Abstract
Compelling public interest is propelling national efforts to advance the evidence base for cancer treatment and control measures and to transform the way in which evidence is aggregated and applied. Substantial investments in health information technology, comparative effectiveness research, health care quality and value, and personalized medicine support these efforts and have resulted in considerable progress to date. An emerging initiative, and one that integrates these converging approaches to improving health care, is “rapid-learning health care.” In this framework, routinely collected real-time clinical data drive the process of scientific discovery, which becomes a natural outgrowth of patient care. To better understand the state of the rapid-learning health care model and its potential implications for oncology, the National Cancer Policy Forum of the Institute of Medicine held a workshop entitled “A Foundation for Evidence-Driven Practice: A Rapid-Learning System for Cancer Care” in October 2009. Participants examined the elements of a rapid-learning system for cancer, including registries and databases, emerging information technology, patient-centered and -driven clinical decision support, patient engagement, culture change, clinical practice guidelines, point-of-care needs in clinical oncology, and federal policy issues and implications. This Special Article reviews the activities of the workshop and sets the stage to move from vision to action.
INTRODUCTION
Multiple intersecting issues have raised public concern about the viability, efficiency, and quality of the US health care system. Health care spending now totals approximately $2.3 trillion, a figure that demands consideration of the value of care purchased (ie, the relative benefit achieved for the national investment),1–4 as well as of the sustainability of the US health care delivery system.5,6 Pervasive problems have been documented with quality of care, which manifest not only in poor performance and outcomes, but also in large variations and disparities in care.7–10 Uncertainty surrounds best practices with respect to treatments and technologies; often evidence is unavailable, inconclusive, or of poor quality and is thus not sufficient to fully inform clinical decisions.11,12 Even when the evidence is robust, a translation gap exists between scientific discovery and clinical practice.13,14 Finally, the promise of personalized medicine cannot be attained until we can match individual patient characteristics with detailed evidence available in real time at the point of care.15–17
In recent months, in an attempt to improve the US health care system, leaders in the biomedical sciences and health policy arenas have proposed and are pursuing new strategies that address these concerns. A cornerstone of these strategies is the use of health information technology (HIT) as the foundation for an improved health care system. A systematic review, which included 257 descriptive and comparative studies and systematic reviews of HIT, demonstrated the efficacy of HIT for improving quality and efficiency.18 Leveraging the capabilities of HIT, diverse approaches to system remodeling focus on health care quality, personalized medicine, translational medicine,19 patient-centered care,20,21 practical clinical trials,22 and the comparative effectiveness of available treatment options.23 Progress in each of these domains requires a common foundation of high-quality data that are available in real time and that can simultaneously be used to improve clinical care, yield quality measurements, and focus research. These approaches are converging at the national level as a movement: “rapid-learning health care.”24 This strategy is seen as driving the process of discovery as a natural outgrowth of patient care, ensuring innovation, quality, safety, and value and serving to reduce the gap between clinical care and research.5,25,26
Many fundamental elements of a rapid-learning health care system are already under development, supported by substantial federal investment in HIT and initially focused on development of electronic health records (EHRs) for all Americans.27 Utilization of the HIT system for clinical and research purposes will require broad adoption of EHRs, exchange of information in an environment of trust, and quality reporting. With $2 billion in federal funds allocated already for a national HIT office,28 to be followed by $45 billion of financing subsidies,29 powerful momentum is pushing the development not only of strategies for achieving a universal EHR that is secure, feasible, usable, and responsive to needs of frontline physicians,30 but also of systems—such as rapid-learning health care—that implement a methodology for, and use of, population-based clinical data.
In October 2009, to gauge progress in the development of this potential new framework for evidence-driven practice, the National Cancer Policy Forum of the Institute of Medicine presented a workshop on a rapid-learning system for cancer care.31,32 Grounded in public desire to advance the evidence base for cancer treatment and control measures and to transform the way in which evidence is generated, aggregated, and applied, this workshop examined the elements that are already available to serve as building blocks of such a system and explored IT components that could support the system's seamless function. This article summarizes the main points and discussion areas of the workshop: How can we make best use of existing capabilities and resources to move forward the vision of rapid-learning health care? What, additionally, do we need to learn and do to progress the vision into reality?
THE RAPID-LEARNING HEALTH CARE VISION
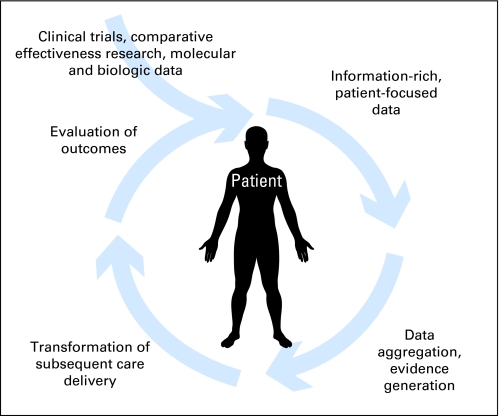
In the rapid-learning health care model, data routinely generated through patient care and clinical research feed into an ever-growing databank or set of coordinated databases. The health care system “learns” by routinely and iteratively (1) collecting data in a planned and strategic manner; (2) analyzing captured data; (3) generating evidence through retrospective analysis of existing data as well as data from prospective studies; (4) implementing new insights into subsequent clinical care; (5) evaluating outcomes of changes in clinical practice; and (6) generating new hypotheses for investigation (Fig 1).33 Thus information, purposefully obtained in real time in the course of routine clinical practice, drives the process of discovery and ensures that a focus on continuous innovation, quality improvement, safety, and value is intrinsic to the health care system. Examples of insights gained through this model, but not through current practice in which clinical data are not routinely collected and analyzed for iterative research, are understanding of clinical effectiveness for the advanced elderly, individuals with multiple comorbidities, and those on concomitant medications frequently excluded from clinical trials; comparison of multiple anticancer drug combinations and of their timing in the trajectory of care; the predictive value of unexpected associations, which could be uncovered through incorporation of a relevance engine into rapid-learning HIT infrastructure (eg, discovery of an association between chemotherapy treatment response and a particular comorbidity); retrieved information on the historical experiences of similar patients that could help guide treatment choices for a current patient (eg, information on management of previous pregnant patients with stage IV breast cancer); and identification of disparities in population-based outcomes that could point toward epidemiologic and/or performance inquiries (eg, a finding that African American patients with prostate cancer in North Carolina have poorer outcomes than comparable patients in the Southeast overall and in surrounding states).
Fig 1.
Cycle of evidence in rapid-learning health care. In a patient-centered system of rapid-learning health care, patient-level data are aggregated to achieve population-based change, and results are applied to care of individual patients to achieve meaningful patient-level practice change.
In which discipline should rapid-learning health care first be developed, tested, and demonstrated? Oncology presents a strong choice. Given its severity, threat to life, costliness, intrinsic and consistent strong patient engagement, and population-wide impact, cancer is one of the most compelling diseases of our time. A sense of urgency surrounds the need for better comparative effectiveness data in general; this urgency is heightened in oncology; where the pace at which new cancer treatments are emerging outstrips the rate of evidence review and dissemination, where many investigational therapies are emerging alongside US Food and Drug Administration–approved therapies; and, in particular, where off-label prescribing constitutes a major proportion of all pharmaceutical treatments currently delivered. From the researcher's perspective, the concepts of continuous investigation, discovery, and evidence implementation are intrinsic to oncology. Moreover, patient-reported outcomes have been well studied in this field, and patient-centeredness has been articulated as a need in, and priority for, cancer care.
Implemented in cancer care, a rapid-learning system would expand the pace and magnitude of evidence generation. It would enable increasingly definitive analyses at both the individual and population levels of the comparative effectiveness of current and future treatment options. Further, it would facilitate and encourage system-wide learning, leveraging the experience of all patients with cancer, as well as of clinical trial participants.
EXISTING RESOURCES AND ACTIVITIES TO SUPPORT RAPID-LEARNING HEALTH CARE
Sound potential for creating a rapid-learning cancer care system already exists; multiple building blocks are currently available, in developmental or early implementation stages. The Department of Health and Human Services (HHS) supports a broad array of resources, including the National Cancer Institute (NCI) and its Cancer Biomedical Informatics Grid (caBIG), state cancer registries, computerized biomedical databases (eg, GenBank), quality measures, regulation of cancer pharmaceutical products and devices, and Web-based consumer health information (eg, Medlineplus.gov). The Veterans Health Administration, functioning as a large-scale delivery system, has been a leading innovator in creating a computerized data capture and analysis system. Similar progress is occurring within other HIT-enabled Integrated Delivery Systems, such as Kaiser Permanente.34 The federal government supports and directs a large technologically advanced cancer care enterprise with a foundation for collaborative learning, comprising the NCI Cancer Centers, Cancer Research Network, and Cancer Clinical Trials Cooperative Groups. National organizations, including professional societies, networked patient groups, research collaborations, and a large biotechnology industry, have all taken steps toward developing a data-focused IT infrastructure and have participated in national discussions of rapid-learning health care.
Recent and pending initiatives leverage and build on these national resources. The American Recovery and Reinvestment Act of 2009 allocated $1.1 billion for comparative effectiveness research (CER). The US Food and Drug Administration Sentinel Network, a nationwide electronic database of adverse-event reports about drugs, biologic products, and medical devices, aims to access 100 million records by 2012. Nearly $45 billion of future Medicare and Medicaid subsidies have been allocated toward the goal of creating an EHR for every American by the year 2014.
The recent upsurge in national support for HIT and CER opens a window of opportunity for advancing the development of rapid-learning health care—beginning with creation of technology infrastructure, demonstration of data linkage and interoperability, and then articulation of next steps in culture change and patient engagement to ensure the system's success. It will be important to address, as well, psychological and practice barriers that might impede implementation and/or use of rapid-learning health care and its technology infrastructure.
TECHNOLOGY AND DATA INFRASTRUCTURE
Existing technologies and infrastructure can provide a foundation for implementation of a rapid-learning health care system. What we face is not an unexplored terra incognita, but rather a challenge of how to best employ new computerized data technologies to enhance research coordination, collaboration, and information exchange. Insights are being generated from several initiatives.
Many critical cancer data sets with longstanding histories now need to be thoughtfully re-engineered, linked, and accessed; if successfully powered by new HIT investments and integrated, they can provide major upgrades in learning resources. The Centers for Disease Control (CDC) is leading the national program of state cancer registries to develop an electronic reporting model and to re-engineer the data collection process to make use of EHRs, thus connecting the cancer surveillance workspace to national HIT efforts.35 A necessary step in development of a rapid-learning system will be further efforts to achieve interoperability between EHRs and multiple other critical databases, including Medicare data sets, Surveillance Epidemiology and End Results (SEER) and National Program of Cancer Registries (NPCR) databases, and state tumor registries supported by SEER and/or NPCR. The National Cancer Data Base and the aggregation of cancer registry data from Commission on Cancer–registered programs provide another system for surveillance of cancer incidence, evaluation of patterns of care, and active quality management. Population-based cancer registries, such as the CDC's NPCR36 and the combined CDC-NPCR and NCI-SEER37 data sets, also represent valuable resources that can support the existing public health data infrastructure and programs on cancer care. When linked with administrative data, like all-payer claims data, there is the opportunity for a “rapid quality-reporting system” that is registry-based and that allows for immediate case acquisition and real-time tracking of care.
As a case example, SEER, a population-based cancer registry currently representing 12 geographic areas and 25% of the US population, is being matched with the Medicare master enrollment file by the NCI. The SEER-Medicare database currently contains more than 3 million cancer cases. Aggregated SEER-Medicare data enable multiple analyses spanning the range of cancer control activities: diagnosis and treatment (eg, studies of patterns of care, outcomes, staging, comorbidities), survivorship, recurrence, health disparities, quality of care, and treatment costs. Although powerful, the SEER-Medicare data set contains limitations that illuminate important considerations when making new HIT investments and learning from linked data sets: (1) potential for selection bias exists; (2) there is a lag time for linkage of 4 years, making such an approach inadequate to support rapid learning; and (3) preliminary studies with the combined data set have highlighted challenges that must be overcome to assess the effectiveness of cancer treatments. Initiatives such as those using the SEER-Medicare data set and other linked data sets would do well to focus on timeliness and improving methodologies to support evidence generation and meaningful use of available and future data.
CANCER DATA SHARING AND EVIDENCE GENERATION
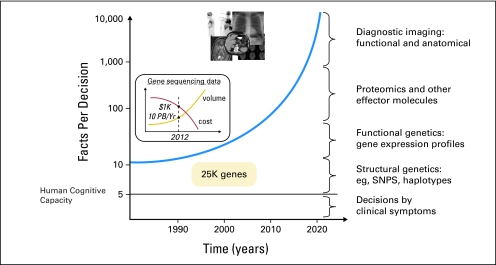
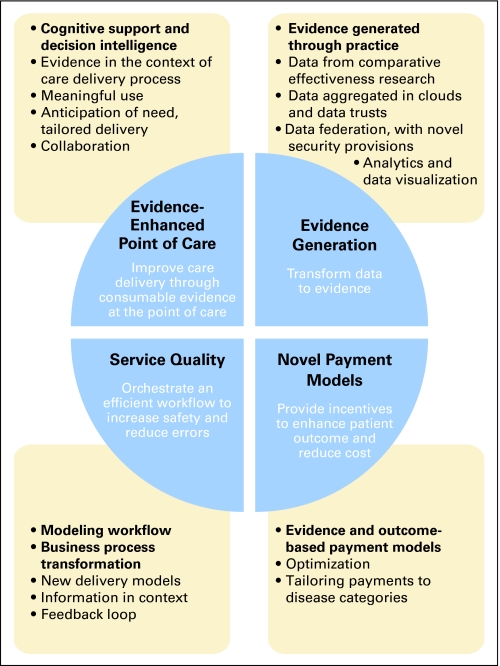
What do we do with all this information? A consequent major challenge of data explosion is the risk of cognitive overload; here, a plethora of raw data, rather than synthesized medical knowledge, confounds the generation of needed information and obscures decision making (Fig 2).32,38,39 However, an evidence-enhanced health care ecosystem will increasingly address and yield appropriate information to appropriate users within the context of the delivery process, provide cognitive and decision support, and interdigitate with service quality, evidence generation, and novel payment mechanisms. Challenges along the path toward creating this ecosystem include data representation, standardized nomenclature, data formats and standards, federated data access, data mining and evidence synthesis approaches, evidence retrieval, reporting, and feedback on use of evidence. Health care optimization through use of HIT is possible (Fig 3), but we must strategically coordinate and test national approaches, or we risk swimming in data soup (or drifting in data clouds) without transforming the health care system into a rational learning system.
Fig 2.
Increase in data required for medical decision making relative to human cognitive capacity. PB, petabytes; Yr, year; SNPs, single-nucleotide polymorphisms.
Fig 3.
Health care optimization through use of technology.
The NCI's caBIG has created many of the tools necessary to support an interoperable IT infrastructure and offers standard rules, a unified architecture, and a common language with which to share information and to collect, analyze, integrate, and disseminate information associated with cancer research. Founded on the core principles of open access, open development, open source, and federation of resources for either local or shared control, caBIG has convened a growing network and established connectivity; it is now working toward widespread deployment and adoption, implementing use cases in the cancer research community.
Although caBIG intentions align directly with our national need for an evidence-enhanced health care ecosystem, its development has underscored the need—associated with any technology change—to simultaneously address cultural and logistical factors. Failure to address cultural barriers imperils large-scale efforts at developing a coordinated data-driven health care system; perhaps at the core, the culture of science, long organized around the needs of the single investigator, is driven by incentives for the individual rather than the collective scientific community.
The culture change required for a national HIT infrastructure requires, first, commitment from all levels within, and often across, organizations.19 Second, community participation in the infrastructure's development is a critical success factor; concept ownership entails community input into system requirements, definition of products, and conventions for sharing information and restricting access. Third, much of what prevents data sharing is not technical challenge, but (1) the affiliation of stakeholders with silos (eg, by discipline, location, or sector), or (2) concerns about intellectual capital and acknowledgment. Federation may provide an acceptable and durable solution for retaining local control of data while permitting aggregation of data from multiple sites into an integrated research data set. Connecting across complex domains demands both leadership and active management; the exchange and use of information requires more than the ability to electronically move data from point to point—it requires human systems to convene to ensure effective and appropriate action. Federal standards will be necessary to require that data can be integrated. Finally, of fundamental importance, we must satisfactorily address data governance and patient privacy concerns.
How do we move from information and data interoperability to evidence generation? In the rapid-learning health care system, evidence is generated at the patient level and available for application to individualized patient care or to evaluation across populations, aggregated at the clinic, institution, health system, or national level. CER and health care quality assessment occur through aggregated analyses; modeling exploits multiple interconnected networks; and finally, personalized CER matches individual patient characteristics to best available evidence in the ecosystem. To make sense of available data, we require tools that include mathematical approaches, software systems, visualization platforms, and education. Existing nascent but evolving systems, such as Adjuvant! Online, model an individual's risk and likely benefit from adjuvant breast cancer treatment options. In a rapid-learning health care system, myriad increasingly complex software tools will connect the large number of personalized patient scenarios with available evidence and ongoing research, and will feed outcomes of treatment back through the system to clinicians and patients, dynamically building the evidence base and guiding clinical decisions.
GUIDELINES, QUALITY, AND HEALTH SYSTEM INTEGRATION
The vision for cancer data sharing, evidence generation, and rapid-learning health care should inform, and be informed by, ongoing clinical care. Currently, clinical practice guidelines and quality metrics generated by respected professional bodies establish a roadmap for contemporary accepted practice. Developed and updated by expert panels, guidelines such as those from the National Comprehensive Cancer Network40 provide a practical starting point for linking evidence and practice in the rapid-learning health care system. The American Society of Clinical Oncology Quality Oncology Practice Initiative (QOPI)41 has established bidirectional information flow: (1) American Society of Clinical Oncology quality guidelines inform care; and (2) clinical sites report on their practices. The QOPI system delivers a report back to the site evaluating its care. (As yet, the QOPI system does not collect outcome data.) The 81 QOPI measures focus on processes of care, are evidence- and consensus-based, and include core measures (eg, documentation of care, pain assessment), disease-specific modules (eg, breast cancer management), and domain-specific modules (eg, end-of-life care, symptom management); linkage with EHRs and creation of QOPI collaboration networks are planned enhancements. In a rapid-learning health care system, this sort of quality assessment would be ongoing; cumulative data would provide insight into best practices, elucidate the value or harm of divergence from accepted standards, and inform the development of new metrics, thus spurring innovation.
Kaiser Permanente has developed an HIT-based approach to pursuing optimal system-based cancer care. Methods include a universally accessible EHR, with focused oncology-relevant functionality, universally accessible patient treatment plans and patient information, a common pathology staging system, integrated clinical/laboratory and pathology modules, a referral system to clinical trials, quality reporting and resource commitment to enable ongoing improvement, assessment of practice patterns, and signal detection for adverse events. Clinical trials are “built in” to the system as available protocols, ensuring reliable data capture and increasing awareness of trials. The intent is for Kaiser clinical data comparable to that collected in a formal clinical trial to be collected on a routine basis for all Kaiser patients, as facilitated by documentation protocols and templates. This model, successfully implemented within a single large organization with more than 8 million enrollees and more than 40,000 new cancer diagnoses annually, encompasses many of the elements of rapid-learning health care.
PATIENT PARTICIPATION IN RAPID-LEARNING HEALTH CARE
Important hallmarks of the last two decades of health care are efforts to promote patient activation and empowerment, and increased patient participation in both decision making and care delivery. IT-savvy and motivated by personal urgency, patients have driven a remarkable advancement of the vision of rapid-learning health care, in conjunction with the efforts of national health care leaders. Patients use the Internet to obtain information about their personal cancer and cancer treatment, investigate providers and institutions, and network with other patients with cancer. Worldwide electronic networks of patients with cancer, organized through sites such as the Association of Cancer Online Resources,42 are promoting participatory medicine by disseminating scientific information in a safe environment, facilitating sharing of personal stories, and supporting patient empowerment and activation. With direct access to and networking of patients with cancer, electronic social networks can accelerate patients' access to relevant information, disseminate clinical trial information, expedite recruitment to trials, and thus foster research opportunities and data collection from within patient communities. For example, daily online conversations about drug adverse effects help to inform patients, preparing them to participate in clinical decision making. PatientsLikeMe43 is using online symptom self-report tools to monitor outcomes of new treatments; here patients themselves are, in effect, conducting massive, structured, rapid cycle observational trials of novel treatments (eg, lithium for amyotrophic lateral sclerosis) and reporting results in the public domain.
An IT-savvy patient population is, in effect, taking control of its own cancer care by using electronic resources for information and communication and by creating a rapid-learning health care process that encircles—though doesn't necessarily engage—the medical community. A draft Declaration of Health Data Rights44 expresses this newfound patient empowerment. The document affirms that patients have the right to own their health data; know the source of each health data element; obtain a complete copy of their personal health data, without delay, at minimal or no cost; access their health data in electronic form (if data exist in that form); and share their health data with others. Through online resources and social networking, patients are creating and pursuing their own version of rapid-learning cancer care that addresses many of the same targets as do the national scientist-led initiatives: symptom awareness and assessment, surveillance for adverse events, patient engagement in clinical decision making, and comparative effectiveness of treatments. The medical establishment and patients need to work together.
LEADERSHIP FOR A RAPID-LEARNING SYSTEM IN CANCER CARE
Federal agencies have a key role to play in advancing rapid-learning health care. The role of HHS, the nation's top health agency and the leading funder of the country's cancer enterprise, is central. Because Medicare finances most cancer care, covering 750,000 new cancer cases each year and sustaining 5 million patients living with cancer, its potential involvement in health care transformation is large. To use its leverage for the advancement of rapid-learning health care, Medicare could do the following: cover new cancer therapies contingent on “evidence development” reporting—to learn, as rapidly as possible, about their best use for personalized care; require reporting of cancer clinical data and quality measures to national cancer registries; pay providers after information is submitted and approved; fund and set standards for EHRs, with Medicare cancer care modules; shift Medicare cancer payments to “pay-for-performance,” paying more for effective, high-quality care; incentivize participation in learning networks; inform Medicare patients and physicians about cancer CER, best practices, and quality performance; and use innovation funds to support new demonstration models of patient-centered, high-quality care.
The 2006 Medicare Oncology Demonstration Project45 reminds us of the advisability of learning from pilot projects about critical hurdles that must be surmounted in order to collect high-quality data that allow us to answer even the most basic questions. We cannot assume that data submitted by “front-line” physicians are of analyzable quality. Although the collection of more data at the clinical encounter is a requirement of rapid-learning health care, adding new data collection tasks to already-busy clinicians' workloads may be unrealistic and/or may result in poor quality of data. New data collection methods must facilitate the accumulation of high-quality information, include mechanisms for routine assessment of data quality, and engage patients whenever possible to ensure that the growing evidence base incorporates patients' experiences. Oncologists' focus will need to shift so that it extends beyond the care of today's patient to also value learning that will benefit the future patient; this perspective shift is essential as an incentive for clinicians to provide data and to strive for highest possible data quality.
In generating evidence through a rapid-learning system, it is important to match study design with the importance and complexity of the research question, balancing rigor against the need to generate timely generalizable evidence. A rapid-learning model lends itself to examination of retrospective, observational, and other warehoused data and makes use of data from nonrandomized trials. This does not eliminate the critical role for prospective, randomized controlled trials (RCTs), but demands that we carefully allocate financial, research, and patient participant resources to RCTs for the right questions. Because, although certain research and clinical questions can be answered through less rigorous study designs, the RCT still presents the most definitive methodology for answering efficacy questions and must therefore hold a central position in the clinical/research system. Choice of study design in the rapid-learning health care model should acknowledge the potential for bias or uncontrolled confounding in observational and nonrandomized studies while judiciously using these designs when they can efficiently and effectively yield the desired information.
STEPS FORWARD: MOVING FROM VISION TO A NATIONAL CANCER STRATEGY
Rapid-learning health care, a compelling model that bridges clinical care and research, is likely to be intrinsic to health care reform. Model development is a work in progress and early in its development. Oncology is a desirable realm in which to explore. Leaders in oncology are developing new and upgraded IT systems and platforms that include millions of patients, defining new CER and other research programs, and making strides in overcoming technological hurdles and institutional inertia. Oncology has a longstanding history of registries and routine data collection and a robust portfolio of clinical guidelines, quality metrics, and templates for care. Research and new discovery are inherent in our culture and woven into accepted clinical practice. At this juncture, our focus needs to be on (1) designing new national data policies and HIT systems that will best serve oncologists and patients, (2) ensuring data interoperability, (3) creating a culture of sharing with simultaneous respect for patient privacy, and (4) developing methods to make sense of information and present it to end users. We cannot expect busy practicing oncologists to become data entry technicians, or to work toward an ivory tower “rapid learning” end goal. Solutions must fit logistically into work flow; the benefits of a rapid-learning environment need to be obvious without additional investments of time. Practical near-term steps include establishment of increased standardized reporting requirements, payment initiatives, common performance measures for public and private sectors, and multiple local, national (and international) collaboratives. EHRs must be enhanced, not only to support data interoperability, but also to ensure that oncology-focused EHRs meet the needs of oncology, improve efficiency, and ensure meaningful, patient-centered communication. Equally valuable is rigorous evaluation of current early adopters, to test whether the vision is ultimately both credible and scalable.
The goal is large-scale collection and aggregation of data, with analytic tools for patients and doctors to learn from cancer information. Longer-range activities will involve instituting reforms that ensure quality improvement, rewarding the “leading edge” through reimbursement, providing better information to patients as consumers of care, prompting new discovery through in silico research, and creating processes that ensure effective use of HIT.
The next step, in our view, should be a national strategy to develop a rapid-learning system for cancer care. HHS, in partnership with those most committed to cancer care in this country, must lead the way. A common vision, commitments, investments—and much hard work—will be required. When we successfully institute, on a widespread scale, real-time HIT systems at the point of care, with these systems linking patient-level data with best evidence and contributing outcomes to a growing national system of clinical research databanks to inform our future, we will have taken a major step toward realizing a vision of a rapid-learning health care system.
Acknowledgment
We thank the workshop speakers and participants.
Footnotes
The activities of the National Cancer Policy Forum of the Institute of Medicine are supported by its sponsoring members, including the National Cancer Institute, Centers for Disease Control and Prevention, US Food and Drug Administration, American Cancer Society, American Society of Clinical Oncology, Association of American Cancer Institutes, and C-Change.
The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the Institute of Medicine, its committees, or its convening activities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Paul Wallace, Permanente Federation, Kaiser Permanente (C); Chalapathy Neti, IBM (C) Consultant or Advisory Role: None Stock Ownership: Chalapathy Neti, IBM Honoraria: None Research Funding: Amy P. Abernethy, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Amy P. Abernethy, Lynn M. Etheredge, Patricia A. Ganz, Paul Wallace, Robert R. German, Chalapathy Neti, Peter B. Bach, Sharon B. Murphy
Administrative support: Amy P. Abernethy, Robert R. German, Peter B. Bach, Sharon B. Murphy
Provision of study materials or patients: Paul Wallace
Collection and assembly of data: Amy P. Abernethy
Data analysis and interpretation: Amy P. Abernethy, Robert R. German
Manuscript writing: Amy P. Abernethy, Lynn M. Etheredge, Patricia A. Ganz, Paul Wallace, Robert R. German, Chalapathy Neti, Peter B. Bach, Sharon B. Murphy
Final approval of manuscript: Amy P. Abernethy, Lynn M. Etheredge, Patricia A. Ganz, Paul Wallace, Robert R. German, Chalapathy Neti, Peter B. Bach, Sharon B. Murphy
REFERENCES
- 1.Skinner J, Staiger D, Fisher ES. Looking back, moving forward. N Engl J Med. 2010;362:569–574. doi: 10.1056/NEJMp1000448. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB. A map to bad policy: Hospital efficiency measures in the Dartmouth Atlas. N Engl J Med. 2010;362:569–573. doi: 10.1056/NEJMp0909947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein MC, Skinner JA. Comparative effectiveness and health care spending: Implications for reform. N Engl J Med. 2010;362:460–465. doi: 10.1056/NEJMsb0911104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orszag PR, Ellis P. The challenge of rising health care costs: A view from the Congressional Budget Office. N Engl J Med. 2007;357:1793–1795. doi: 10.1056/NEJMp078190. [DOI] [PubMed] [Google Scholar]
- 5.Eden J, Wheatley B, McNeil B, et al. Washington, DC: National Academies Press; 2008. Knowing What Works in Health Care: A Roadmap for the Nation. [Google Scholar]
- 6.Hartman M, Martin A, Nuccio O, et al. Health spending growth at a historic low in 2008. Health Aff (Millwood) 2010;29:147–155. doi: 10.1377/hlthaff.2009.0839. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Washington, DC: National Academies Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality. National Healthcare Quality Report. 2008. http://www.ahrq.gov/qual/nhqr08/Key.htm. [DOI] [PubMed]
- 9.Wennberg JE, Fisher ES, Goodman DC, et al. Tracking the Care of Patients with Severe Chronic Illness: The Dartmouth Atlas of Health Care 2008. http://www.dartmouthatlas.org/downloads/atlases/2008_Chronic_Care_Atlas.pdf. [PubMed]
- 10.Sirovich BE, Gottlieb DJ, Welch HG, et al. Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144:641–649. doi: 10.7326/0003-4819-144-9-200605020-00007. [DOI] [PubMed] [Google Scholar]
- 11.Phillips KA. Closing the evidence gap in the use of emerging testing technologies in clinical practice. JAMA. 2008;300:2542–2544. doi: 10.1001/jama.2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury MJ, Berg A, Coates R, et al. The evidence dilemma in genomic medicine. Health Aff (Millwood) 2008;27:1600–1611. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- 13.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 14.Scott NA, Moga C, Harstall C, et al. Using health technology assessment to identify research gaps: An unexploited resource for increasing the value of clinical research. Healthc Policy. 2008;3(3) http://www.longwoods.com/product.php?productid=19558&cat=529&page=1. [PMC free article] [PubMed] [Google Scholar]
- 15.Lesko LJ. Personalized medicine: Elusive dream or imminent reality? Clin Pharmacol Ther. 2007;81:807–816. doi: 10.1038/sj.clpt.6100204. [DOI] [PubMed] [Google Scholar]
- 16.Sadée W, Dai Z. Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet. 2005;14:R207–R214. doi: 10.1093/hmg/ddi261. [DOI] [PubMed] [Google Scholar]
- 17.Burke W, Psaty BM. Personalized medicine in the era of genomics. JAMA. 2007;298:1682–1684. doi: 10.1001/jama.298.14.1682. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742–752. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 19.Dougherty D, Conway PH. The “3T's” road map to transform US health care: The “how” of high-quality care. JAMA. 2008;299:2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- 20.Stewart M, Brown JB, Weston WW, et al. Abingdon, United Kingdom: Radcliffe Medical Press; 2003. Patient-Centered Medicine: Transforming the Clinical Method (ed 2) [Google Scholar]
- 21.Bergeson SC, Dean JD. A systems approach to patient-centered care. JAMA. 2006;296:2848–2851. doi: 10.1001/jama.296.23.2848. [DOI] [PubMed] [Google Scholar]
- 22.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 23.VanLare JM, Conway PH, Sox HC. Five next steps for a new national program for comparative-effectiveness research. N Engl J Med. 2010;362:970–973. doi: 10.1056/NEJMp1000096. [DOI] [PubMed] [Google Scholar]
- 24.Slutsky JR. Moving closer to a rapid-learning health care system. Health Aff (Millwood) 2007;26:122–124. doi: 10.1377/hlthaff.26.2.w122. [DOI] [PubMed] [Google Scholar]
- 25.Olsen LA, Aisner D, McGinnis JM, editors. Washington, DC: National Academies Press; 2007. The Learning Healthcare System: Workshop Summary (IOM Roundtable on Evidence-Based Medicine) [PubMed] [Google Scholar]
- 26.Etheredge LM. A rapid-learning health system. Health Aff (Millwood) 2007;26:w107–w118. doi: 10.1377/hlthaff.26.2.w107. [DOI] [PubMed] [Google Scholar]
- 27.McCullagh D. U.S. stimulus bill pushes e-health records for all. CNET News, 2009. http://news.cnet.com/8301-13578_3-10161233-38.html. [Google Scholar]
- 28.Telecommunications Industry Association. Stimulus Package: What's in the Bill? - Funding Breakdown, Health IT, 2010. http://www.tiaonline.org/gov_affairs/stimulus_package/whats_in_the_bill/funding_breakdown/funding_breakdown-HealthIT.cfm.
- 29.Nagesh G. NextGov: Technology and the Business of Government; 2009. House stimulus plan packed with $45 billion in technology spending. http://www.nextgov.com/nextgov/ng_20090116_8081.php. [Google Scholar]
- 30.Sittig DF, Singh H. Eight rights of safe electronic health record use. JAMA. 2009;302:1111–1113. doi: 10.1001/jama.2009.1311. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine. A Foundation for Evidence-Driven Practice: A Rapid-Learning System for Cancer. http://www.iom.edu/Activities/Disease/NCPF/2009-OCT-09.aspx.
- 32.Institute of Medicine. Washington, DC: National Academies Press; A Foundation for Evidence-Driven Practice: A Rapid Learning System for Cancer Care: Workshop Summary. in press. [PubMed] [Google Scholar]
- 33.Abernethy AP, Ahmad A, Zafar SY, et al. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. in press. [DOI] [PubMed]
- 34.Chen C, Garrido T, Chock D, et al. The Kaiser Permanente electronic health record: Transforming and streamlining modalities of care. Health Aff (Millwood) 2009;28:323–333. doi: 10.1377/hlthaff.28.2.323. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention, National Program of Cancer Registries. Advancing E-Cancer Reporting and Registry Operations. http://www.cdc.gov/cancer/npcr/informatics/aerro.
- 36.Centers for Disease Control and Prevention: National Program of Cancer Registries. http://www.cdc.gov/cancer/npcr/
- 37.Centers for Disease Control and Prevention, National Program of Cancer Registries: United States Cancer Statistics. http://apps.nccd.cdc.gov/uscs/
- 38.Stead WW, Lin HS. Washington, DC: National Academies Press; 2009. Computational Technology for Effective Healthcare: Immediate Steps and Strategic Directions. [PubMed] [Google Scholar]
- 39.Stead WW. Washington, DC: National Academies Press; 2008. Beyond Expert-Based Practice: Evidence-Based Medicine and the Changing Nature of Health Care: 2007 IOM Annual Meeting Summary, Introduction and Overview—2007 Institute of Medicine Annual Meeting. [PubMed] [Google Scholar]
- 40.National Comprehensive Cancer Network. http://www.nccn.org/index.asp.
- 41.Quality Oncology Practice Initiative. http://qopi.asco.org/
- 42.Association of Cancer Online Resources. http://www.acor.org/
- 43.PatientsLikeMe. http://www.patientslikeme.com/
- 44.HealthDataRights.org. http://www.healthdatarights.org/
- 45.US Department of Health and Human Services: Center for Medicare and Medicaid Services. http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=1717.