Abstract
Purpose
To estimate the maximum-tolerated dose, dose-limiting toxicities (DLTs), and pharmacokinetic properties of lapatinib, a selective epidermal growth factor receptor (EGFR) and ERBB2 inhibitor, in children with refractory or recurrent CNS malignancies.
Patients and Methods
Lapatinib was administered orally twice daily at escalating doses starting at 300 mg/m2 to patients who were not (stratum I) or were (stratum II) receiving steroids. Pharmacokinetic studies were performed during the first two courses. Expression of the four ERBB receptors and downstream signaling elements in tumor tissue was evaluated by immunohistochemistry.
Results
Fifty-nine patients were enrolled (stratum I, n = 32; stratum II, n = 27). Of 29 patients evaluable for toxicity in stratum I, one experienced a DLT (diarrhea) at 520 mg/m2 twice daily, and all three receiving 1,150 mg/m2 twice daily experienced DLTs (one each of rash, diarrhea, and fatigue). Two of 21 patients evaluable for toxicity in stratum II experienced DLTs of rash at 900 mg/m2 twice daily. Lapatinib dosage was related linearly to area under the [concentration-time] curve from start time to 12 hours later (AUC0-12) and dose-normalized maximum serum concentration and AUC values for patients in stratum II were both significantly higher (P = .001) than those for patients in stratum I. Frequent, high-level expression of activated (phosphorylated) EGFR and ERBB2 receptors and downstream signal intermediates were observed in tumors, particularly in ependymomas that displayed prolonged stable disease on lapatinib therapy.
Conclusion
Lapatinib is well tolerated in children with recurrent CNS malignancies, with rash, diarrhea, and fatigue identified as DLTs. The recommended phase II dose, regardless of steroid use, is 900 mg/m2 twice daily.
INTRODUCTION
Aberrant cell signaling via the four members of the epidermal growth factor receptor (EGFR) family (also called ERBB receptors) has been implicated as a fundamental mediator of tumorigenesis, and they may serve as targets for novel therapies.1–3 EGFR, ERBB2, ERBB3, and ERBB4 interact to form a complex signaling network of transmembrane homo- and heterodimers.3–6 Receptor dimerization promotes autophosphorylation and triggers downstream signaling via the mitogen-activated protein kinase (MAPK), phosphatidylinositol 3–kinase (PI3K)/AKT, and signal transducers and activator of transcription (STAT) pathways. Amplification, mutation, and/or overexpression of various members of the EGFR receptor family have been reported in aggressive forms of a variety of cancers including breast, non–small-cell lung, head and neck, and colon cancer and glioblastoma.5,7–9 We have reported that ERBB2 and ERBB4 are highly expressed in aggressive forms of medulloblastoma10 and ependymoma,11 and EGFR is amplified and overexpressed in brainstem glioma.12 These observations have led to efforts to develop pharmacologic inhibitors of EGFR and ERBB2 receptors, including humanized anti-ERBB2 monoclonal antibodies (eg, trastuzumab13 and pertuzumab14), small-molecule inhibitors of the EGFR tyrosine kinases (eg, erlotinib15 and gefitinib16), and combined EGFR and ERBB2 inhibitors (eg, lapatinib17).
Lapatinib, a member of the 4-anilinoquinazoline class of tyrosine kinase inhibitors, blocks the EGFR and ERBB2 tyrosine kinase with an IC50 [concentration that causes 50% inhibition of growth] of 10 nmol/L (6 ng/mL) and the ERBB4 tyrosine kinase at a higher concentration. Lapatinib has demonstrated activity against breast as well as head and neck carcinoma xenografts17–19 and is approved in combination with capecitabine for the treatment of ERBB2-positive advanced breast cancer. Its main toxicities are rash, diarrhea, fatigue, and nausea20,21 with recommended doses of 1,500 mg (approximately 880 mg/m2) once a day or 500 to 750 mg twice a day. Published data indicate that lapatinib can penetrate brain tumor tissue.22 In one study in patients with progressive glioblastoma multiforme,22 in which patients were pretreated with lapatinib for 7 to 10 days before resection, lapatinib was shown to have significant uptake in glioma tissue with an average tumor to plasma ratio of 13:1 (range, 0.65 to 39; n = 15). Moreover, lapatinib has demonstrated modest activity against CNS metastases from breast cancer.23,24
We report the results of a phase I trial of lapatinib in children with recurrent or refractory malignant CNS tumors. The primary objectives were to estimate the maximum-tolerated dose (MTD) and to describe the dose-limiting toxicities (DLTs) of lapatinib administered twice daily continuously for 28 days when patients were stratified on the basis of steroid use (stratum I: no steroids; stratum II: receiving steroids). The secondary objectives were to characterize lapatinib plasma pharmacokinetics, to assess the effect of steroids on lapatinib pharmacokinetics, and to determine the incidence of EGFR, ERBB2, ERBB3, and ERBB4 expression and pathway activation in children with recurrent or refractory CNS malignancies.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients were age ≤ 21 years with a histologically verified malignant CNS tumor (histology was not required for diffuse intrinsic pontine gliomas) that was refractory to conventional therapy and had a Lansky or Karnofsky performance score ≥ 50. Patients were required to have recovered from the acute toxic effects of prior therapy and not to have received any of the following: growth factors within 2 weeks of study entry, myelosuppressive chemotherapy within 3 weeks (6 weeks if prior nitrosourea or mitomycin therapy), craniospinal or total-body irradiation within 3 months, local radiotherapy to the primary tumor within 4 weeks, or focal irradiation to symptomatic metastatic sites within 2 weeks. Patients who were receiving enzyme-inducing anticonvulsants at the time of registration were excluded, as were pregnant or lactating women or patients with uncontrolled infections. Patients who had received CYP3A4 inducers within 7 days or CYP3A4 inhibitors (with the exception of steroids) within 14 days before registration were excluded from the study. Patients in stratum II who were taking corticosteroids must have had a stable or decreasing dose for ≥ 1 week before registration. Other requirements included adequate bone marrow (peripheral absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 100,000/μL, transfusion independent hemoglobin ≥ 8.0 g/dL), renal (serum creatinine ≤ 1.5× upper limit of normal [ULN] for age, or glomerular filtration rate ≥ 70 mL/min/1.73 m2), liver (total bilirubin ≤ 1.5× institutional ULN for age, ALT ≤ 2.5× institutional ULN for age, and albumin ≥ 2 g/dL), cardiac (shortening fraction ≥ 27% by echocardiogram or left ventricular ejection fraction > 50% by gated radionuclide study), and pulmonary (no evidence of dyspnea at rest, no exercise intolerance, and a pulse oximetry > 94% if there was clinical indication for determination) function. Patients had to have displayed stable neurologic deficits for at least 1 week. Informed consent was obtained from patients, parents, or guardians, and assent was obtained as appropriate at the time of protocol enrollment. The institutional review boards of each Pediatric Brain Tumor Consortium (PBTC) institution approved the protocol before initial patient enrollment, and continuing approval was maintained throughout the study.
Drug Administration and Study Design
Lapatinib, supplied by the Cancer Therapy Evaluation Program (National Cancer Institute [NCI], Bethesda, MD) as a 250-mg oval film-coated tablet, was administered orally twice daily. Each course was 28 days long. Tablets could be cut in half; total daily doses were rounded to the nearest 125 mg. For patients who had difficulty swallowing, lapatinib tablets were added to 2 to 4 oz of water or Kool-Aid or 3 oz of chocolate milk and stirred to form a suspension. A dosing nomogram based on body surface area and dose level (rounded to the nearest 125 mg) was used to minimize interpatient dosing variability. The starting lapatinib dosage was 300 mg/m2 twice daily (approximately 70% of the adult recommended dose of up to 750 mg orally twice daily). Dose levels for subsequent patient cohorts were escalated in 30% increments after at least two patients were treated and monitored for one course at each dose level. Patients could receive up to 26 courses in the absence of disease progression.
The MTD, which was defined as the dose level at which 25% of patients were expected to experience a DLT, was estimated via the modified continual reassessment method (CRM).25 The CRM is comparable to the traditional phase I design in terms of study duration and proportion of patients treated at a dose greater than the MTD; however, it can minimize the frequency and duration of unnecessary accrual closures and makes dose escalation/de-escalation decisions on the basis of the actual doses received. This latter point is important when flexibility of dosing is limited by pill size. Toxicities were graded according to the NCI Common Toxicity Criteria version 3.0.
Hematologic DLT was defined as grade 4 neutropenia or grade 3 or 4 thrombocytopenia related to lapatinib. Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity with the specific exclusion of grade 3 nausea and vomiting controlled with adequate antiemetics; grade 3 fever or infection; grade 3 diarrhea responsive to optimal use of loperamide; or grade 3 or 4 hypokalemia, hypophosphatemia or hypomagnesemia that resolved to grade ≤ 2 by supplementation within 7 days. Any grade 2 nonhematologic toxicity that persisted for > 7 days and was considered sufficiently medically significant or sufficiently intolerable by patients to warrant treatment interruption and/or dose reduction was considered dose-limiting.
Pretreatment evaluations included a history, physical examination, performance status, disease evaluation, CBC, electrolyte measurement, renal and liver function tests, pregnancy test for female patients of childbearing age, and echocardiogram or multigated acquisition scan. CBCs were obtained weekly during course 1, every 2 weeks during course 2, and before each subsequent course. History, physical examinations, and serum chemistries were obtained weekly in course 1 and before each subsequent course. Echocardiogram or multigated angiogram was obtained at the end of course 2 and every 12 weeks thereafter.
Disease evaluations were obtained at baseline, after course 2, and for every other course thereafter. Tumor response was defined as follows: complete response, disappearance of all measurable lesions on magnetic resonance imaging; partial response, ≥ 50% reduction in tumor size by bidimensional measurement on a stable or decreasing dose of corticosteroids accompanied by a stable or improving neurologic examination and maintained for at least 6 weeks; progressive disease, worsening neurologic status or > 25% increase in the bidimensional measurement, appearance of new lesions, or increasing corticosteroids doses; and stable disease (SD), magnetic resonance imaging response not meeting the criteria for other categories with stable neurologic examination and corticosteroid dose.26
Pharmacokinetic Studies
Pharmacokinetic studies were performed in consenting patients with the first dose of courses 1 and 2. Serial whole blood samples (2 mL) were collected in heparinized tubes before the dose, and at 0.5, 1, 1.5, 3, 6, and 8 hours after administration. Plasma samples were prepared using solid-phase extraction, and their lapatinib concentrations were analyzed by the liquid chromatography electrospray ionization tandem mass spectrometry method.27 The lower limit of quantitation of lapatinib was 15 ng/mL, the interday coefficient of variation was ≤ 7%, and the intraday coefficient of variation was ≤ 3%.
Lapatinib concentration-time data were modeled by nonlinear mixed effects modeling as implemented in nonlinear mixed effects modeling (NONMEM) software (version V, double precision level 1.1) using the first-order conditional estimation (FOCE) method with interaction (FOCE-INTER).28 The base model describing lapatinib pharmacokinetics was a one-compartment model with first-order elimination (ADVAN 2). After estimation of the population parameters, individual pharmacokinetic parameters were obtained by using a post hoc analysis. Estimated pharmacokinetic parameters included apparent oral clearance (Cl/F) and apparent volume of distribution (V/F) where F is the bioavailability factor and absorption rate constant (ka). The model parameters for each patient in each course were used to simulate the plasma concentration-time points from which the area under the [concentration-time] curve (AUC0→12) was calculated using the log-linear trapezoidal method.29 Maximum plasma concentration (Cmax) and the time to maximum plasma concentration (tmax) were determined by visual inspection of the data. Log-transformed dose-normalized AUC0→12, Cmax, and ka were compared between steroid use strata by using analysis of variance with strata as a fixed effect.
ERBB Receptor Expression and Signal Activity
Expression and activation of the ERBB signaling network was analyzed in pretreatment and relapse tumor samples available from trial patients by using standard immunohistochemical (IHC) techniques exactly as described previously.10,11
RESULTS
Patient Characteristics
Fifty-nine patients were enrolled on the study; 32 on stratum I (no steroids) and 27 on stratum II (receiving steroids). The distribution of age, sex, and diagnoses were similar between the two strata. Table 1 summarizes the characteristics of the eligible patients. Fifty patients (29 in stratum I, 21 in stratum II) were evaluable for toxicity. Nine patients were not evaluable for toxicity for the following reasons: lapatinib was not administered because of worsening medical condition (n = 1), withdrawal of consent before treatment (n = 2), failure to complete course 1 because of consent withdrawal (n = 1), insufficient drug dosing (n = 2), or progressive disease (n = 3). The median number of courses in both strata was two (range, one to 26).
Table 1.
Characteristics of Eligible Patients (N = 59)
| Characteristic | No. of Patients in Stratum I(n = 32) | No. of Patients in Stratum II(n = 27) |
|---|---|---|
| Male:female ratio | 14:18 | 16:11 |
| Age, years | ||
| Median | 9.3 | 9.7 |
| Range | 1.2-20.9 | 1.1-21.2 |
| Diagnosis | ||
| Astrocytoma (not otherwise specified) | 0 | 1 |
| Anaplastic ganglioglioma | 0 | 1 |
| Brain stem glioma | 2 | 8 |
| Ependymoma | 14 | 2 |
| High-grade glioma (glioblastoma multiforme, anaplastic astrocytoma) | 5 | 7 |
| Gliomatosis cerebri | 1 | 0 |
| Medulloblastoma/primitive neuroectodermal tumor | 9 | 6 |
| Pineoblastoma | 0 | 1 |
| Pleomorphic xanthoastrocytoma | 0 | 1 |
| Atypical teratoid rhabdoid tumor | 1 | 0 |
| Prior therapy | ||
| Chemotherapy only | 2 | 4 |
| Radiotherapy only | 2 | 1 |
| Chemotherapy and radiotherapy | 26 | 21 |
| Chemotherapy, radiotherapy, and stem-cell transplantation | 2 | 1 |
| Courses of lapatinib | ||
| Median | 2 | 2 |
| Range | 1-26 | 1-26 |
Toxicity
The observed DLTs are summarized in Table 2. In stratum I, one patient experienced grade 3 diarrhea at 520 mg/m2 twice daily. Accrual of an expanded cohort at the planned maximum dose of 700 mg/m2 twice daily showed no further DLTs in either stratum; therefore, two additional dose levels (900 and 1,150 mg/m2 twice daily) were added. In stratum I at 1,150 mg/m2 twice daily, all three patients experienced DLTs (rash, diarrhea, and fatigue). No DLTs were observed at 900 mg/m2 twice daily in six patients, making this the recommended MTD for this stratum. In stratum II, dose levels of 300 to 700 mg/m2 twice daily were well tolerated; however, at 900 mg/m2 twice daily, one of three patients had a DLT of grade 3 rash. The cohort was expanded to enroll three more evaluable patients among whom another patient experienced a DLT of grade 3 rash. The CRM-estimated MTD was 905.11 mg/m2 twice daily; hence, this dose was declared the MTD for patients taking steroids (stratum II). Appendix Table A1 (online only) summarizes all adverse events at least possibly attributable to lapatinib in the 50 patients evaluable for toxicity according to stratum.
Table 2.
DLT Summary for Course 1
| Stratum | Dose (mg/m2)* | No. of Patients Entered | No. of Assessable Patients | No. of Patients With DLT | Grade 3 DLT |
|---|---|---|---|---|---|
| I ( no steroids) | |||||
| 300 | 4 | 3 | 0 | ||
| 400 | 3 | 3 | 0 | ||
| 520 | 9 | 7 | 1 | Diarrhea (n = 1) | |
| 700 | 7 | 7 | 0 | ||
| 900 | 6 | 6 | 0 | ||
| 1,150 | 3 | 3 | 3 | Diarrhea (n = 2), rash (n = 1), fatigue (n = 1) | |
| II (receiving steroids) | |||||
| 300 | 3 | 2 | 0 | ||
| 400 | 3 | 3 | 0 | ||
| 520 | 3 | 2 | 0 | ||
| 700 | 9 | 8 | 0 | ||
| 900 | 9 | 6 | 2 | Rash (n = 2) |
Abbreviation: DLT, dose-limiting toxicity.
Dose administered twice daily.
Responses
No objective responses were reported. In stratum I, prolonged SD (≥ four courses of therapy) was observed in four patients with ependymoma (four to 26 courses) and one patient each with glioblastoma multiforme (four courses), anaplastic astrocytoma (four courses) and a primitive neuroectodermal tumor (four courses). In stratum II, six patients experienced prolonged SD (four to 26 courses): one patient each with anaplastic astrocytoma (four courses), diffuse intrinsic pontine glioma (four courses), pineoblastoma (four courses), pleomorphic xanthoastrocytoma (four courses), medulloblastoma (26 courses), and ependymoma (26 courses).
Pharmacokinetics
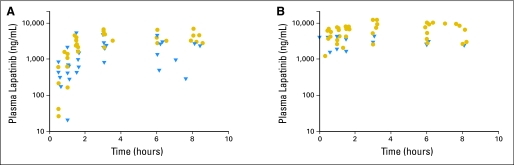
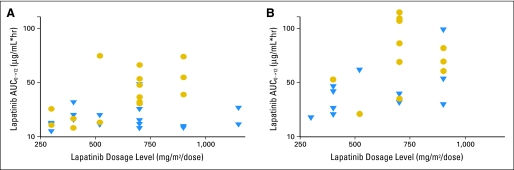
Pharmacokinetic studies were performed on 35 consenting patients, but only 33 were evaluable for pharmacokinetic modeling (ie, there were too few samples in two patients). Of these 33 patients, 21 had repeat studies during the second course. Depicted in Figure 1 are the lapatinib plasma concentration-time data for patients given the 700 mg/m2 twice daily dose with the best-fit line from model-predicted parameters. A summary of the pharmacokinetic parameters determined during courses 1 and 2 is presented in Table 3. The median (range) lapatinib apparent Cl/F values for courses 1 and 2 were 18.3 L/h/m2 (range, 4.6 to 142.0 L/h/m2) and 11.3 L/h/m2 (range, 5.8 to 27.5 L/h/m2), respectively. As depicted in Figure 2, the lapatinib AUC0→12 increases with increasing lapatinib dose.
Fig 1.
Lapatinib concentration-time data for patients studied at the 700 mg/m2 twice daily dose level. Pharmacokinetic studies for course 1 day 1 (A) and course 2 day 1 (B). Circles indicate patients receiving steroids; triangles indicate patients not receiving steroids.
Table 3.
Summary of Lapatinib Pharmacokinetic Parameters in Relation to Lapatinib Dose for Day 1 of Courses 1 and 2
| Variable | Lapatinib Dose (mg/m2) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 300 |
400 |
520 |
700 |
900 |
||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| Course 1, day 1 | ||||||||||
| No. of patients | 5 | 5 | 4 | 12 | 5 | |||||
| Actual dose, mg/m2 | 379 | 313-399 | 457 | 385-532 | 619 | 493-688 | 726 | 379-926 | 921 | 893-987 |
| Cmax, μg/mL | 1.9 | 0.7-2.9 | 2.4 | 1.2-3.9 | 2.1 | 1.3-12.5 | 3.9 | 0.8-6.9 | 4.3 | 1.5-7.5 |
| tmax, hours | 3.0 | 1.5-8.0 | 3.1 | 2.7-7.9 | 3.1 | 3.0-6.0 | 3.3 | 1.5-8.1 | 8.0 | 3.2-8.1 |
| AUC0-12, μg/mL × hours | 12.1 | 5.0-26.2 | 17.3 | 8.7-31.7 | 17.0 | 11.1-75.3 | 31.9 | 7.9-66.7 | 39.2 | 8.3-74.4 |
| 300 | 400 | 500 | 700 | 900 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Course 2, day 1 | ||||||||||
| No. of patients | 2 | 5 | 2 | 8 | 4 | |||||
| Actual dosage mg/m2 | 324 | 323-326 | 457 | 385-532 | 582 | 507-658 | 707 | 383-813 | 919 | 899-987 |
| Cmax, μg/mL | 2.3 | 1.9-2.7 | 4.1 | 3.1-6.5 | 4.5 | 2.3-6.6 | 7.1 | 2.9-12.1 | 6.2 | 3.1-10.3 |
| tmax, hours | 2.3 | 1.5-3.0 | 3.0 | 1.0-3.2 | 3.3 | 0.5-6.0 | 6.0 | 1.5-8.1 | 5.6 | 3.0-6.2 |
| AUC0-12, μg/mL × hours | 15.2 | 15.2–N/A | 37.5 | 18.1-48.0 | 37.5 | 19.5-55.4 | 70.4 | 28.5-104.3 | 55.2 | 26.2-88.8 |
NOTE. The pharmacokinetic parameters for the two patients studied at the 1,150 mg/m2 dosage level on course 1 day 1 for lapatinib were Cmax = 2.4 and 3.2 μg/mL, tmax = 3.1 and 3.1, and AUC0-12 = 11.5 and 26.7 μg/mL × hours.
Abbreviations: Cmax, maximum plasma concentration; tmax, time to maximum plasma concentration; AUC, area under the [plasma concentration-time] curve; N/A, not available.
Fig 2.
Relation between lapatinib dosage and AUC0→12 for course 1 day 1 (A) and course 2 day 1 (B). Circles indicate area under the (concentration-time) curve (AUC) values for patients receiving dexamethasone; triangles indicate AUC values for patients not receiving dexamethasone.
The effect of dexamethasone was evaluated in 31 patients studied during course 1 (two patients treated at 1,150 mg/m2 in stratum I were omitted because no patients were studied at that dose level in stratum II), and in 18 patients during course 2 (three patients had their dexamethasone status change from course 1 to course 2 and were omitted from the analysis). During course 1, the dose-normalized Cmax and AUC0-12 were 1.9- and 2.1-fold greater in patients treated with dexamethasone than in those not receiving dexamethasone (P = .001 for both comparisons). Interestingly, ka in the dexamethasone-treated group (stratum II) was more rapid compared with that in patients not receiving dexamethasone (eg, at the MTD of 900 mg/m2, estimated geometric means for strata I and II were 0.19 and 0.35 hours−1). During course 2, the dose-normalized AUC0-12 was 1.5-fold greater in the patients treated with dexamethasone than in those not treated with dexamethasone, but the difference was not statistically significant (P = .08).
ERBB Receptor Expression and Signal Activity
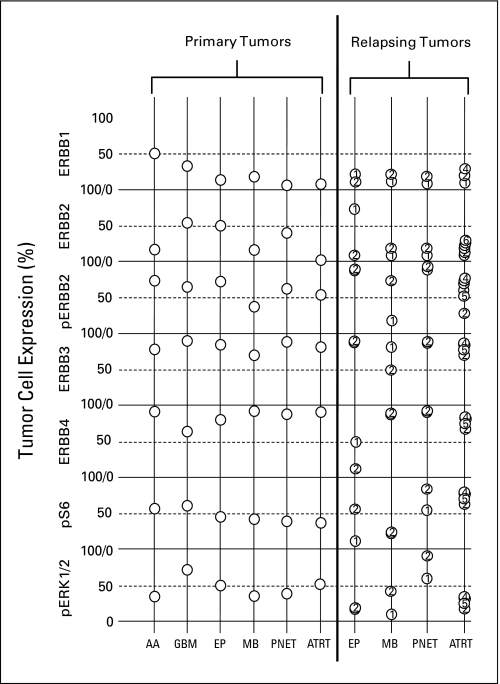
Pretrial tumor samples were available from 25 patients, including in four patients (one each with ependymoma, medulloblastoma, primitive neuroectodermal tumor [PNET], and atypical teratoid/rhabdoid tumor [AT/RT] with primary and at least one subsequent relapsed sample. Patterns of expression of the ERBB receptors and downstream mediators among these samples are summarized in Figure 3. IHC analysis demonstrated percentages of cells expressing the ERBB receptors similar to those reported by us30 in a prior analysis of a similar phase I population that included ependymoma and medulloblastoma patients. Although limited by number of samples, EGFR does appear to be expressed by a higher percentage of glioma cells than other types of brain tumor cells. In general, detectable levels of phosphorylated ERBB2 paralleled those of the total receptor. ERBB3 and ERRB4 were highly expressed in all pediatric brain tumors with little difference among the various histologic tumor types.
Fig 3.
The left side of the graph displays the average percentage expression of ERBB receptors and downstream signaling elements for patients with primary tumors (anaplastic astrocytoma [AA; n = 2]; glioblastoma multiforme [GBM; n = 4]; ependymoma [EP; n = 10]; medulloblastoma [MB; n = 5]; primitive neuroectodermal tumor [PNET; n = 3]; and atypical teratoid/rhabdoid tumor [ATRT; n = 1]). The right side of the graph displays expression in primary (1) and subsequent relapses numbered chronologically.
DISCUSSION
This pediatric phase I trial establishes the MTD of lapatinib as 900 mg/m2 orally twice daily, regardless of steroid use. Similar to findings in adult studies, observed DLTs included diarrhea, rash, and fatigue. Although no objective responses were reported, 12 patients, including five with ependymoma, experienced prolonged SD (four to 26 courses).
To the best of our knowledge, this is the first description of lapatinib disposition in children with cancer and one of the few reports of lapatinib pharmacokinetics using a twice-daily dosing schedule. The disposition of lapatinib in children was similar to that reported in adults receiving twice-daily lapatinib.31 As observed in adults, the maximum lapatinib plasma concentration and AUC0-12 increased with dosage. Interpatient variability was significant with an approximate five-fold variation in apparent oral clearance at steady state (5.8 to 27.5 L/h/m2). It is difficult to directly compare our pharmacokinetic data with published adult data, since the twice-daily dosing regimen has been reported to lead to an increased systemic exposure (AUC) for the same total dose given once daily.32 However, our AUC values are greater than those reported from once-daily body surface area–normalized adult doses.20,33,34
Since many children with CNS tumors are treated with steroids, we assessed the effect of steroids on lapatinib disposition. The dose-normalized Cmax and AUC values for patients treated with dexamethasone were significantly higher than those for patients not receiving dexamethasone. Since dexamethasone is a well-established inducer of the cytochrome P450 (CYP) 3A subfamily35 and since lapatinib undergoes extensive metabolism, primarily by CYP3A4 or CYP3A5, with minor contributions from CYP2C19 and CYP2C8, we expected that lapatinib systemic exposure in the dexamethasone-treated group would be lower. Although the study was designed to evaluate the effect of dexamethasone on lapatinib pharmacokinetics, it was clearly not designed to elucidate the mechanism of this interaction. So whether the mechanism is a direct effect of dexamethasone on lapatinib disposition (eg, absorption, metabolism, or elimination) or an indirect mechanism (eg, patients on dexamethasone have increased appetite and increased oral intake leading to an increased oral bioavailability) cannot be determined from this study. However, this interaction could be viewed as a positive one from a therapeutic standpoint, leading to increased lapatinib systemic exposure and possibly therapeutic effect.
IHC analyses confirmed frequent and high-level expression of the EGFR family and active downstream signal intermediates in pediatric brain tumors at a level similar to that previously observed.30 Importantly, expression levels of the ERBB receptors and downstream signaling intermediates remained remarkably stable from primary CNS tumor through relapse and reflected levels observed across the phase I population. Thus, future phase I or II trials of ERBB inhibitors are unlikely to be confounded by variability of brain tumor ERBB receptor levels among eligible patients.
This study demonstrates that lapatinib is well tolerated in children and may induce prolonged disease stabilization in some patients with recurrent CNS malignancies. Although lapatinib showed little activity in the Pediatric Preclinical Testing Program in vitro and in vivo panels,36 lapatinib has demonstrated synergy with agents such as capecitabine37 and bevacizumab38 in patients with breast cancer. Moreover, we have previously demonstrated that depletion of blood vessels from orthotopic brain tumor xenografts using bevacizumab can ablate self-renewing cells from tumors and arrest tumor growth.39 On the basis of these data, a phase II trial of lapatinib and bevacizumab is currently being conducted in children with recurrent ependymoma.
Acknowledgment
We acknowledge the clinical research assistant support of Helen Gallagher and Christopher Smith and the technical assistance of Inga Luckett and Radhika Thiruvenkatam.
Appendix
Table A1.
All Toxicities Attributed to Therapy
| Toxicity | Stratum I |
Stratum II |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Total |
Grade |
Total |
|||||||||||||||
| 1 |
2 |
3 |
1 |
2 |
3 |
4 |
||||||||||||
| No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | No. of Events | No. of Patients | |
| Diarrhea | 26 | 17 | 12 | 10 | 7 | 5 | 45 | 22 | 15 | 14 | 6 | 6 | 2 | 2 | 23 | 17 | ||
| Vomiting | 13 | 12 | 2 | 2 | 15 | 13 | 11 | 6 | 11 | 6 | ||||||||
| Nausea | 10 | 9 | 1 | 1 | 11 | 9 | 5 | 4 | 1 | 1 | 6 | 5 | ||||||
| Flatulence | 3 | 3 | 3 | 3 | ||||||||||||||
| Constipation | 1 | 1 | 1 | 1 | ||||||||||||||
| Anorexia | 3 | 3 | 1 | 1 | 4 | 4 | 2 | 2 | 1 | 1 | 3 | 3 | ||||||
| Heartburn/dyspepsia | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | ||||||||||
| Distension/bloating, abdominal | 1 | 1 | 1 | 1 | ||||||||||||||
| Mucositis/stomatitis | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||||||||||
| Fatigue (asthenia, lethargy, malaise) | 10 | 9 | 1 | 1 | 1 | 1 | 12 | 11 | 6 | 6 | 2 | 2 | 8 | 8 | ||||
| Weight loss | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Neutrophils/granulocytes (ANC/AGC) | 2 | 1 | 1 | 1 | 3 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 5 | 4 | ||||
| Lymphopenia | 9 | 5 | 2 | 2 | 2 | 2 | 13 | 8 | 12 | 5 | 8 | 5 | 10 | 5 | 1 | 1 | 31 | 10 |
| Leukocytes (total WBC) | 7 | 5 | 7 | 5 | 10 | 5 | 3 | 3 | 2 | 2 | 15 | 7 | ||||||
| Platelets | 1 | 1 | 1 | 1 | 8 | 5 | 2 | 1 | 1 | 1 | 1 | 1 | 12 | 5 | ||||
| Hemoglobin | 9 | 8 | 2 | 2 | 11 | 10 | 9 | 7 | 5 | 2 | 2 | 1 | 16 | 7 | ||||
| Hypernatremia | 2 | 2 | 2 | 2 | ||||||||||||||
| Hyponatremia | 5 | 4 | 1 | 1 | 6 | 5 | ||||||||||||
| Hypocalcemia | 6 | 6 | 6 | 6 | ||||||||||||||
| Hypercalcemia | 1 | 1 | 1 | 1 | ||||||||||||||
| Hypokalemia | 9 | 7 | 1 | 1 | 10 | 7 | 7 | 6 | 1 | 1 | 2 | 1 | 10 | 8 | ||||
| Hyperkalemia | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | ||||||||||
| Hypophosphatemia | 3 | 3 | 3 | 3 | 8 | 6 | 2 | 2 | 10 | 8 | ||||||||
| ALT | 8 | 6 | 8 | 6 | 6 | 5 | 4 | 2 | 10 | 7 | ||||||||
| AST | 7 | 6 | 7 | 6 | 7 | 3 | 1 | 1 | 8 | 4 | ||||||||
| Hypermagnesemia | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | ||||||||||
| Hypomagnesemia | 1 | 1 | 1 | 1 | ||||||||||||||
| Hyperglycemia | 2 | 2 | 2 | 2 | 5 | 5 | 5 | 5 | ||||||||||
| Hypoglycemia | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||||
| Hypoalbuminemia | 2 | 2 | 2 | 2 | 6 | 5 | 3 | 2 | 9 | 7 | ||||||||
| Serum bicarbonate, low | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||||||||||
| Hyperuricemia | 3 | 2 | 3 | 2 | ||||||||||||||
| Hyperbilirubinemia | 2 | 1 | 2 | 1 | 5 | 3 | 5 | 3 | ||||||||||
| High creatinine | 1 | 1 | 1 | 1 | ||||||||||||||
| Alkaline phosphatase | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Proteinuria | 1 | 1 | 1 | 1 | ||||||||||||||
| PTT | 1 | 1 | 1 | 1 | ||||||||||||||
| Rash/desquamation | 7 | 7 | 2 | 2 | 9 | 9 | 7 | 6 | 1 | 1 | 8 | 7 | ||||||
| Rash, acne/acneiform | 2 | 2 | 3 | 3 | 1 | 1 | 6 | 5 | 3 | 2 | 3 | 2 | 1 | 1 | 7 | 3 | ||
| Pruritus/itching | 2 | 2 | 1 | 1 | 3 | 3 | ||||||||||||
| Flushing | 1 | 1 | 3 | 3 | 4 | 4 | 1 | 1 | 1 | 1 | ||||||||
| Dry skin | 2 | 2 | 2 | 2 | ||||||||||||||
| Ulceration | 1 | 1 | 1 | 1 | ||||||||||||||
| Bruising (in absence of Grade 3 or 4 thrombocytopenia) | 2 | 2 | 2 | 2 | ||||||||||||||
| Dermatology/skin, legs much cooler than rest of body | 1 | 1 | 1 | 1 | ||||||||||||||
| Dermatology/skin, local irritation | 1 | 1 | 1 | 1 | ||||||||||||||
| Hair loss/alopecia (scalp or body) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Nail changes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Hematuria | 1 | 1 | 1 | 1 | ||||||||||||||
| Hemorrhage, CNS | 1 | 1 | 1 | 1 | 2 | 2 | ||||||||||||
| Hemorrhage, pulmonary/upper respiratory (nose) | 6 | 4 | 6 | 4 | 2 | 2 | 2 | 2 | ||||||||||
| Allergic reaction/hypersensitivity (including drug fever) | 1 | 1 | 1 | 1 | ||||||||||||||
| Allergic rhinitis (including sneezing, nasal stuffiness, postnasal drip) | 5 | 5 | 5 | 5 | ||||||||||||||
| Cough | 2 | 2 | 2 | 2 | 3 | 1 | 3 | 1 | ||||||||||
| Hypoxia | 1 | 1 | 1 | 1 | ||||||||||||||
| Pain (abdomen, head/headache, chest/thorax, throat/pharynx/larynx, stomach, other) | 13 | 11 | 1 | 1 | 14 | 12 | 7 | 5 | 1 | 1 | 8 | 6 | ||||||
| Edema: head and neck, limb | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | ||||||||
| Infection with normal ANC or grade 1 or 2 neutrophils | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 2 | ||||||||
| Infection (documented clinically or microbiologically) with grade 3 or 4 neutrophils (ANC < 1.0 × 109/L; urinary tract NOS) | 1 | 1 | 1 | 1 | ||||||||||||||
| Dizziness | 2 | 2 | 2 | 2 | ||||||||||||||
| Neuropathy: motor | 2 | 2 | 2 | 2 | ||||||||||||||
| Taste alteration (dysgeusia) | 2 | 2 | 2 | 2 | ||||||||||||||
| Seizure | 1 | 1 | 1 | 1 | ||||||||||||||
| Urinary frequency/urgency | 1 | 1 | 1 | 1 | ||||||||||||||
| Dysuria | 1 | 1 | 1 | 1 | ||||||||||||||
| Incontinence, urinary | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Obstruction, genitourinary (ureter) | 1 | 1 | 1 | 1 | ||||||||||||||
| Left ventricular hypertrophy with normal function | 1 | 1 | 1 | 1 | ||||||||||||||
| Watery eye (epiphora, tearing) | 1 | 1 | 1 | 1 | ||||||||||||||
| Ocular surface disease | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| Vision-blurred vision | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
Abbreviations: ANC, absolute neutrophil count; AGC, absolute granulocyte count; PTT, partial thromboplastin time; NOS, not otherwise stated.
Footnotes
Supported in part by Grant No. U01 CA81457 from the National Institutes of Health to the Pediatric Brain Tumor Consortium and by the American Lebanese Syrian Associated Charities.
Presented in part at the 13th Annual International Society on Pediatric Neuro-Oncology, June 29-July 2, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00095940.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Maryam Fouladi, Clinton F. Stewart, Arzu Onar-Thomas, Roger J. Packer, Amar Gajjar, Larry E. Kun, James M. Boyett, Richard J. Gilbertson
Administrative support: Amar Gajjar, Larry E. Kun, James M. Boyett
Provision of study materials or patients: Maryam Fouladi, Susan M. Blaney, Amar Gajjar
Collection and assembly of data: Maryam Fouladi, Clinton F. Stewart, Richard J. Gilbertson
Data analysis and interpretation: Maryam Fouladi, Clinton F. Stewart, Susan M. Blaney, Arzu Onar-Thomas, Paula Schaiquevich, Roger J. Packer, Amar Gajjar, James M. Boyett, Richard J. Gilbertson
Manuscript writing: Maryam Fouladi, Clinton F. Stewart, Susan M. Blaney, Arzu Onar-Thomas, Roger J. Packer, Amar Gajjar, Larry E. Kun, James M. Boyett, Richard J. Gilbertson
Final approval of manuscript: Maryam Fouladi, Clinton F. Stewart, Susan M. Blaney, Arzu Onar-Thomas, Paula Schaiquevich, Roger J. Packer, Amar Gajjar, Larry E. Kun, James M. Boyett,Richard J. Gilbertson
REFERENCES
- 1.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Burgess AW, Cho HS, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 4.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 6.Linggi B, Carpenter G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 8.Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 9.Egloff AM, Grandis JR. Targeting epidermal growth factor receptor and SRC pathways in head and neck cancer. Semin Oncol. 2008;35:286–297. doi: 10.1053/j.seminoncol.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajjar A, Hernan R, Kocak M, et al. Clinical, histopathologic, and molecular markers of prognosis: Toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson RJ, Bentley L, Hernan R, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- 12.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 13.Hudziak RM, Lewis GD, Winget M, et al. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 16.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: A review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 17.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 18.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 19.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 20.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 21.Burris HA., 3rd Dual kinase inhibition in the treatment of breast cancer: Initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist. 2004;9(suppl 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn J, Robins I, Mehta M, et al. Tumor sequestration of lapatinib (NABTC 04-01) Neuro Oncol. 2008;10:759–915. abstr ET-05. [Google Scholar]
- 23.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 24.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onar A, Kocak M, Boyett JM. Continual reassessment method vs. traditional empirically based design: Modifications motivated by Phase I trials in pediatric oncology by the Pediatric Brain Tumor Consortium. J Biopharm Stat. 2009;19:437–455. doi: 10.1080/10543400902800486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieran MW, Packer RJ, Onar A, et al. Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: A Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2007;25:3137–3143. doi: 10.1200/JCO.2006.09.4243. [DOI] [PubMed] [Google Scholar]
- 27.Bai F, Freeman BB, 3rd, Fraga CH, et al. Determination of lapatinib (GW572016) in human plasma by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:169–175. doi: 10.1016/j.jchromb.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Beal SL. San Francisco, CA: 1998. NONMEM Users' Guide Parts I-VIII. [Google Scholar]
- 29.D'Argenio DZ, Schumitzky A. Los Angeles, CA: Biomedical Simulations Resource, University of Southern California; 1997. ADAPT II User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. [Google Scholar]
- 30.Jakacki RI, Hamilton M, Gilbertson RJ, et al. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: A Children's Oncology Group Phase I Consortium Study. J Clin Oncol. 2008;26:4921–4927. doi: 10.1200/JCO.2007.15.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiessen B, Stewart C, Tsao M, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: Clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother Pharmacol. doi: 10.1007/s00280-009-1041-6. [epub ahead of print on June 5, 2009] [DOI] [PubMed] [Google Scholar]
- 32.Ratain MJ, Cohen EE. The value meal: How to save $1,700 per month or more on lapatinib. J Clin Oncol. 2007;25:3397–3398. doi: 10.1200/JCO.2007.12.0758. [DOI] [PubMed] [Google Scholar]
- 33.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 34.Chu QS, Schwartz G, de Bono J, et al. Phase I and pharmacokinetic study of lapatinib in combination with capecitabine in patients with advanced solid malignancies. J Clin Oncol. 2007;25:3753–3758. doi: 10.1200/JCO.2007.11.1765. [DOI] [PubMed] [Google Scholar]
- 35.McCune JS, Hawke RL, LeCluyse EL, et al. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–366. doi: 10.1067/mcp.2000.110215. [DOI] [PubMed] [Google Scholar]
- 36.Houghton PJ, Maris JM, Courtright J, et al. European Organisation for Research and Treatment of Cancer; 2007. Pediatric Preclinical Testing Program (PPTP): Evaluation of the EGFR and ErbB2 Inhibitor Lapatinib. [Google Scholar]
- 37.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 38.Falchook GS, Moulder S, Wheler JJ, et al. Combination trastuzumab, lapatinib, and bevacizumab in HER2+ breast cancer and other malignancies. 2009 Breast Cancer Symposium; October 8-10; San Francisco, CA. (abstr 244) [Google Scholar]
- 39.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]