Abstract
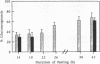
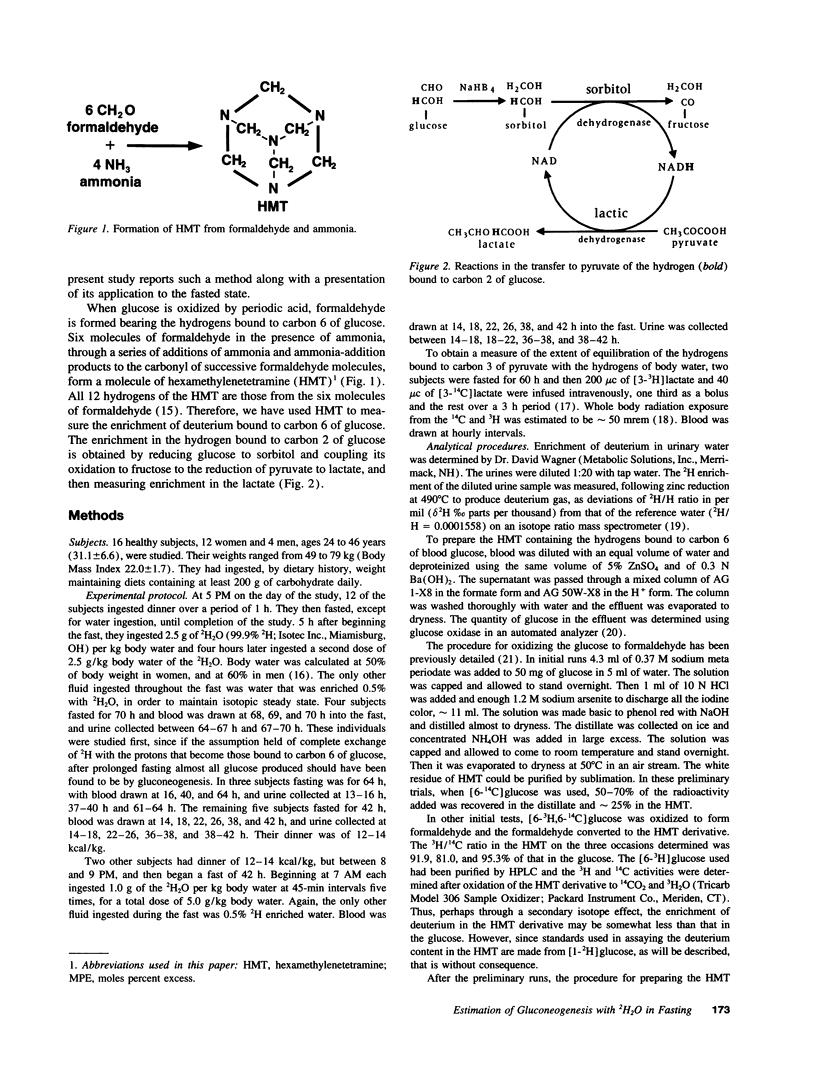
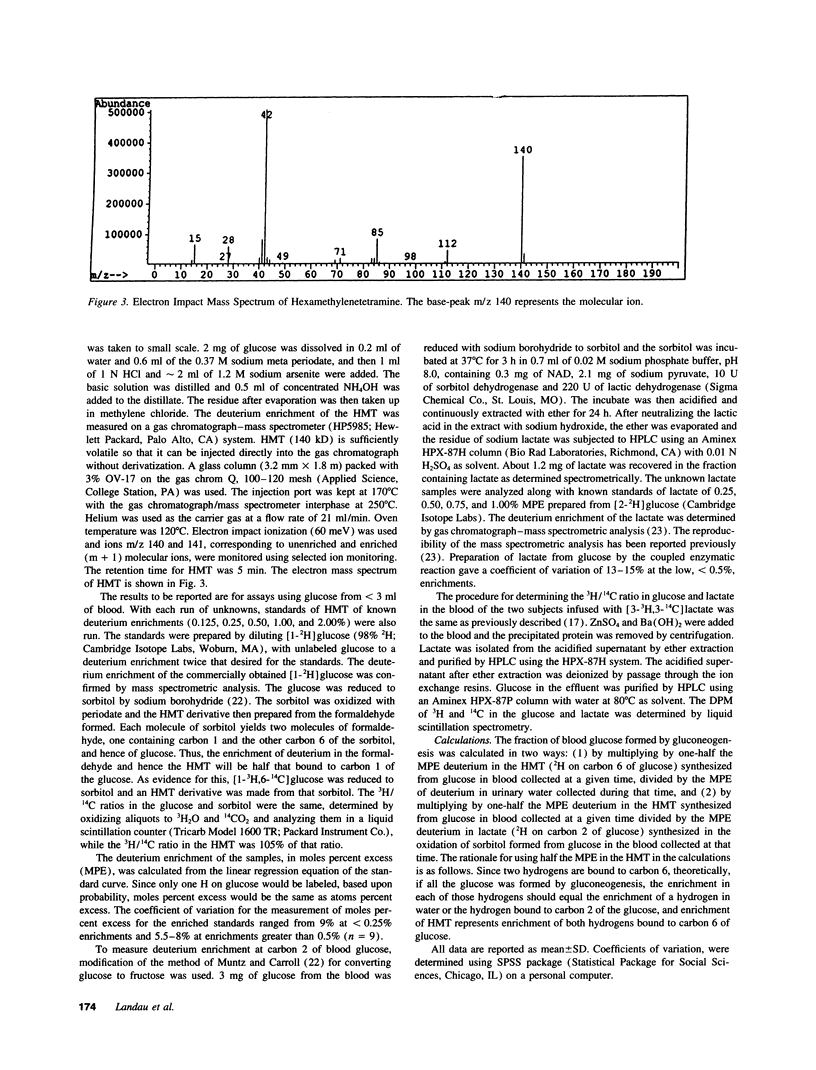
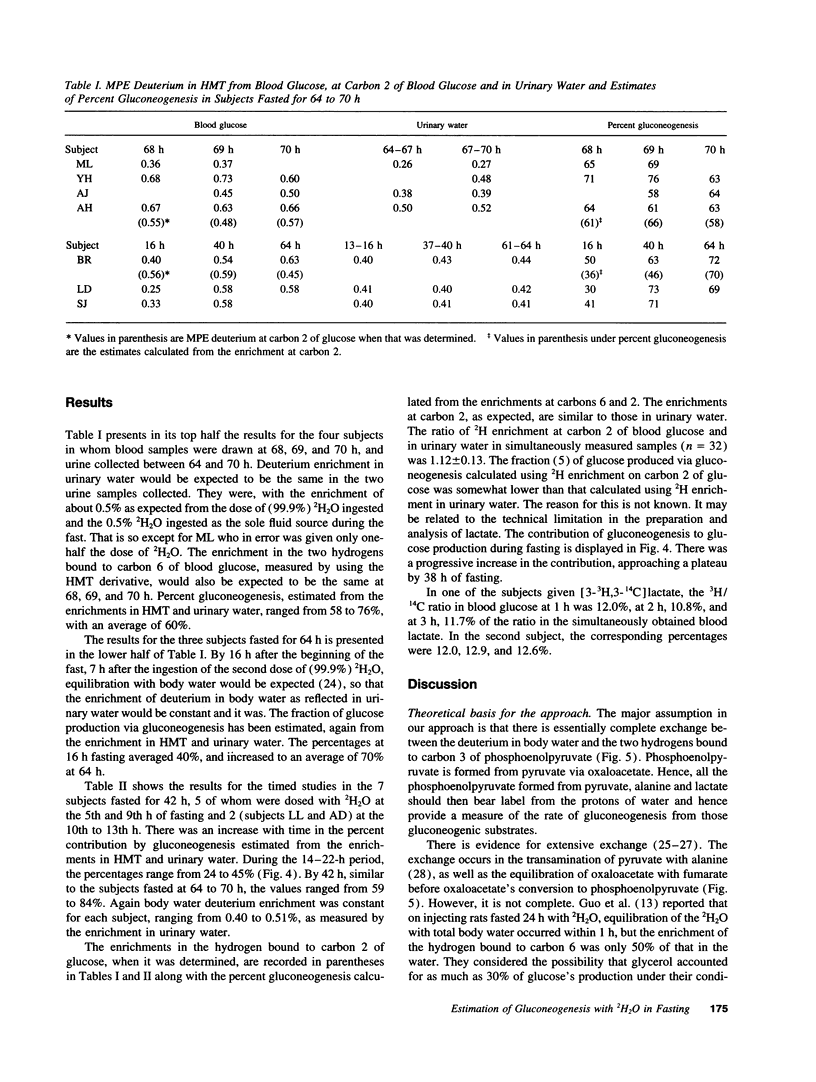
A method is introduced for estimating the contribution of gluconeogenesis to glucose production. 2H2O is administered orally to achieve 0.5% deuterium enrichment in body water. Enrichments are determined in the hydrogens bound to carbons 2 and 6 of blood glucose and in urinary water. Enrichment at carbon 6 of glucose is assayed in hexamethylenetetramine, formed from formaldehyde produced by periodate oxidation of the glucose. Enrichment at carbon 2 is assayed in lactate formed by enzymatic transfer of the hydrogen from glucose via sorbitol to pyruvate. The fraction gluconeogenesis contributes to glucose production equals the ratio of the enrichment at carbon 6 to that at carbon 2 or in urinary water. Applying the method, the contribution of gluconeogenesis in healthy subjects was 23-42% after fasting 14 h, increasing to 59-84% after fasting 42 h. Enrichment at carbon 2 to that in urinary water was 1.12 +/- 0.13. Therefore, the assumption that hydrogen equilibrated during hexose-6-P isomerization was fulfilled. The 3H/14C ratio in glucose formed from [3-3H,3-14C]lactate given to healthy subjects was 0.1 to 0.2 of that in the lactate. Therefore equilibration during gluconeogenesis of the hydrogen bound to carbon 6 with that in body water was 80-90% complete, so that gluconeogenesis is underestimated by 10-20%. Glycerol's contribution to gluconeogenesis is not included in these estimates. The method is applicable to studies in humans of gluconeogenesis at safe doses of 2H2O.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkman O., Felig P., Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980 Aug;29(8):610–616. doi: 10.2337/diab.29.8.610. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Pangburn T., Lapenna D., Gerich J. Limitations in the use of [2-14C]acetate for measuring gluconeogenesis in vivo. Diabetes. 1993 May;42(5):732–737. doi: 10.2337/diab.42.5.732. [DOI] [PubMed] [Google Scholar]
- Esenmo E., Chandramouli V., Schumann W. C., Kumaran K., Wahren J., Landau B. R. Use of 14CO2 in estimating rates of hepatic gluconeogenesis. Am J Physiol. 1992 Jul;263(1 Pt 1):E36–E41. doi: 10.1152/ajpendo.1992.263.1.E36. [DOI] [PubMed] [Google Scholar]
- Gay L. J., Schneiter P., Schutz Y., Di Vetta V., Jéquier E., Tappy L. A non-invasive assessment of hepatic glycogen kinetics and post-absorptive gluconeogenesis in man. Diabetologia. 1994 May;37(5):517–523. doi: 10.1007/s001250050141. [DOI] [PubMed] [Google Scholar]
- Guo Z. K., Lee W. N., Katz J., Bergner A. E. Quantitation of positional isomers of deuterium-labeled glucose by gas chromatography/mass spectrometry. Anal Biochem. 1992 Aug 1;204(2):273–282. doi: 10.1016/0003-2697(92)90238-3. [DOI] [PubMed] [Google Scholar]
- Klein S., Young V. R., Blackburn G. L., Bistrian B. R., Wolfe R. R. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest. 1986 Oct;78(4):928–933. doi: 10.1172/JCI112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M., Golden S., Katz J., Unger R. H., Foster D. W., McGarry J. D. Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate. J Biol Chem. 1986 Feb 25;261(6):2632–2637. [PubMed] [Google Scholar]
- LANDAU B. R., HASTINGS A. B., NESBETT F. B. Origin of glucose and glycogen carbons formed from C14-labeled pyruvate by livers of normal and diabetic rats. J Biol Chem. 1955 Jun;214(2):525–535. [PubMed] [Google Scholar]
- Landau B. R. Estimating gluconeogenic rates in NIDDM. Adv Exp Med Biol. 1993;334:209–220. doi: 10.1007/978-1-4615-2910-1_15. [DOI] [PubMed] [Google Scholar]
- Landau B. R. Measuring glucose and fructose-6-phosphate cycling in liver in vivo. Metabolism. 1993 Apr;42(4):457–462. doi: 10.1016/0026-0495(93)90103-u. [DOI] [PubMed] [Google Scholar]
- Landau B. R., Shreeve W. W. Radiation exposure from long-lived beta emitters in clinical investigation. Am J Physiol. 1991 Sep;261(3 Pt 1):E415–E417. doi: 10.1152/ajpendo.1991.261.3.E415. [DOI] [PubMed] [Google Scholar]
- MUNTZ J. A., CARROLL R. E. A method for converting glucose to fructose. J Biol Chem. 1960 May;235:1258–1260. [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Schumann W. C., Bartsch G. E., Chandramouli V., Kumaran K., Wahren J., Landau B. R. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem. 1991 Apr 15;266(11):6975–6984. [PubMed] [Google Scholar]
- Nurjhan N., Consoli A., Gerich J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Jan;89(1):169–175. doi: 10.1172/JCI115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHIMA T., TAMIYA N. Mechanism of transaminase action. Biochem J. 1961 Jan;78:116–119. doi: 10.1042/bj0780116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Chenoweth M., Rognstad R., Dunn A., Katz J. Metabolism of 3H- and 14C-labelled lactate in starved rats. Biochem J. 1981 Feb 15;194(2):525–540. doi: 10.1042/bj1940525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. K., Ho K. J., Taylor C. B. Biologic effects of prolonged exposure to deuterium oxide. A behavioral, metabolic, and morphologic study. Arch Pathol. 1972 Jul;94(1):81–89. [PubMed] [Google Scholar]
- Postle A. D., Bloxham D. P. The use of tritiated water to measure absolute rates of hepatic glycogen synthesis. Biochem J. 1980 Oct 15;192(1):65–73. doi: 10.1042/bj1920065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Clark G., Katz J. Glucose synthesis in tritiated water. Eur J Biochem. 1974 Sep 1;47(2):383–388. doi: 10.1111/j.1432-1033.1974.tb03703.x. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Estimation of gluconeogenesis and glycogenolysis in vivo using tritiated water. Biochem J. 1991 Nov 1;279(Pt 3):911–911. doi: 10.1042/bj2790911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991 Oct 25;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- Schumann W. C., Magnusson I., Chandramouli V., Kumaran K., Wahren J., Landau B. R. Metabolism of [2-14C]acetate and its use in assessing hepatic Krebs cycle activity and gluconeogenesis. J Biol Chem. 1991 Apr 15;266(11):6985–6990. [PubMed] [Google Scholar]
- Shalwitz R. A., Beth T. J., MacLeod A. M., Tucker S. J., Rolison G. G. Use of 2H2O to study labeling in plasma glucose and hepatic glycogen during a hyperglycemic clamp. Am J Physiol. 1994 Mar;266(3 Pt 1):E433–E437. doi: 10.1152/ajpendo.1994.266.3.E433. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Gilfillan C. A., Kalhan S. C. Determination of carbon-13 labeled lactate in blood by gas chromatography/mass spectrometry. Anal Chem. 1984 Mar;56(3):517–523. doi: 10.1021/ac00267a049. [DOI] [PubMed] [Google Scholar]
- Virkamäki A., Puhakainen I., Nurjhan N., Gerich J. E., Yki-Järvinen H. Measurement of lactate formation from glucose using [6-3H]- and [6-14C]glucose in humans. Am J Physiol. 1990 Sep;259(3 Pt 1):E397–E404. doi: 10.1152/ajpendo.1990.259.3.E397. [DOI] [PubMed] [Google Scholar]
- Wahren J., Efendić S., Luft R., Hagenfeldt L., Björkman O., Felig P. Influence of somatostatin on splanchnic glucose metabolism in postabsorptive and 60-hour fasted humans. J Clin Invest. 1977 Feb;59(2):299–307. doi: 10.1172/JCI108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Wennlund A., Nilsson L. H., Felig P. Influence of hyperthyroidism on splanchnic exchange of glucose and gluconeogenic precursors. J Clin Invest. 1981 Apr;67(4):1056–1063. doi: 10.1172/JCI110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajngot A., Chandramouli V., Schumann W. C., Kumaran K., Efendić S., Landau B. R. Testing of the assumptions made in estimating the extent of futile cycling. Am J Physiol. 1989 May;256(5 Pt 1):E668–E675. doi: 10.1152/ajpendo.1989.256.5.E668. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Peters E. J. Lipolytic response to glucose infusion in human subjects. Am J Physiol. 1987 Feb;252(2 Pt 1):E218–E223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Lee L. S., Klein P. D. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987 May;45(5):905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]