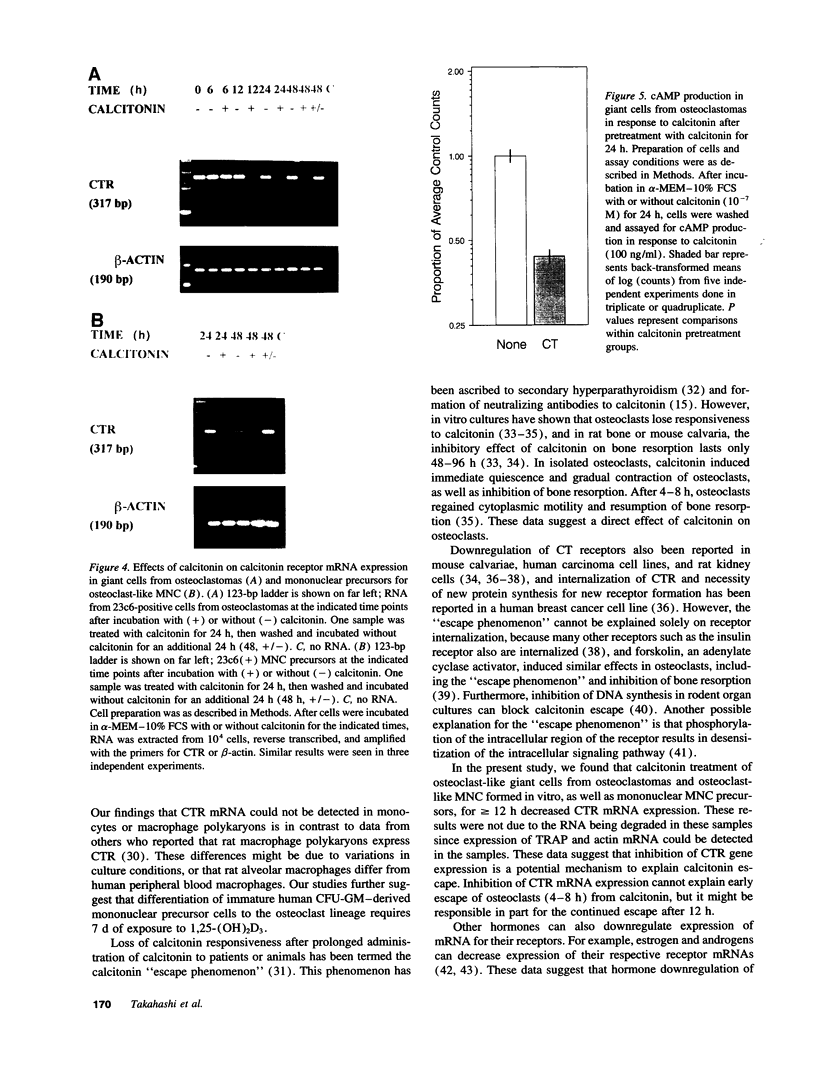
Abstract
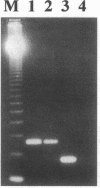
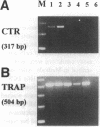
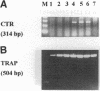
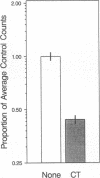
Calcitonin inhibits both osteoclast formation and bone resorption, and is a primary treatment for patients with hypercalcemia and increased bone turnover. However, the clinical utility of calcitonin is limited because patients become refractory to calcitonin after several days (the calcitonin "escape phenomenon"). The molecular basis for calcitonin "escape" is unclear. To determine the regulatory mechanisms controlling calcitonin receptor (CTR) expression in osteoclasts and their precursors, we treated immature mononuclear precursors for human osteoclast-like multinucleated cells (MNC) formed in vitro with 1,25-(OH)2D3, to induce their differentiation to committed mononuclear precursors, and mature multinucleated osteoclasts, and used reverse transcriptase (RT)-PCR to assess expression of CTR mRNA in both committed mononuclear precursors and MNC. The PCR fragment produced was cloned and sequenced to confirm that it was derived from CTR mRNA. CTR mRNA expression was detected in mononuclear MNC precursors after 7 d of 1,25-(OH)2D3 treatment. It was also present in osteoclast-like MNC and highly purified giant cells from osteoclastomas, but not in monocytes or macrophage polykaryons formed in vitro. Calcitonin markedly decreased CTR but not actin mRNA expression in giant cells and MNC after 12 h, and removal of calcitonin restored CTR mRNA expression. Similarly, calcitonin decreased calcitonin-induced adenylate cyclase activity. These data suggest: (a) downregulation of CTR gene expression by calcitonin may in part explain the calcitonin "escape phenomenon"; and (b) expression of CTR mRNA occurs in mononuclear osteoclast precursors within 7 d after exposure to 1,25-(OH)2D3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara O., Reddy S. V., Roodman G. D., Boldt D. H. Transcriptional regulation of the tartrate-resistant acid phosphatase (TRAP) gene by iron. Biochem J. 1994 Mar 1;298(Pt 2):421–425. doi: 10.1042/bj2980421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Dong X. F., Roux-Dossetto M., Martin P. M. Expression of estrogen receptor and its messenger ribonucleic acid in the MCF-7 cell line: multiparametric analysis of its processing and regulation by estrogen. Mol Cell Endocrinol. 1990 Nov 12;74(1):11–20. doi: 10.1016/0303-7207(90)90201-i. [DOI] [PubMed] [Google Scholar]
- Binstock M. L., Mundy G. R. Effect of calcitonin and glutocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980 Aug;93(2):269–272. doi: 10.7326/0003-4819-93-2-269. [DOI] [PubMed] [Google Scholar]
- Bouizar Z., Rostène W. H., Milhaud G. Down-regulation of rat kidney calcitonin receptors by salmon calcitonin infusion evidenced by autoradiography. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5125–5128. doi: 10.1073/pnas.84.15.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouizar Z., Rostène W. H., Milhaud G. Down-regulation of rat kidney calcitonin receptors by salmon calcitonin infusion evidenced by autoradiography. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5125–5128. doi: 10.1073/pnas.84.15.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., McSheehy P. M., Thomson B. M., Fuller K. The effect of calcium-regulating hormones and prostaglandins on bone resorption by osteoclasts disaggregated from neonatal rabbit bones. Endocrinology. 1985 Jan;116(1):234–239. doi: 10.1210/endo-116-1-234. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., Martin T. J. Relationship between internalization and calcitonin-induced receptor loss in T 47D cells. Endocrinology. 1984 Jul;115(1):78–83. doi: 10.1210/endo-115-1-78. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Moseley J. M., Martin T. J. Calcitonin binding and degradation by two cultured human breast cancer cell lines (MCF 7 and T 47D). Biochem J. 1981 May 15;196(2):513–520. doi: 10.1042/bj1960513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Martin T. J. Independent down-regulation of insulin and calcitonin receptors on a human tumour cell line. J Endocrinol. 1981 Feb;88(2):271–281. doi: 10.1677/joe.0.0880271. [DOI] [PubMed] [Google Scholar]
- Friedman J., Au W. Y., Raisz L. G. Responses of fetal rat bone to thyrocalcitonin in tissue culture. Endocrinology. 1968 Jan;82(1):149–156. doi: 10.1210/endo-82-1-149. [DOI] [PubMed] [Google Scholar]
- Glajchen N., Thomas S., Jowell P., Epstein S., Ismail F., Fallon M. The effect of high-dose salmon calcitonin on bone mineral metabolism in the normal rat. Calcif Tissue Int. 1990 Jan;46(1):28–32. doi: 10.1007/BF02555821. [DOI] [PubMed] [Google Scholar]
- Gorn A. H., Lin H. Y., Yamin M., Auron P. E., Flannery M. R., Tapp D. R., Manning C. A., Lodish H. F., Krane S. M., Goldring S. R. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. J Clin Invest. 1992 Nov;90(5):1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G. E., Mundy G. R., Derynck R., Hewlett E. L., Katz M. S. Inhibition of parathyroid hormone-responsive adenylate cyclase in clonal osteoblast-like cells by transforming growth factor alpha and epidermal growth factor. J Biol Chem. 1987 Nov 25;262(33):15845–15850. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Heersche J. N. Calcitonin effects on osteoclastic resorption: the 'escape phenomenon' revisited. Bone Miner. 1992 Mar;16(3):174–177. doi: 10.1016/0169-6009(92)90895-k. [DOI] [PubMed] [Google Scholar]
- Kanehisa J. Time course of "escape" from calcitonin-induced inhibition of motility and resorption of disaggregated osteoclasts. Bone. 1989;10(2):125–129. doi: 10.1016/8756-3282(89)90010-0. [DOI] [PubMed] [Google Scholar]
- Krieger N. S., Feldman R. S., Tashjian A. H., Jr Parathyroid hormone and calcitonin interactions in bone: irradiation-induced inhibition of escape in vitro. Calcif Tissue Int. 1982 Mar;34(2):197–203. doi: 10.1007/BF02411233. [DOI] [PubMed] [Google Scholar]
- Kukita T., McManus L. M., Miller M., Civin C., Roodman G. D. Osteoclast-like cells formed in long-term human bone marrow cultures express a similar surface phenotype as authentic osteoclasts. Lab Invest. 1989 Apr;60(4):532–538. [PubMed] [Google Scholar]
- Kurihara N., Chenu C., Miller M., Civin C., Roodman G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology. 1990 May;126(5):2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Gluck S., Roodman G. D. Sequential expression of phenotype markers for osteoclasts during differentiation of precursors for multinucleated cells formed in long-term human marrow cultures. Endocrinology. 1990 Dec;127(6):3215–3221. doi: 10.1210/endo-127-6-3215. [DOI] [PubMed] [Google Scholar]
- Lerner U. H., Ransjö M., Klaushofer K., Hörandner H., Hoffmann O., Czerwenka E., Koller K., Peterlik M. Comparison between the effects of forskolin and calcitonin on bone resorption and osteoclast morphology in vitro. Bone. 1989;10(5):377–387. doi: 10.1016/8756-3282(89)90134-8. [DOI] [PubMed] [Google Scholar]
- Liggett S. B. Desensitization of the beta-adrenergic receptor: distinct molecular determinants of phosphorylation by specific kinases. Pharmacol Res. 1991 Aug;24 (Suppl 1):29–41. doi: 10.1016/1043-6618(91)90119-i. [DOI] [PubMed] [Google Scholar]
- Lin M. C., Rajfer J., Swerdloff R. S., González-Cadavid N. F. Testosterone down-regulates the levels of androgen receptor mRNA in smooth muscle cells from the rat corpora cavernosa via aromatization to estrogens. J Steroid Biochem Mol Biol. 1993 May;45(5):333–343. doi: 10.1016/0960-0760(93)90002-e. [DOI] [PubMed] [Google Scholar]
- Lord D. K., Cross N. C., Bevilacqua M. A., Rider S. H., Gorman P. A., Groves A. V., Moss D. W., Sheer D., Cox T. M. Type 5 acid phosphatase. Sequence, expression and chromosomal localization of a differentiation-associated protein of the human macrophage. Eur J Biochem. 1990 Apr 30;189(2):287–293. doi: 10.1111/j.1432-1033.1990.tb15488.x. [DOI] [PubMed] [Google Scholar]
- MacDonald B. R., Takahashi N., McManus L. M., Holahan J., Mundy G. R., Roodman G. D. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987 Jun;120(6):2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Mundy G. R., Wilkinson R., Heath D. A. Comparative study of available medical therapy for hypercalcemia of malignancy. Am J Med. 1983 Mar;74(3):421–432. doi: 10.1016/0002-9343(83)90961-0. [DOI] [PubMed] [Google Scholar]
- Murrills R. J., Shane E., Lindsay R., Dempster D. W. Bone resorption by isolated human osteoclasts in vitro: effects of calcitonin. J Bone Miner Res. 1989 Apr;4(2):259–268. doi: 10.1002/jbmr.5650040219. [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Gunning P., Eddy R., Ponte P., Leavitt J., Shows T., Kedes L. Evolution of the functional human beta-actin gene and its multi-pseudogene family: conservation of noncoding regions and chromosomal dispersion of pseudogenes. Mol Cell Biol. 1985 Oct;5(10):2720–2732. doi: 10.1128/mcb.5.10.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson G. C., Horton M. A., Sexton P. M., D'Santos C. S., Moseley J. M., Kemp B. E., Pringle J. A., Martin T. J. Calcitonin receptors of human osteoclastoma. Horm Metab Res. 1987 Nov;19(11):585–589. doi: 10.1055/s-2007-1011887. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., Moseley J. M., Sexton P. M., Mendelsohn F. A., Martin T. J. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest. 1986 Aug;78(2):355–360. doi: 10.1172/JCI112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaki Y., Takahashi S., Scarcez T., Demulder A., Nishihara T., Williams R., Roodman G. D. Evidence for an autocrine/paracrine role for interleukin-6 in bone resorption by giant cells from giant cell tumors of bone. Endocrinology. 1992 Nov;131(5):2229–2234. doi: 10.1210/endo.131.5.1425421. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Singer F. R., Fredericks R. S., Minkin C. Salmon calcitonin therapy for Paget's disease of bone. The problem of acquired clinical resistance. Arthritis Rheum. 1980 Oct;23(10):1148–1154. doi: 10.1002/art.1780231012. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Sasaki T., Nicholson G. C., Moseley J. M., Martin T. J., Suda T. Induction of calcitonin receptors by 1 alpha, 25-dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells. Endocrinology. 1988 Sep;123(3):1504–1510. doi: 10.1210/endo-123-3-1504. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kukita T., MacDonald B. R., Bird A., Mundy G. R., McManus L. M., Miller M., Boyde A., Jones S. J., Roodman G. D. Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest. 1989 Feb;83(2):543–550. doi: 10.1172/JCI113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Wright D. R., Ivey J. L., Pont A. Calcitonin binding sites in bone: relationships to biological response and "escape". Recent Prog Horm Res. 1978;34:285–334. doi: 10.1016/b978-0-12-571134-0.50012-0. [DOI] [PubMed] [Google Scholar]
- Vignery A., Raymond M. J., Qian H. Y., Wang F., Rosenzweig S. A. Multinucleated rat alveolar macrophages express functional receptors for calcitonin. Am J Physiol. 1991 Dec;261(6 Pt 2):F1026–F1032. doi: 10.1152/ajprenal.1991.261.6.F1026. [DOI] [PubMed] [Google Scholar]
- Wilkinson R. Treatment of hypercalcaemia associated with malignancy. Br Med J (Clin Res Ed) 1984 Mar 17;288(6420):812–813. doi: 10.1136/bmj.288.6420.812. [DOI] [PMC free article] [PubMed] [Google Scholar]