Abstract
Purpose
To determine the prognostic importance of p16 and human papillomavirus (HPV) in patients with oropharyngeal cancer treated on a phase III concurrent chemoradiotherapy trial.
Patients and Methods
Patients with stage III or IV head and neck squamous cell cancer were randomly assigned to concurrent radiotherapy and cisplatin with or without tirapazamine. In this substudy, analyses were restricted to patients with oropharyngeal cancer. p16 was detected by immunohistochemistry, and HPV was detected by in situ hybridization and polymerase chain reaction.
Results
Slides were available for p16 assay in 206 of 465 patients, of which 185 were eligible, and p16 and HPV were evaluable in 172 patients. One hundred six (57%) of 185 were p16-positive, and in patients evaluable for both p16 and HPV, 88 (86%) of 102 p16-positive patients were also HPV-positive. Patients who were p16-positive had lower T and higher N categories and better Eastern Cooperative Oncology Group (ECOG) performance status. p16-positive tumors compared with p16-negative tumors were associated with better 2-year overall survival (91% v 74%; hazard ratio [HR], 0.36; 95% CI, 0.17 to 0.74; P = .004) and failure-free survival (87% v 72%; HR, 0.39; 95% CI, 0.20 to 0.74; P = .003). p16 was a significant prognostic factor on multivariable analysis (HR, 0.45; 95% CI, 0.21 to 0.96; P = .04). p16-positive patients had lower rates of locoregional failure and deaths due to other causes. There was a trend favoring the tirapazamine arm for improved locoregional control in p16-negative patients (HR, 0.33; 95% CI, 0.09 to 1.24; P = .13).
Conclusion
HPV-associated oropharyngeal cancer is a distinct entity with a favorable prognosis compared with HPV-negative oropharyngeal cancer when treated with cisplatin-based chemoradiotherapy.
INTRODUCTION
An increasing proportion of oropharyngeal cancer is associated with the human papillomavirus (HPV).1 In some countries, the majority of oropharyngeal cancer cases are now associated with HPV.2,3 The HPV 16 subtype is present in 90% to 95% of HPV-associated oropharyngeal cancers compared with only 50% in cervical cancers.4 HPV-positive oropharyngeal cancer is associated with several types of sexual behavior, but not tobacco or alcohol use.5 HPV-positive oropharyngeal cancer is associated with wild-type p536 and downregulation of cyclin D and the retinoblastoma protein pRb. The E7 viral oncoprotein of HPV functionally inactivates pRb leading to upregulation of CDKN2A and increased expression of p16INK4A referred to hereafter as p16. p16 overexpression has been reported to strongly correlate with HPV expression in several studies.7–10
In retrospective and small prospective studies, HPV-associated oropharyngeal cancer has been associated with an improved prognosis.1,7,8,10–13 We sought to determine the prognostic significance of p16 and HPV in oropharyngeal cancer in patients treated with cisplatin-based concurrent chemoradiotherapy on a large international phase III trial (Trans Tasman Radiation Oncology Group [TROG] 02.02).
PATIENTS AND METHODS
Study Design and Eligibility
The original trial was an open-label, randomized study of radiation and cisplatin with or without the hypoxic cytotoxin tirapazamine that was conducted in 82 centers in 16 countries in Australia, New Zealand, North America, Europe, and South America between April 2002 and September 2005. We have previously reported that there were no statistically significant differences in overall survival, failure-free survival, or time to locoregional failure between the two treatment arms.14
Eligibility criteria for the phase III trial were as previously described14 and included previously untreated squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx or larynx; stage III or IV disease (excluding T1-2N1 and distant metastases); Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2; adequate hematologic and liver function; calculated creatinine clearance (Cockcroft-Gault) > 55 mL/min; and no prior radiotherapy for head and neck cancer. Written informed consent was obtained from all patients, and the institutional ethics committees approved the protocol.
For the HPV substudy, additional eligibility criteria were oropharyngeal cancer, slides available for p16 testing, receipt of ≥ 60 Gy, and being assessed as having no major radiation deviations predicted to have an impact on tumor control.15
Pretreatment and Follow-Up Evaluations
All patients underwent a full history, physical examination, blood tests, fiberoptic endoscopy, computed tomography (CT) scan or magnetic resonance imaging of the head and neck, and CT scan of the chest. Tumor assessment by clinical examination and CT scanning took place at 2, 6, 10, and 14 months after completion of treatment and then every 6 months. Current smoking status was ascertained from the baseline Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N) quality-of-life questionnaire, which includes a question about whether they had smoked in the last 7 days. Information about previous smoking history was not available.
Treatment
Arm 1: Cisplatin (100 mg/m2) was administered over 1 hour on day 1 of weeks 1, 4, and 7, followed by radiotherapy. Arm 2: Tirapazamine (290 mg/m2) was administered over 2 hours on day 1 of weeks 1, 4, and 7, followed 1 hour later by cisplatin (75 mg/m2) over 1 hour, followed immediately by radiotherapy. In addition, tirapazamine (160 mg/m2) was given before radiation three times per week in weeks 2 and 3.
Radiation Therapy
Radiation therapy was the same in both arms. A dose of 70 Gy in 35 fractions over 7 weeks was delivered to gross disease using a shrinking field technique. Sites potentially harboring subclinical disease (including a minimum of two nodal echelons beyond gross disease) were treated to 50 Gy in 25 fractions over 5 weeks, and areas adjacent to gross disease were treated to 60 Gy.
Randomization and Stratification
Treatment assignment was done centrally by stratifying for disease stage (III v IV), primary site (oropharynx/larynx v hypopharynx/oral cavity), and hemoglobin (≥ 13.5 g/dL for men and ≥ 12.5 g/dL for women).
End Points
For this study, times to events were measured from the end of radiotherapy. Overall survival was measured to the date of death. Failure-free survival was measured to the date of first treatment failure or death. Failure was defined as persistent disease in the primary site (other than a stable radiologic abnormality without clinical evidence of disease), progression of disease in the neck in patients not undergoing neck dissection, residual disease left behind following neck dissection, locoregional relapse following complete response, or distant metastasis. Time to locoregional failure was measured to the date of locoregional failure and censored by distant metastasis and death without preceding failure.
Statistical Methods
The Kaplan-Meier method was used to estimate time-to-event curves. Patterns of first failure were analyzed using a competing risks analysis for the events: locoregional failure only, distant failure only, simultaneous (within 1 month) locoregional and distant failure, and death without a preceding failure.
The groups were compared with respect to overall survival, failure-free survival, and time to locoregional failure using the log-rank test, and the Cox proportional hazards model was used to assess p16 status in the presence of the prognostic factors of ECOG performance status (0 v 1, 2), T category (1, 2 v 3, 4), N category (0, 1 v 2, 3), and hemoglobin (≥ 13.5 v < 13.5 g/dL for men and ≥ 12.5 v < 12.5 g/dL for women) and to test for interaction between p16 status and treatment arm.
p16 Immunohistochemistry
p16 immunohistochemistry (IHC) was performed on formalin-fixed paraffin embedded (FFPE) tissue sections using a Dako autostainer (Dako, Copenhagen, Denmark). After antigen retrieval, sections were incubated with mouse monoclonal anti-p16 (Lab Vision/NeoMarkers, Fremont, CA), and then EnVision+ System HRP anti-mouse (Dako), followed by diaminobenzidine chromogen (Dako) and counterstaining with hematoxylin. Cervical cancer sections known to be HPV-positive were used as a positive control, and omission of primary antibody was used as a negative control. All p16 IHC slides were semiquantitatively scored by a pathologist (S.B.F.) for intensity of staining in the cell nucleus and cytoplasm. Intensity was scored as 0 (none), 1 (weak), 2 (moderate), or 3 (strong), with 0 or 1 scores defined as negative and 2 or 3 defined as positive. p16 scoring was performed without knowledge of HPV status.
HPV Chromogenic In Situ Hybridization
High-risk HPV subtypes 16 and 18 in FFPE tissue sections were detected by using the in situ hybridization (ISH) -catalyzed signal amplification method for biotinylated probes (Dako). After target retrieval and digestion with pepsin, sections were dehydrated with graded ethanols and air dried, and HPV 16/18 biotinylated DNA probe (Dako) was applied. Sections were then denatured for 5 minutes at 92°C, hybridized for 3 hours at 37°C on a StatSpin Hybridizer (Dako), stringently washed, and examined by IHC on a Dako autostainer. Sections were counterstained with hematoxylin. Cervical cancer sections known to be HPV positive were used as a positive control. HPV ISH slides were scored by a pathologist (S.B.F.) as detected (if brown punctate signals were visualized specifically within the nucleus of tumor cells, typically one to two per cell) or undetected (if no signals were present). HPV ISH scoring was performed without knowledge of p16 IHC status.
HPV Polymerase Chain Reaction
Detection and subtyping of HPV by polymerase chain reaction (PCR) was performed using a PapType human papillomavirus detection test (Genera Biosystems, Scoresby, Victoria, Australia). This assay detects 14 high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and two low-risk HPV subtypes (6 and 11). FFPE tissue sections were assessed by a pathologist to determine percent tumor; then DNA was extracted using a Qiagen QIAamp DNA Blood mini kit (Qiagen, Valencia, CA). Extracted DNA was used in PCR with general primers to detect all HPV subtypes; the PCR reaction mix was then hybridized to genotype (HPV subtype) -specific hybridization beads, which were analyzed on a BD FACSArray (BD Biosciences, San Jose, CA) to determine the HPV status of each sample.
RESULTS
Patient Characteristics
In total, 861 patients were randomly assigned in this trial, of which 465 (54%) had oropharyngeal cancer. Slides were available for p16 analysis in 206 patients with oropharyngeal cancer; 21 were excluded because they received < 60 Gy or had radiotherapy deviations, leaving 185 patients for p16 analysis (Fig 1). One hundred seventy-two patients were able to be tested for both p16 and HPV status. The mean potential follow-up time from end of treatment to the closeout date was 29 months (range, 18 to 42 months). Slides were available only from sites in Australia, New Zealand, North America, and Western Europe (Table 1). The patients with slides available for p16 testing differed from the patients without available slides, with better performance status, lower T category, and higher hemoglobin, and they were less likely to be current smokers.
Fig 1.
CONSORT diagram. HPV, human papillomavirus; +ve, positive; -ve, negative; ISH, in situ hybridization; PCR, polymerase chain reaction. (*) One patient not evaluable.
Table 1.
Patient Characteristics
| Characteristic | p16-Negative(n = 79) |
p16-Positive(n = 106) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | .31 | ||||
| Male | 64 | 81 | 92 | 87 | |
| Female | 15 | 19 | 14 | 13 | |
| Age, years | |||||
| Median | 58 | 54 | |||
| Range | 39 to 74 | 32 to 80 | |||
| ECOG performance status | .002 | ||||
| 0 | 45 | 57 | 83 | 78 | |
| 1-2 | 34 | 43 | 23 | 22 | |
| Stage | .12 | ||||
| III | 10 | 13 | 6 | 6 | |
| IV | 69 | 87 | 100 | 94 | |
| T category | .001 | ||||
| 1-2 | 12 | 15 | 39 | 37 | |
| 3-4 | 67 | 85 | 67 | 63 | |
| N category | .001 | ||||
| 0-1 | 28 | 35 | 15 | 14 | |
| 2-3 | 51 | 65 | 91 | 86 | |
| Hemoglobin | .060 | ||||
| Low | 20 | 25 | 15 | 14 | |
| High | 59 | 75 | 91 | 86 | |
| Geographic region | |||||
| Australia and New Zealand | 43 | 54 | 53 | 50 | |
| Eastern Europe | 0 | 0 | 0 | 0 | |
| North America | 14 | 18 | 44 | 42 | |
| South America | 0 | 0 | 0 | 0 | |
| Western Europe | 22 | 28 | 9 | 8 | |
| Current smoker | < .001 | ||||
| Yes | 35 | 45 | 16 | 15 | |
| No | 42 | 55 | 89 | 85 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
One hundred six (57%) of 185 patients were p16 positive. Baseline patient characteristics of p16-positive and p16-negative patients are shown in Table 1. Patients who were p16 positive had less advanced T category, more advanced nodal category, and better performance status, and they were less likely to be current smokers.
Efficacy
Overall survival was superior in the p16-positive group compared with that in the p16-negative group, with 2-year survival rates of 91% and 74%, respectively (HR, 0.36; 95% CI, 0.17 to 0.74; P = .004; Fig 2). Failure-free survival was also superior, with 2-year survival rates of 87% and 72%, respectively (HR, 0.39; 95% CI, 0.20 to 0.74; P = .003; Fig 3). The 2-year time-to-locoregional-failure rates were 93% in p16-positive patients and 86% in p16-negative patients (HR, 0.43; 95% CI, 0.17 to 1.11; P = .091). Analysis within each treatment arm demonstrated that, on the control arm, the 2-year overall survival rates were 89% in p16-positive patients and 68% in p16-negative patients (HR, 0.34; 95% CI, 0.14 to 0.86; P = .021). On the tirapazamine arm, the corresponding 2-year overall survival rates were 94% and 80%, respectively (HR, 0.36; 95% CI, 0.11 to 1.18; P = .094). The test for interaction between p16 and study arm was not statistically significant (P = .95).
Fig 2.
Overall survival by p16 status. HR, hazard ratio; OS, overall survival.
Fig 3.
Failure-free survival by p16 status. HR, hazard ratio.
Analyses that included all patients with slides available (including patients who were excluded because they received < 60 Gy or they had radiotherapy deviations predicted to have an impact on tumor control) showed similar results. Overall survival in the p16-positive group compared with that in the p16-negative group showed 2-year rates of 91% versus 77% (HR, 0.35; 95% CI, 0.18 to 0.69; P = .002). Fourteen (67%) of 21 excluded patients were p16-positive compared with 106 (57%) of 185 who were included in the main analysis (P = .49).
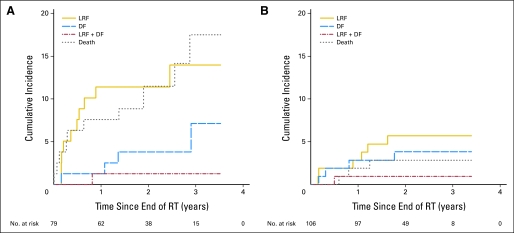
The cumulative incidence at site of first failure is shown in Figure 4. Locoregional failure and death without failure were lower in the p16-positive population, but the rates of distant failure were similar, albeit low, in both arms.
Fig 4.
Cumulative incidence curves of site of first failure by p16 status for (A) p16-negative and (B) p16-positive patients. LRF, locoregional failure; DF, distant failure; RT, radiotherapy.
Cox regression analysis of overall survival, including the prognostic factors of hemoglobin, T category, N category, and ECOG performance status, demonstrated that p16 status was the only significant factor in multivariable analysis (Table 2).
Table 2.
Multivariate Analysis of Overall Survival
| Factor | Levels | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| p16 | Positive v negative | 0.43 | 0.20 to 0.93 | .031 |
| Performance status | 0 v 1, 2 | 0.65 | 0.32 to 1.34 | .25 |
| Hemoglobin | High v Low | 0.51 | 0.24 to 1.07 | .075 |
| T category | 1, 2 v 3, 4 | 0.38 | 0.13 to 1.12 | .079 |
| N category | 0, 1 v 2, 3 | 0.51 | 0.22 to 1.22 | .13 |
NOTE. Cox regression analysis of p16, adjusting for four prognostic factors (185 patients, 33 deaths).
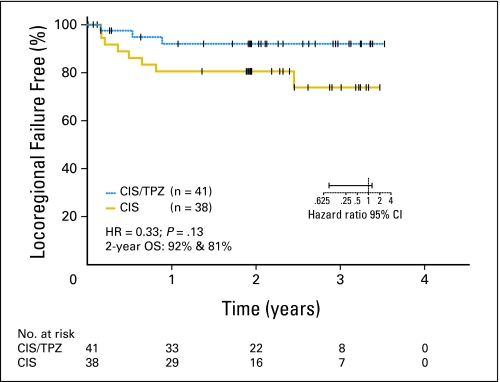
We also sought to determine whether there was any differential impact in the two treatment arms between p16-positive and p16-negative patients. There was a trend for improved locoregional control with the tirapazamine regimen in the p16-negative patients (Fig 5). The 2-year time-to-locoregional-failure rates were 92% on the cisplatin and tirapazamine arm and 81% on the cisplatin arm (HR, 0.33; 95% CI, 0.09 to 1.24; P = .13).
Fig 5.
Time to locoregional failure by treatment arm. CIS, cisplatin; TPZ, tirapazamine; HR, hazard ratio; OS, overall survival.
HPV Analysis
Assessment of HPV status by ISH demonstrated a large group of HPV-negative, p16-positive patients representing 57% of the p16-positive patients (Fig 1). This raised concerns about the sensitivity of this assay, so we sought to measure HPV by PCR, focusing on the patients in whom there was discordance between the p16 and HPV ISH results; we assessed HPV by PCR only in a subset of the patients where the ISH and p16 results were concordant (Fig 1). We were able to assess HPV by PCR in 56 of the 58 HPV-negative, ISH-negative but p16-positive patients. Forty-four (79%) of these 56 patients were positive by PCR. The results of the HPV PCR were concordant with the HPV ISH results in the ISH-positive, p16-positive patients and also in the ISH-negative, p16-negative patients with one exception. Interestingly, only one of three HPV-positive, ISH-positive, p16-negative patients could be confirmed on PCR.
DISCUSSION
In this large, international, multicenter phase III trial, we have demonstrated the prognostic significance of p16 and HPV status in patients treated with cisplatin-based concurrent chemoradiotherapy. Retrospective studies have reported improved outcomes in HPV-associated oropharyngeal cancer.1,8,10,11 However, these studies need to be interpreted with caution because many of these studies are small, patients may not have been treated in a uniform fashion, and there are significant limitations to retrospectively collected clinical data. There have been recent reports of clinical trial results based on HPV status, but in many of these studies, the number of oropharyngeal cancer patients included is relatively small, and the treatment given may have been different compared with current standard practice. To the best of our knowledge, the trial reported by Fakhry et al7 was the first clinical trial to report an improved outcome in HPV-positive oropharyngeal cancer. In that study, there were 62 oropharyngeal cancer patients with 38 (61%) being HPV-positive, and they were treated with induction carboplatin and paclitaxel chemotherapy followed by radiation with concurrent paclitaxel. More recently, an analysis of the Danish Head and Neck Cancer (DAHANCA) Study Group 5 trial12 reported improved survival in p16-positive patients treated with radiotherapy alone. In that study, conducted in the late 1980s, there were 74 oropharyngeal cancer patients among whom only 24 (32%) were p16 positive. Settle et al16 reported that in the TAX 324 trial, in which patients received induction chemotherapy (cisplatin and fluorouracil with or without docetaxel) followed by radiation and weekly low-dose carboplatin, there was improved survival in the HPV-positive patients. In that substudy, there were 119 oropharyngeal patients among whom 59 (50%) were HPV positive. Apart from our own study, the only trial results on the prognostic significance of HPV in patients treated with cisplatin-based concurrent chemoradiotherapy have been presented by the Radiation Therapy Oncology Group in a preliminary report.17 The combined results from these five clinical trials have unequivocally confirmed the importance of HPV status as a prognostic variable in oropharyngeal cancer.
In our study, the improved survival in p16-positive patients seems to be due to lower rates of locoregional failure and deaths without failure. A proportion of the deaths without failure in the p16-negative group are likely to be related to a higher incidence of comorbidities in this group. The rate for distant metastases as a site of first failure was similar in p16-positive and p16-negative patients, despite the more advanced nodal stage in the p16-positive patients.
Our study has clearly shown that HPV-associated oropharyngeal cancer is associated with less advanced T category, more advanced N category, and better performance status. The T and N category associations, although reported in previous studies, have not been consistently identified, probably because of the small sample size in many of these studies.7,9,10,12 Not surprisingly, p16-positive patients were much less likely to be current smokers. The differences in baseline characteristics that we found between patients tested for p16 and patients who could not be tested may reflect geographic variation inthe proportion of oropharyngeal cancer that is associated with HPV (Table 1).
A variety of assays have been used to assess the HPV status of head and neck cancers. In general, p16 has correlated well with HPV and is recognized as a robust, reproducible assay suitable for use in routine clinical care and clinical trials.9,11,12 Our study, which has examined p16 in conjunction with both HPV ISH and HPV PCR would support p16 as an effective surrogate for HPV. While most studies report a proportion of HPV-negative, p16-positive tumors,7 the high proportion identified with the commercially available HPV ISH assay used initially in our study probably reflects the low sensitivity of the assay. We were able to demonstrate that 79% of these p16-positive, HPV ISH –negative patients were actually HPV-positive by PCR, resulting in 86% of all p16-positive patients overall being confirmed to be HPV positive. While there are false positives with both p16 and HPV PCR, the combination of the two tests has been advocated by some.18 Our findings of overexpression of p16 in 60% of oropharyngeal cancer patients, with 52% being p16-positive and HPV-positive, are consistent with recent reports.7,9
The phase III trial—an analysis limited to patients who had received ≥ 60 Gy and radiotherapy without major deviations predicted to have an adverse impact on tumor control—demonstrated a trend for improved locoregional control in the tirapazamine arm (79% v 75% at 2 years; P = .07).15 Thus, we were interested to see whether there was any differential impact of the two treatment arms in the p16-positive and p16-negative groups. While there was no difference in the p16-positive group, there was a trend for improved locoregional control with tirapazamine in the p16-negative patients. This finding is consistent with the recent reanalysis of the DAHANCA trial of the hypoxic cell sensitizer nimorazole, which found that improved locoregional control when this drug was added to radiotherapy was restricted to the p16-negative patients.19 These results suggest that interventions targeting hypoxia may still be worth pursuing in HPV-negative head and neck cancer. On the basis of our previous work with hypoxic positron emission tomography imaging,20,21 the group most likely to benefit may be HPV-negative patients with hypoxic primary tumors.
In conclusion, our study clearly demonstrates that HPV-associated oropharyngeal cancer treated with a standard regimen of concurrent cisplatin and radiation has a superior outcome compared with HPV-negative oropharyngeal cancer. Furthermore, HPV (or p16) is the most important prognostic variable in multivariable analysis. Interpretation of past trials in oropharyngeal cancer may be confounded by the absence of information about HPV status; at the very least, HPV status must be included as a stratification factor in future trials. Separate trials in HPV-positive and HPV-negative oropharyngeal cancers will be required to determine the optimal treatment for each of these distinct entities. The focus in HPV-positive oropharyngeal cancer will be on determining whether the intensity of treatment and the consequential toxicity can be decreased without compromising the excellent outcomes currently achieved with chemoradiotherapy.
Acknowledgment
We thank Richard McCoy, PhD, at Gribbles Pathology for performing the human papillomavirus polymerase chain reaction assays.
Footnotes
See accompanying editorial on page 4103
Supported by a project grant from the Australian National Health and Medical Research Council and by Grant No. 1 R01 CA118582 from the National Institutes of Health.
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00094081.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Danny Rischin, Richard J. Young, Richard Fisher, Grant A. McArthur
Provision of study materials or patients: Danny Rischin, Quynh-Thu Le, Lester J. Peters, Brian O'Sullivan, Lizbeth M. Kenny, Grant A. McArthur
Collection and assembly of data: Danny Rischin, Richard J. Young, Richard Fisher, Stephen B. Fox, Ben Solomon, Grant A. McArthur
Data analysis and interpretation: Danny Rischin, Richard J. Young, Richard Fisher, Stephen B. Fox, Ben Solomon, Jimin Choi, Grant A. McArthur
Manuscript writing: Danny Rischin, Richard J. Young, Richard Fisher, Stephen B. Fox, Quynh-Thu Le, Lester J. Peters, Ben Solomon, Jimin Choi, Brian O'Sullivan, Lizbeth M. Kenny, Grant A. McArthur
Final approval of manuscript: Danny Rischin, Richard J. Young, Richard Fisher, Stephen B. Fox, Quynh-Thu Le, Lester J. Peters, Ben Solomon, Jimin Choi, Brian O'Sullivan, Lizbeth M. Kenny, Grant A. McArthur
REFERENCES
- 1.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 3.Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 4.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 6.Westra WH, Taube JM, Poeta ML, et al. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 7.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 8.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 9.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 10.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 12.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 13.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 14.Rischin D, Peters LJ, O'Sullivan B, et al. Tirapazamine, cisplatin and radiation versus cisplatin and radiation for advance squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial in the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28:2989–2995. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 15.Peters LJ, O'Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of head and neck cancer: Results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 16.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila Pa) 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillison ML, Harris J, Westra W, et al. Survival outcomes by tumor human papillomavirus (HPV) status in stage III-IV oropharyngeal cancer (OPC) in RTOG 0129. J Clin Oncol. 2009; 27(suppl; abstr 6003):301s. [Google Scholar]
- 18.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 19.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94:30–35. doi: 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Rischin D, Fisher R, Peters L, et al. Hypoxia in head and neck cancer: Studies with hypoxic positron emission tomography imaging and hypoxic cytotoxins. Int J Radiat Oncol Biol Phys. 2007;69:S61–S63. doi: 10.1016/j.ijrobp.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: A substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]