Abstract
To determine the cause of spotted fever cases in the southern United States, we screened Gulf Coast ticks (Amblyomma maculatum) collected in Arkansas for rickettsiae. Of the screened ticks, 30% had PCR amplicons consistent with Rickettsia parkeri or Candidatus Rickettsia amblyommii.
Keywords: Rickettsiae, vector-borne infections, Rocky Mountain spotted fever, Amblyomma maculatum, Rickettsia parkeri, Rickettsia amblyommii, Gulf Coast, ticks, Arkansas, dispatch
The Centers for Disease Control and Prevention identified Arkansas as a leading state for the incidence of Rocky Mountain spotted fever (causative agent Rickettsia rickettsii) and reported >15 cases per 1,000,000 persons in 2002 (1). Given the known cross-reactivity of serologic testing results for spotted fever group (SFG) rickettsia, it is unclear if cases outside the natural range of the vectors for R. rickettsii are misdiagnosed, if the pathogen is less virulent than previously suggested, or if additional rickettsiae are responsible for pathogenesis (2).
Recently, the Gulf Coast tick (Amblyomma maculatum) was identified as the primary vector of R. parkeri, a newly described pathogen that causes disease symptoms similar to Rocky Mountain spotted fever (3). R. parkeri has previously been identified in A. maculatum tick specimens collected in the southeastern United States (4) and from a human biopsy specimen in Virginia, USA (5). We have identified A. maculatum ticks collected from canids, felids, white-tailed deer, and a cow from locations throughout Arkansas (6). Notably, R. amblyommii has been identified as a potential pathogen and is found in lone star ticks (A. americanum) (7,8). We report the presence of DNA consistent with that of Candidatus Rickettsia amblyommii and R. parkeri in A. maculatum ticks in Arkansas.
The Study
We screened 112 A. maculatum ticks collected during March 2006–January 2008 from 22 dogs (Canis lupus familiaris) and 95 A. maculatum ticks collected during the 2008 hunting season from 52 white-tailed deer (Odocoileus virginianus Boddaert) for rickettsial DNA. Collectors removed specimens; stored them in vials containing 100% ethanol; and recorded tick collection date, location, and host (6). Ticks were identified by species, sex, life stage, and engorgement (9). Each sample was bisected longitudinally with a razor blade and subjected to the extraction procedure by using QIAGEN DNeasy (QIAGEN, Valencia, CA, USA) following the manufacturer’s protocols.
Tick DNA extracts were screened for SFG Rickettsia spp. DNA by PCR by using genus-specific primers for the citrate synthase (gltA) (10) and rickettsial outer membrane protein B (rompB) (11) genes. Reaction products were analyzed (12), and positive amplicons for gltA (513 bp) and rompB (578 bp) were sent to the University of North Texas Health Science Center (Fort Worth, TX, USA) for sequence determination. At least 1 amplicon from each host was sequenced to determine the Rickettsia species identity. PCR products were hydrolyzed with ExoSAP-IT (USB Corporation, Cleveland, OH, USA), and sequence determination was performed by using a BigDYE Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, Inc., Foster City, CA, USA) followed by capillary electrophoresis on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Inc.) (13).
Sequences were edited, aligned, and analyzed with Sequencher 4.7 (Gene Codes Corporation, Ann Arbor, MI, USA) and compared with sequences in GenBank (National Center for Biotechnology Information, Bethesda, MD, USA). BEAST version 1.4.2 software (http://beast.bio.ed.ac.uk/Main_Page) was used to infer phylogenetic relationships and create dendrograms (14). The consensus tree ran for 106 generations with a burn-in of 2 × 104. Established methods were used (12) to conduct parsimony bootstrap and maximum-likelihood analyses. Maximum-likelihood and unweighted parsimony analyses on the alignments were performed by using the branch and bound algorithm of PAUP* 4.0b10 (http://paup.csit.fsu.edu). Outgroup taxa were obtained from GenBank.
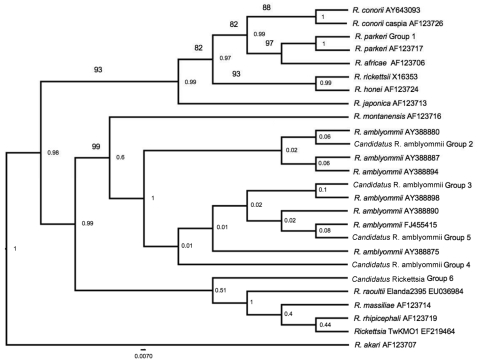
Of the 207 ticks, 62 were positive for Rickettsia spp. DNA by PCR. Nineteen ticks were positive for gltA only, 12 were positive for rompB only, and 31 were positive for both genes (Table). Of the ticks collected from white-tailed deer, 28 were positive, and those amplicons were 100% homologous with Candidatus Rickettsia amblyommii from GenBank (FJ455415, EU7228827, AY388899) (Table, Figure). Of the positive ticks collected from dogs, 3 had sequences with 100% similarity to either rompB (AF123717) or gltA (EF102236) of R. parkeri. A single tick (unengorged male) had a sequence 98% similar to GenBank sequences EF219464 (rompB) and EF451001 (gltA). The remaining 30 ticks collected from dogs that were positive all produced amplicons with 100% sequence identity to Candidatus R. amblyommii gltA (EF450708). However, rompB sequences generated from the same sample set demonstrated greater diversity (Table, Figure).
Table. Gulf Coast ticks (Amblyomma maculatum) collected from white-tailed deer and dogs, Arkansas, USA, 2006–2008*.
| Source | No. tested | No. (%) positive | Blood meal | gltA positive |
rompB-positive groups |
gltA- and rompB-positive groups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 6 | NS | ||||||
| White-tailed deer | |||||||||||||||
| Nymph | 8 | 0 | Yes | ||||||||||||
| No | |||||||||||||||
| Male | 46 | 15 (33) | Yes | 2 | 2 | ||||||||||
| No | 5 | 2 | 4 | ||||||||||||
| Female | 41 | 13 (32) | Yes | 2 | 2 | ||||||||||
|
|
|
|
No |
3 |
3 |
|
|
|
|
|
3 |
|
|
|
|
| Total collected from deer |
95 |
28 (29) |
|
10 |
7 |
0 |
0 |
0 |
|
0 |
9 |
0 |
0 |
0 |
2 |
| Dogs | |||||||||||||||
| Nymph | 8 | 2 (25) | Yes | ||||||||||||
| No | 1 | 1 | |||||||||||||
| Male | 95 | 28 (29) | Yes | 1 | 1 | ||||||||||
| No | 8 | 1 | 1 | 1 | 1 | 8 | 2 | 3 | 1 | ||||||
| Female | 9 | 4 (44) | Yes | ||||||||||||
|
|
|
|
No |
|
1 |
|
|
|
|
3 |
|
|
|
|
|
| Total collected from dogs |
112 |
34 (30) |
|
9 |
2 |
1 |
1 |
1 |
|
3 |
10 |
2 |
3 |
1 |
1 |
| Total ticks collected | 207 | 62 (30) | 19 | 9 | 1 | 1 | 1 | 3 | 19 | 2 | 3 | 1 | 3 | ||
*Of 207 ticks collected, 30% were positive for rickettsiae. Ticks were positive for citrate synthase (gltA) gene only, rickettsial outer membrane protein B (rompB) gene only, or both genes; rompB sequences fell within 6 sequence groups. Ticks were collected from canids during March 2006–January 2008 and from deer during the 2008 deer hunting season. NS, not sequenced.
In total, 3 ticks collected from 3 different canine hosts produced sequences 100% identical to those of R. parkeri rompB (AF123717) and gltA (EF102236). Candidatus R. amblyommii sequences were identified in 29 ticks collected from 13 dogs and 25 ticks collected from 25 deer. The resulting Bayesian tree showed weak support (consistency index 0.792, tree length 159) (Figure). Neighbor-joining and maximum-likelihood trees supported the GenBank homologies.
Figure.
Phylogenetic relationship of 6 rickettsial outer membrane protein B rickettsiae groups (578 bp) identified in Amblyomma maculatum ticks collected in Arkansas and similar rickettsiae identified from GenBank. The tree was constructed by using the maximum-likelihood and maximum-parsimony analysis in BEAST 9 (http://beast.bio.ed.ac.uk/Main_Page) Numbers on lines are bootstrap support values >75 and numbers at nodes are posterior values. Scale bar indicates nucleotide substitutions per site.
Conclusions
We report the identification of SFG rickettsiae in A. maculatum ticks collected from Arkansas, specifically R. parkeri, Candidatus R. amblyommii, and an uncharacterized Rickettsia sp. sequence with high homology to GenBank sequence no. EF219464. Identification of these rickettsiae may be a public health concern given their recent association with cases of spotted fever (4,7,8). The risk for spotted fever transmission to humans is unknown but may be of concern to public health officials in Arkansas because of canid–human relationships and habitat fragmentation that has moved deer ranges closer to human habitation. Additional investigations of the distribution of A. maculatum ticks, the pathogenesis of Rocky Mountain spotted fever, and the ticks’ relationship to human disease should be conducted.
Acknowledgments
We thank the personnel associated with the Arkansas Game and Fish Commission and Arkansas Veterinary Medical Association involved with collecting tick samples.
This research was supported in part by the University of Arkansas, Arkansas Agricultural Experiment Station.
Biography
Ms Trout is a PhD candidate in the Department of Entomology at the University of Arkansas. Her dissertation focuses on the spatial identification and genetic characterization of ticks and their relationship with Borrelia and Rickettsia species within Arkansas.
Footnotes
Suggested citation for this article: Trout R, Steelman CD, Szalanski AL, Williamson PC. Rickettsiae in Gulf Coast ticks, Arkansas, USA. Emerg Infect Dis [serial on the Internet]. 2010 May [date cited]. http://www.cdc.gov/EID/content/16/5/830.htm
References
- 1.Centers for Disease Control and Prevention. Tickborne rickettsial diseases: statistics. 2008. [cited 2009 Aug 1]. http://www.cdc.gov/ticks/diseases/rocky_mountain_spotted_fever/statistics.html
- 2.Raoult D, Parola P. Rocky Mountain spotted fever in the USA: a benign disease or a common diagnostic error? Lancet Infect Dis. 2008;8:587–9. 10.1016/S1473-3099(08)70210-X [DOI] [PubMed] [Google Scholar]
- 3.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:812–3.Medline 10.1086/381894 [DOI] [PubMed] [Google Scholar]
- 4.Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, et al. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, et al. Rickettsia parkeri infection after tick bite, Virginia. Emerg Infect Dis. 2007;13:334–6. 10.3201/eid1302.061295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trout RT, Steelman CD, Szalanski AL, Loftin K. Establishment of Amblyomma maculatum (Gulf Coast tick) in Arkansas, USA. Fla Entomol. 2010;93:120–2. 10.1653/024.093.0117 [DOI] [Google Scholar]
- 7.Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 2007;7:607–10.Medline 10.1089/vbz.2007.0121 [DOI] [PubMed] [Google Scholar]
- 8.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, et al. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. 10.1089/vbz.2007.0271 [DOI] [PubMed] [Google Scholar]
- 9.Lancaster JL. A guide to ticks of Arkansas. Fayetteville (AR): Agricultural Experiment Station, Division of Agriculture, University of Arkansas; 1973. Bulletin 779. [Google Scholar]
- 10.Kollars TM Jr, Kengluecha A. Spotted fever group Rickettsia in Dermacentor variabilis (Acari: Ixodidae) infesting raccoons (Carnivora: Procyonidae) and opossums (Marsupialia: Didelphimorphidae) in Tennessee. J Med Entomol. 2001;38:601–2. 10.1603/0022-2585-38.4.601 [DOI] [PubMed] [Google Scholar]
- 11.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trout RT, Steelman CD, Szalanski AL. Population genetics and phylogeography of Ixodes scapularis from canines and deer in Arkansas. Southwest Entomologist. 2009;34:273–87. 10.3958/059.034.0308 [DOI] [Google Scholar]
- 13.Stromdahl EY, Vince MA, Billingsley PM, Dobbs NA, Williamson PC. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15–24. 10.1089/vbz.2007.0138 [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. 10.2307/2408678 [DOI] [PubMed] [Google Scholar]