Abstract
Purpose
The goal of this study was to determine the frequency and clinical features of early treatment failure during induction chemotherapy before protocol radiation therapy for children with intermediate-risk rhabdomyosarcoma (RMS).
Patients and Methods
Patients with intermediate-risk RMS enrolled onto the Intergroup Rhabdomyosarcoma Study-IV and the Children's Oncology Group D9803 study were reviewed for an early treatment failure. Early failure was defined as failure caused by progressive disease, death as a result of progressive disease, or death as a result of other causes occurring fewer than 120 days from study entry. Patients with parameningeal site RMS with high-risk features who received radiation therapy at week 1 were excluded from analysis. Overall survival (OS) was estimated using the Kaplan-Meier method. Fisher's exact test was used to compare differences between groups. Cumulative incidence of progression was estimated.
Results
Of 916 patients, 20 (2.2%) were found to have an early disease progression and did not receive planned protocol radiotherapy. Three additional early failures resulted from treatment-related death without progression. Median time to failure was 48 days (range, 7 to 106 days). Nineteen (95%) of the 20 patients experienced progression at their primary site. Five-year OS was 32% (95% CI, 12% to 54%) for patients experiencing an early progression.
Conclusion
A small proportion of patients with intermediate-risk RMS suffer an early failure as a result of early progression (2.2%) or treatment-related mortality (0.3%). The majority of patients with early progression had a local failure. Earlier radiotherapy could potentially improve outcome by preventing early local progression.
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood.1 A multimodality approach is used in the treatment of RMS, including a combination of surgery and radiotherapy for local therapy. The goal of radiation therapy is to provide local and regional control. The optimal timing of radiation therapy is not well established. For intermediate-risk RMS, the Intergroup Rhabdomyosarcoma Study Group and Children's Oncology Group (COG) have incorporated radiation at weeks 9 or 12 for most patients after induction chemotherapy in Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) and COG D9803, respectively. In contrast, patients with a parameningeal primary site and higher risk features, such as intracranial extension, cranial neuropathy, or base of skull erosion, were recommended to receive radiotherapy at week 1 of therapy.
Although RMS is a chemotherapy-sensitive disease, Burke et al2 reported that 77% of patients will demonstrate a reduction in the size of the mass after induction chemotherapy, with the majority of these patients having only a partial response (PR) before planned local control. Unless patients demonstrate disease progression to induction chemotherapy, local control with radiation therapy, surgery, or both and chemotherapy is continued according to protocol-prescribed treatment. Despite the aggressive treatment approach, 25% of patients with intermediate-risk RMS experience treatment failure.3 A better understanding of the natural history and failure patterns may lead to a more effective sequence and timing of multimodality therapy. This study seeks to define the frequency and pattern of early failure, particularly before protocol-recommended radiation therapy. Our study also evaluated which, if any, prognostic factors may predict early failure to identify a subset of patients who would benefit from early radiation therapy.
PATIENTS AND METHODS
Patient Population
Patients considered for this study were those with a diagnosis of intermediate-risk RMS under the age of 21 years enrolled onto IRS-IV or under the age of 50 years enrolled onto D9803. Inclusion criteria included patients with embryonal, botryoid, or spindle cell histology, stage 2 or 3, group III; and all group I, II, or III alveolar RMS. Excluded from analysis were patients with parameningeal site RMS with high-risk features who received radiation therapy at week 1, patients with histology classified as not otherwise specified or undifferentiated subtypes, and patients with metastatic intermediate-risk disease (patients with group IV embryonal RMS < 10 years old were eligible for D9803 but were excluded in this analysis). Pathologic material was confirmed as to diagnosis and histologic subtype by members of the study pathology subcommittee. Signed written informed consent was obtained by the patient or guardian according to institutional guidelines with institutional review board approval according to the Declaration of Helsinki.
An early failure was defined as a failure caused by progressive disease, death as a result of progressive disease, or death as a result of other causes occurring less than 120 days from study entry resulting in discontinuation of study protocol and thus no receipt of protocol-required radiation therapy. A period of 120 days was chosen to encompass all failures during induction chemotherapy (week 8 in IRS-IV and week 12 in D9803) and included patients who experienced failure in the interim between the completion of induction chemotherapy and the start of protocol-specified radiation therapy. Response to induction chemotherapy was determined before the initiation of radiation therapy and was compared with pretreatment (baseline) status. A complete response (CR) was defined as the disappearance of all tumors with no evidence of disease. A PR was a ≥ 50% decrease in the sum of the products of the maximum perpendicular diameters. No response (NR) was a less than 50% decrease in the sum of the products of the maximum perpendicular diameters of all measurable lesions. Progressive disease was defined as a ≥ 25% increase in the sum of the products of the maximum perpendicular diameters of measureable lesions at any involved site and/or the appearance of new lesions. Medical records were reviewed to abstract date of birth, sex, race/ethnicity, date of diagnosis, date of enrollment onto protocol, date of treatment start, clinical group, stage, pathologic subtype, primary tumor site, initial tumor size, chemotherapy regimen, date of failure, failure site, and survival status.
Patients enrolled onto IRS-IV were randomly assigned to vincristine, dactinomycin, and cyclophosphamide (VAC); vincristine, ifosfamide, and etoposide; or vincristine, dactinomycin, and ifosfamide during weeks 0 to 28.4 All patients then received VAC chemotherapy until the end of therapy.4 Tumor response to induction chemotherapy was evaluated at week 8, and radiation therapy was delivered at week 9. Patients on D9803 received VAC or VAC alternating with vincristine, cyclophosphamide, and topotecan.3 Tumor response to induction chemotherapy was evaluated at week 12 with the option of second-look surgery or biopsy before the initiation of radiotherapy at week 12.
Statistical Analysis
Fisher's exact test was used to compare the difference in proportions for baseline characteristics and treatment response. Time to failure was calculated from treatment start date to the failure date (progression or death from any cause), at which time patients were taken off the study protocol. Overall survival (OS) was defined as the interval from date of early failure to death from any cause. Patients alive at their last follow-up date were regarded as observations censored at that time. To focus on the outcome of patients who experienced early progression, the OS analysis excluded patients who had early failure as a result of treatment-related death without prior progression. Time to event distributions were estimated using the Kaplan-Meier method5 and were compared using the log-rank test. The cumulative incidence estimator was used to estimate the rate of progression during induction therapy for all patients included in the analysis. Patients who experienced death as a result of causes other than progression of disease were treated as having competing events. Site of failure was defined as local if the tumor recurred only at the site of primary disease or the patient experienced a local failure as a component of regional or metastatic failure, as regional if regional lymph nodes were involved, and as distant if any metastatic disease was present. Only the first failure was analyzed, not subsequent failures.
RESULTS
Of 916 patients, 23 patients (2.5%) experienced an early failure and did not start protocol radiation therapy. Twenty patients (2.2%) experienced early progression (seven before protocol radiation therapy at week 9 on IRS-IV; six before protocol radiotherapy at week 12 on D9803; three after week 9 but before protocol radiotherapy on IRS-IV; and four after week 12 but before protocol radiotherapy on D9803). Of the three patients on IRS-IV who were taken off protocol after week 9, one patient developed a primary brain tumor (week 11), a second patient did not receive radiation therapy at the discretion of the patient's physician (week 15.1), and a third patient experienced local progression (week 9.1). Of the four patients on D9803 who experienced failure after week 12, all had approximately a 1-week delay in completion of chemotherapy and were taken off of protocol by weeks 13 to 14.3 as a result of local and/or distant progressive disease. Three of these four patients had documented adverse effects that extended chemotherapy beyond 12 weeks (eg, neutropenia, anemia, and/or thrombocytopenia); it is unclear why the fourth patient's chemotherapy was extended beyond 12 weeks. Median age at diagnosis for the patients with early failures was 4.1 years (range, 0.3 to 20.3 years). Median time to failure was 48 days (range, 7 to 106 days) for patients with early failure. Only four patients experienced failure before week 4, three with tumor progression and one as a result of treatment- related infection.
Table 1 lists the patient characteristics for all patients enrolled onto IRS-IV and D9803. Patients experiencing early failure were similar to other eligible intermediate-risk patients with respect to patient characteristics and disease staging with the exception of age and histology. Patients with embryonal, botryoid, or spindle cell histology tumors had higher rates of early failure (4.3%) than patients with alveolar tumors (0.7%; P < .001). Age less than 1 year was associated with a higher early failure rate (n = 3, 9.1%) compared with patients age 1 to 9 years (n = 15, 2.4%) and patients ≥ 10 years of age (n = 5, 1.9%; P = .04). The primary sites of disease that suggested higher rates of early failure included extremity (3.7%), parameningeal (2.5%), and retroperitoneal (6.1%) sites (P = .12). Only three patients with an early failure had regional nodal disease at diagnosis. All patients with early failures, except for one patient, had group III disease.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Patients Without Early Failure (n = 893) |
Patients With Early Failure (n = 23) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Protocol | |||||
| IRS-IV | 423 | 97.7 | 10 | 2.3 | |
| D9803 | 470 | 97.3 | 13 | 2.7 | |
| Sex | .18 | ||||
| Male | 551 | 98.0 | 11 | 2.0 | |
| Female | 342 | 96.6 | 12 | 3.4 | |
| Race | .58 | ||||
| Other | 263 | 97.0 | 8 | 3.0 | |
| White | 630 | 97.7 | 15 | 2.3 | |
| Age, years | .04 | ||||
| < 1 | 30 | 90.9 | 3 | 9.1 | |
| 1 to 9 | 606 | 97.6 | 15 | 2.4 | |
| ≥ 10 | 257 | 98.1 | 5 | 1.9 | |
| Pathology | < .001 | ||||
| Alveolar | 446 | 99.3 | 3 | 0.7 | |
| Embryonal/spindle cell/botryoid | 447 | 95.7 | 20 | 4.3 | |
| Group | .24 | ||||
| I | 58 | 100.0 | 0 | 0 | |
| IIa | 77 | 100.0 | 0 | 0 | |
| IIb | 13 | 100.0 | 0 | 0 | |
| IIc | 14 | 93.3 | 1 | 6.7 | |
| III | 722 | 97.0 | 22 | 3.0 | |
| Stage | .1 | ||||
| 1 | 110 | 100.0 | 0 | 0 | |
| 2 | 249 | 98.0 | 5 | 2.0 | |
| 3 | 525 | 96.7 | 18 | 3.3 | |
| Tumor invasion | .22 | ||||
| T1 | 379 | 98.2 | 7 | 1.8 | |
| T2 | 500 | 96.9 | 16 | 3.1 | |
| Size, cm | .12 | ||||
| ≤ 5 | 373 | 98.4 | 6 | 1.6 | |
| > 5 | 506 | 96.8 | 17 | 3.3 | |
| Node status | .47 | ||||
| N0 | 676 | 97.1 | 20 | 2.9 | |
| N1 | 182 | 98.4 | 3 | 1.6 | |
| Nx | 22 | 100.0 | 0 | 0 | |
| Primary site | |||||
| Extremity | 157 | 96.3 | 6 | 3.7 | |
| GI | 6 | 85.7 | 1 | 14.3 | |
| GU bladder/prostate | 134 | 99.3 | 1 | 0.7 | |
| GU non-bladder/prostate | 39 | 100.0 | 0 | 0 | .12 |
| Head and neck | 51 | 100.0 | 0 | 0 | |
| Intrathoracic | 6 | 100.0 | 0 | 0 | |
| Orbit | 22 | 100.0 | 0 | 0 | |
| Other | 15 | 100.0 | 0 | 0 | |
| Parameningeal | 315 | 97.5 | 8 | 2.5 | |
| Perineum | 24 | 100.0 | 0 | 0 | |
| Retroperitoneum | 92 | 93.9 | 6 | 6.1 | |
| Trunk | 22 | 95.6 | 1 | 4.4 | |
Abbreviations: IRS-IV, Intergroup Rhabdomyosarcoma Study-IV; GU, genitourinary.
Table 2 shows the pattern of failures. Nearly all patients with early progressive disease (n = 19, 95%) had progression at their primary site. One patient on IRS-IV achieved a CR at week 8, did not receive protocol-mandated radiation therapy, and subsequently developed local progression at week 15. Two patients with local progression also developed distant metastatic disease. One patient developed local progression at an extremity primary site and a second primary malignancy (CNS primitive neuroectodermal tumor). Only one patient developed an early, isolated distant progression (bone). The early failures in three patients were treatment-related deaths without prior progression, including two as a result of veno-occlusive disease and one as a result of respiratory syncytial virus (Table 3).
Table 2.
Patterns and Sites of Failure
| Pattern and Site of Failure | No. of Failures |
|---|---|
| Type of failure* | |
| Local | 19 |
| Distant | 3 |
| Second primary malignancy | 1 |
| VOD/RSV | 3 |
| Failure site* | |
| Extremity | 5 |
| GU bladder/prostate | 1 |
| Parameningeal | 7 |
| Retroperitoneum | 5 |
| Trunk | 1 |
| Other | 3 |
Abbreviations: VOD, veno-occlusive disease; RSV, respiratory syncytial virus; GU, genitourinary.
Some patients experienced failure at more than one site.
Table 3.
Characteristics of Patients With Early Failure Who Experienced Treatment-Related Death
| Type of Failure | Histology | Primary Site of Disease | Protocol | Status |
|---|---|---|---|---|
| VOD | Spindle cell | Nasopharynx | D9803 | Dead |
| VOD | Spindle cell | Shoulder girdle | D9803 | Dead |
| RSV | Embryonal | Retroperitoneal | D9803 | Dead |
Abbreviations: VOD, veno-occlusive disease; RSV, respiratory syncytial virus.
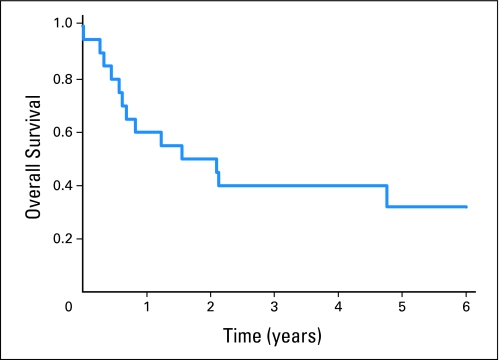
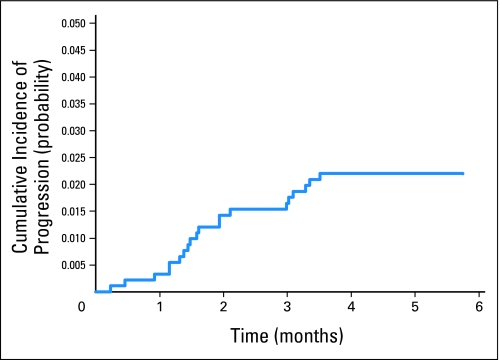
The 5-year OS rate for patients with early progression (and excluding the three patients with early treatment-related deaths) was 32% (95% CI, 12% to 54%; Fig 1). Figure 2 shows the cumulative incidence of early progression. At 4 months, the cumulative incidence of early progression alone was 2.2% (95% CI, 1.2% to 3.2%), and the estimated probability of early progression or death was 2.5% (95% CI, 1.7% to 3.8%). The estimated cumulative incidence of early death as a first event at 4 months was 0.3%. At last follow-up, seven of 20 patients with early progression were alive, including three patients without evidence of disease after retrieval therapy at last contact (range, 2.4 to 10.1 years of follow-up) and four patients with unknown disease status at last contact (range, 2.2 to 6.9 years of follow-up).
Fig 1.
Overall survival (OS) for the 20 patients who developed early progression. Patients with treatment-related deaths without prior progression were excluded from this analysis. Three-year OS was 40% (95% CI, 19% to 60%), and 5-year OS was 32% (95% CI, 12% to 54%).
Fig 2.
Cumulative incidence of early progression. At 4 months, the cumulative progression was 2.2% (95% CI, 1.2% to 3.2%).
DISCUSSION
When radiotherapy was delayed for 9 to 12 weeks as a component of protocol therapy for intermediate-risk RMS, early treatment failure was relatively uncommon (2.5%) and included 2.2% of patients with early disease progression and 0.3% of patients with early treatment-related death. The median time to failure was short (< 7 weeks), with only four patients developing failure before week 4. The vast majority of the early progressions occurred at the primary site (19 of 20 patients). Because almost all early progressions were local only, it is possible that earlier radiotherapy could have prevented these early treatment failures. Some patients had chemotherapy-refractory disease, as seen in three patients who developed distant metastases during induction therapy. Alternate treatment strategies are needed for this subset of patients.
Because early local progression was relatively uncommon, identification of clinical features that were associated with a higher risk for early progression might be used to select patients for early rather than delayed radiotherapy. Younger age is an independent poor prognostic factor.6 We observed that patients younger than 1 year of age had a higher rate of early failure. In D9803, patients with alveolar histology were at an increased risk of failure compared with patients with embryonal histology.3 Paradoxically, our study observed a higher frequency of early failure among patients with embryonal, botryoid, or spindle cell histology compared with alveolar histology. This finding is not likely to be a result of an increased sensitivity to chemotherapy in patients with alveolar histology RMS, because the early CR and PR rates for alveolar and embryonal, botryoid, or spindle cell histology RMS were similar in IRS-IV.2 Qualman et al7 have shown that in some cases, the presence of anaplasia may predict for a worse outcome, particularly in intermediate-risk patients with embryonal histology. It is possible that the difference in early failure by histology was a result of the relatively small study population.
Progressive disease was assessed by imaging studies, using computed tomography and/or magnetic resonance imaging. However, measuring tumor response to induction chemotherapy by imaging does not predict outcome2; therefore, induction chemotherapy response should not be used to guide timing of radiotherapy. The response rate for induction chemotherapy at week 8 for IRS-IV was 77% (21% CR and 56% PR).2 Response had no influence on 5-year failure-free survival for group III RMS.2 Forty patients received no radiation treatment at all (protocol violation), and response to induction therapy had no effect on outcome.2 These data do not support the practice of modifying therapy after induction for patients who do not achieve a CR, PR, or NR to induction therapy. In addition, CR status at the end of 1 year of protocol therapy in group III patients was not associated with a reduction in disease recurrence and death.8 Surprisingly, the OS after early progression is better (5-year OS, 32%) than that observed in IRS Group historical trials after recurrence (20% for all comers)9 and better than that seen in the German Cooperative Soft Tissue Sarcoma Study Group sarcoma trials (12% for recurrence within 6 months).10
Histologic assessment of percent tumor necrosis has prognostic significance and is an important tool to determine therapy for patients with osteosarcoma and Ewing family of tumors.11,12 For RMS, histologic response to treatment has not shown similar prognostic value. Rodeberg et al8 identified 16 patients who underwent resection for residual mass (PR/NR) on imaging at the end of IRS-IV therapy and had available pathology. Only 50% showed viable tumor in the resected specimen, suggesting that radiographic analysis alone is not sufficient to determine the presence of active disease. However, this is a selected population and may not reflect the overall population. Alternatively, surrogate markers such as functional imaging with positron emission tomography may prove useful in monitoring response to therapy and distinguishing viable tumor necrosis from fibrosis, particularly in patients in whom surgical resection is not feasible.13 Positron emission tomography is being studied in the current COG intermediate-risk treatment protocol (ARST0531).
The timing of radiation therapy may be important in controlling local disease that demonstrates an aggressive local course. Our study excluded patients with parameningeal primary disease who required radiation at the time of diagnosis. However, in patients with parameningeal primary site and higher risk factors such as meningeal impingement, initiating radiation therapy within 2 weeks of diagnosis was associated with lower rates of local failure when compared with delayed radiation therapy (18% v 33%, respectively; P = .03).14 Among patients who received radiation therapy within 2 weeks of diagnosis versus those with delayed radiation therapy, lower local failure rates were seen in patients with intracranial extension (16% v 37%, respectively; P = .07) compared with patients with cranial nerve palsy or cranial base bone erosion without intracranial extension (21% v 30%, respectively; P = .23).14 Thus, there may be a subset of patients who may benefit from earlier radiotherapy. Delayed radiotherapy leads to worse local control in other malignancies such as breast and head and neck cancers.15 For medulloblastoma, a randomized trial showed that event-free survival was worse for patients who received delayed radiation.16 In our cohort, in 19 patients with early progression (95% of all early progressions), failure was a result of progression at the primary site, suggesting that local control might have been achieved with earlier radiation therapy. Moving the radiation forward to week 4 may result in fewer early failures and provide improved local control. The current COG study (ARST0531) incorporates radiation therapy at week 4 for intermediate-risk patients with an analytic plan to compare local control rates and failure-free survival to IRS-IV as a historical control. We await the results of this study to determine whether routine early radiotherapy, as a strategy, will improve the outcome for intermediate-risk RMS. However, the potential impact on improved local control must be weighed against the downside of earlier radiation therapy, including late effects in infants.
One limitation of this study is that it depended on retrospective analysis of data from a controlled trial evaluating different end points. We found relatively few patients with early failure, making it difficult to identify a subset of children who would benefit from the routine use of early radiation therapy.
In conclusion, a small proportion of patients experienced failure early, predominantly as a result of local progression before planned radiation therapy. The optimal sequence and timing of radiation therapy is not established. The current COG intermediate-risk RMS clinical trial incorporates radiation therapy early in treatment, testing the hypothesis that earlier radiation therapy will reduce early failure and improve overall outcome.
Footnotes
Supported by Grants No. U10 CA98543, CA98413, CA24507, CA30138, CA30969, CA29139, and CA13539 from the National Cancer Institute/National Institutes of Health, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003958.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Carola A. Arndt, National Cancer Institute/Children's Oncology Group Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: A. Yuriko Minn, Lynn Million, Carola A. Arndt, Sarah S. Donaldson
Provision of study materials or patients: Carola A. Arndt, Kenneth Brown, Sarah S. Donaldson
Collection and assembly of data: A. Yuriko Minn, James R. Anderson, Carola A. Arndt
Data analysis and interpretation: A. Yuriko Minn, Elizabeth R. Lyden, James R. Anderson, Lynn Million, Carola A. Arndt, Douglas S. Hawkins, Sarah S. Donaldson
Manuscript writing: A. Yuriko Minn, Elizabeth R. Lyden, James R. Anderson, Lynn Million, Carola A. Arndt, Kenneth Brown, Douglas S. Hawkins, Sarah S. Donaldson
Final approval of manuscript: A. Yuriko Minn, Elizabeth R. Lyden, James R. Anderson, Lynn Million, Carola A. Arndt, Kenneth Brown, Douglas S. Hawkins, Sarah S. Donaldson
REFERENCES
- 1.Gurney JG, Young JL, Roffers SD, et al. Bethesda, MD: National Cancer Institute; 1999. Soft Tissue Sarcomas, SEER Pediatric Monograph. [Google Scholar]
- 2.Burke M, Anderson JR, Kao SC, et al. Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: The Intergroup Rhabdomyosarcoma Study-IV experience—A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2007;25:4909–4913. doi: 10.1200/JCO.2006.10.4257. [DOI] [PubMed] [Google Scholar]
- 3.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 6.Joshi D, Anderson JR, Paidas C, et al. Age is an independent prognostic factor in rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2004;42:64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 7.Qualman S, Lynch J, Bridge J, et al. Prevalence and clinical impact of anaplasia in childhood rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Cancer. 2008;113:3242–3247. doi: 10.1002/cncr.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeberg DA, Stoner JA, Hayes-Jordan A, et al. Prognostic significance of tumor response at the end of therapy in group III rhabdomyosarcoma: A report from the Children's Oncology Group. J Clin Oncol. 2009;27:3705–3711. doi: 10.1200/JCO.2008.19.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- 10.Mattke AC, Bailey EJ, Schuck A, et al. Does the time-point of relapse influence outcome in pediatric rhabdomyosarcomas? Pediatr Blood Cancer. 2009;52:772–776. doi: 10.1002/pbc.21906. [DOI] [PubMed] [Google Scholar]
- 11.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 12.Wunder JS, Paulian G, Huvos AG, et al. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80:1020–1033. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Peng F, Rabkin G, Muzik O. Use of 2-deoxy-2-[F-18]-fluoro-D-glucose positron emission tomography to monitor therapeutic response by rhabdomyosarcoma in children: Report of a retrospective case study. Clin Nucl Med. 2006;31:394–397. doi: 10.1097/01.rlu.0000222954.38724.be. [DOI] [PubMed] [Google Scholar]
- 14.Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys. 2004;59:1027–1038. doi: 10.1016/j.ijrobp.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CC, Gnekow A, Wellek S, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma: International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO)—SIOP II. Med Pediatr Oncol. 1995;25:166–178. doi: 10.1002/mpo.2950250303. [DOI] [PubMed] [Google Scholar]