Abstract
BACKGROUND:
Left atrial (LA) systolic force (LASF) is significantly increased in chronic heart failure (CHF), arterial hypertension (HT) and aortic stenosis (AS). The increase is proportional to the degree of left ventricular hypertrophy and diastolic dysfunction.
OBJECTIVES:
To assess the magnitude of changes in maximal LA volume (LAVmax) and LASF in systolic CHF compared with other cardiac diseases, and to assess whether the left atrium remodels differently and works in response to specific conditions affecting diastolic function and to individual factors associated with LA alterations.
METHODS:
LAVmax and LASF were measured and evaluated by two-dimensional Doppler echocardiography in 94 patients with systolic CHF and normal left ventricular filling pressure, 100 control patients, 181 patients with HT, 40 patients with idiopathic hypertrophic cardiomyopathy (HCMP) and 85 patients with AS. The prevalence of LA dilation and supernormal LASF (defined as values of LAVmax and LASF exceeding two SDs of the mean of controls) was measured in all groups.
RESULTS:
LAVmax and LASF were 7.1±2 mL/m3 and 7.8±4 kdynes in controls, and 11.0±4 mL/m3 and 19.7±11 kdynes in systolic CHF patients, respectively (both P<0.001). These values were significantly higher than in patients with HT, but similar to those with AS and HCMP. LA dilation and supernormal LASF were detected in 13% and 11% of patients with HT, 32% and 59% of patients with AS, 26% and 43% of patients with HCMP, and 41% and 56% of patients with systolic CHF, respectively (all P<0.01). In multiple logistic analysis, systolic CHF represented the strongest predictor of supernormal LASF. It was not independently associated with LA dilation, which was mainly related to indexes of volume load.
CONCLUSIONS:
LAVmax and LASF were markedly increased in patients with systolic CHF, with a magnitude that was significantly higher than that of HT patients, but similar to that measured in HCMP and AS patients. In the present community population with various cardiac diseases, systolic CHF represented the most powerful stimulus for increasing LASF and was not related to LA dilation.
Keywords: Aortic stenosis, Arterial hypertension, Chronic heart failure, Dilated cardiomyopathy, Hypertrophic cardiomyopathy, Left atrial systolic function
In patients with systolic chronic heart failure (CHF), systemic arterial hypertension (HT), aortic stenosis (AS) or idiopathic hypertrophic cardiomyopathy (HCMP), left atrial (LA) size and function increase to counterbalance the impairment of left ventricular (LV) diastolic function (1–8). Thus, LA size and function change in all conditions of increased LV hemodynamic load and/or LV mass, irrespective of aortic valve gradient, LV outflow tract obstruction, degree of HT and distribution of hypertrophy, with differences among various etiologies related to LA preload and/or intrinsic LA contractility (9,10).
We demonstrated that in patients with AS and HT, concentric LV geometry increased LA size and function more than eccentric geometry (1,4). The spectrum of LA modifications may be different when systolic CHF coexists. Indeed, patients with systolic CHF have an eccentric LV geometry resulting from the myocardial remodelling process and the need for recruiting the maximal LA work for maintaining LV filling pressures near to normal values (6,7).
In the present study, we measured LA size and force in a group of patients with systolic CHF, and compared these variables with those measured in controls and in patients with HT, AS and HCMP (typically pressure or combined pressure-volume overload states). The aims of the present study were to assess the magnitude of changes in LA size and force in systolic CHF and in the other cardiac diseases, and to assess whether the left atrium remodels differently and works in response to specific conditions affecting diastolic function and to individual factors associated with LA alterations.
METHODS
Patients were referred to the echocardiography laboratory at Villa Bianca Hospital in Trento, Italy, by their general practitioner. Patients who were older than 50 years of age and in sinus rhythm were selected for the study. Patients were consecutively enrolled from December 2005 to May 2007. They underwent a clinical and echocardiographic examination performed on the same day between 08:00 and 12:00.
Patients with HT (n=181) (defined as receiving pharmacological treatment for high blood pressure), AS (n=85) (defined as aortic valve thickening accompanied by a Doppler-measured aortic peak flow velocity of 2.5 m/s or greater) (11), HCMP (n=40) (identified according to Maron’s criteria [12]) and systolic CHF (n=94) were included in the study. Among the systolic CHF patients, subjects were selected if they were in stable clinical condition (New York Heart Association [NYHA] class II or III) and had experienced at least one episode of congestive heart failure requiring hospitalization, and were receiving tailored pharmacological treatment for heart failure (13), with an LV end-diastolic volume of greater than 28 mL/m3 (corresponding to 78 mL/m2) and an LV ejection fraction of 50% or less (14). For entry into the study, subjects needed to be classified as group I or II according to Redfield’s classification of LV diastolic function (see below) (15).
For all study groups, the exclusion criteria were the recognition of signs of heart failure at clinical evaluation, any grade of mitral stenosis, any grade of atrioventricular block, moderate-severe mitral regurgitation, any procedure involving coronary artery revascularization and/or documented episodes of sustained atrial arrhythmias occurring within three months from the echocardiogram. Patients taking medications to prevent atrial arrhythmias were also not eligible to participate. Patients with systolic CHF were excluded if their pulmonary artery wedge pressure (PAWP) measured by echo-Doppler technique exceeded 12 mmHg. One hundred healthy subjects who underwent echocardiography for a check-up and whose echocardiographic features were interpreted as normal formed the control group. These subjects did not take any medication, and did not have HT, diabetes mellitus, or previous atrial arrhythmias or cardiovascular events.
Echocardiography
Echocardiographic studies were performed by an experienced sonographer (GC) using commercially available equipment (Megas machine, Esaote Biomedica, Italy). LV chamber dimensions (normalized for high), septum and posterior wall thickness, and mass were measured by M-mode tracings according to the American Society of Echocardiography recommendations (16,17). LV mass was normalized for height to the 2.7 power (18), and LV hypertrophy was defined as an LV mass of 49.2 g/m2.7 or greater for men and 46.7 g/m2.7 or greater for women (19). Relative wall thickness was calculated as two times the posterior wall thickness/LV diastolic diameter ratio and used as an index of LV geometry (values of 0.44 or greater were considered to be indicative of concentric geometry) (19). Wall LV mechanics were assessed by computation of midwall fractional shortening according to previously reported methods (20). LV volumes were calculated by two-dimensional mode apical four-chamber view using the area-length method, which were then normalized for height to the third power. In patients with AS, aortic valve area was measured by the continuity equation method. Mitral regurgitation was diagnosed by colour Doppler and quantified using a 1 to 4+ grading system (21).
Mitral flow velocities (in all patients) and pulmonary venous flow velocities (measured only in systolic CHF patients for estimating PAWP according to the equations proposed by Pozzoli et al [22]) were assessed by pulsed-wave Doppler as previously described. Tissue Doppler was also used for measuring peak systolic mitral annular velocity (E′ wave) obtained from the septal site of the mitral annulus to confirm the presence of normal PAWP (defined as an E/E′ ratio of less than 8) (23). Patients were categorized into four classes according to the progression of diastolic dysfunction as follows: class I, normal; class II, mild dysfunction (impaired relaxation without evidence of increased PAWP; class III, moderate dysfunction (impaired relaxation associated with mild to moderate elevation of PAWP); and class IV, severe dysfunction (restrictive filling with increased PAWP) (15). Cardiac output was determined by pulsed Doppler echocardiography as previously reported (24).
LA size was evaluated as maximal LA volume (LAVmax) from two-dimensional apical four-chamber view using the area-length method (25). LA systolic force (LASF) – an index of LA systolic function – was determined using a formula previously validated by Manning et al (26), with calculation of the mitral valve area from the mitral diameter assuming a circular annular geometry:
where MOA is the mitral orifice area and A is the velocity of the late diastolic wave of mitral flow (atrial systole). MOA was calculated from the mitral annulus diameter measured from the apical four-chamber view during the LA mechanical contraction (LV end-diastolic period, at the beginning of the R wave of electrocardiography); transmitral peak A velocity was measured as detailed above.
The reproducibility of M-mode, two-dimensional mode and pulsed-wave Doppler echocardiographic measurements has been previously reported (1,4).
Statistical analysis
Statistical analysis was performed using SPSS software (version 11.0, SPSS Inc, USA). Data are reported as mean values ± one SD. Between-group comparisons of categorical and continuous variables were performed by χ2 test and ANOVA, with post hoc comparison between each group by Scheffé’s test and Tukey’s honestly significant difference test (Spjotvoll-Stoline) for unequal sample sizes, as appropriate. Age and height were also considered to be covariates in the analyses for their influence on LASF and LAVmax. Patients were categorized according to the presence of LA dilation and supernormal LASF using the cut-off values of 11.3 mL/m3 and 15.4 kdynes, corresponding to the mean LAVmax and LASF plus two SDs of the mean in controls, respectively.
The associations of LAVmax and LASF with clinical and echocardiographic variables were assessed using least squares linear regression. Multiple linear regression analysis was used to evaluate independent relations of LAVmax and LASF with variables that were significantly associated (P<0.05) in the univariate analysis. In-model tolerance was calculated to evaluate multicollinearity. Pretest minimal accepted tolerance was 0.80 or greater. These analyses were initially performed to include the total population, then in patients with systolic CHF, controls and in the remaining patients belonging to the HT, AS and HCMP groups (they had similar LV geometry and were considered collectively). Multivariate logistic regression analyses were performed to assess the factors associated with LA dilation and supernormal LASF. A two-tailed P<0.05 was considered to be statistically significant.
RESULTS
During the recruitment period, 500 subjects fulfilled the enrollment criteria (mean age was 72±10 years, 56% women). Patients with AS had a mean transaortic peak gradient of 39±17 mmHg, a mean gradient of 27±14 mmHg and a valve area of 1.15±0.3 cm2. The prevalence of LV hypertrophy was 60% and 36% in patients with AS and HT, respectively. LV diameters and mass were progressively greater in patients with HT (n=181), AS (n=85), HCMP (n=40) and systolic CHF (n=94). The main characteristics of the study groups are reported in Table 1.
TABLE 1.
Main characteristics of the study patients
| Variable | Systolic CHF (n=94) | Arterial hypertension (n=181) | HCMP (n=40) | Aortic stenosis (n=85) |
|---|---|---|---|---|
| Age, years | 73±11 | 72±8 | 73±10 | 75±8 |
| Body mass index, kg/m2 | 24±5 | 26±4 | 25±4 | 26±4 |
| Heart rate, beats/min | 67±11 | 68±10 | 58±5 | 66±11 |
| Systolic blood pressure, mmHg | 126±11 | 150±19 | 138±11 | 134±10 |
| Relative wall thickness | 0.32±0.07 | 0.43±0.07 | 0.47±0.07 | 0.49±0.08 |
| LV mass, g/m2.7 | 77±27 | 55±14 | 77±20 | 67±19 |
| LV end-diastolic volume, mL/m3 | 35±15 | 20±5 | 22±7 | 22±7 |
| LV ejection fraction, % | 37±13 | 64±7 | 60±6 | 61±6 |
| LV stroke volume, mL/beat | 58±17 | 60±18 | 59±21 | 70±25 |
| Peak E wave velocity MF, cm/s | 62±20 | 56±16 | 64±15 | 61±18 |
| Peak A wave velocity MF, cm/s | 74±21 | 70±16 | 78±23 | 83±19 |
| E/A ratio | 0.84±0.28 | 0.80±0.21 | 0.82±0.26 | 0.73±0.20 |
| Deceleration time of MF, ms | 232±86 | 185±53 | 315±113 | 243±93 |
Data presented as mean ± SD. CHF Chronic heart failure; HCMP Hypertrophic cardiomyopathy; LV left ventricular; MF Mitral flow
In systolic CHF patients, the mean PAWP was 9.9±1.9 mmHg and cardiac output was 2.25±0.71 L/min/m2. The etiology of systolic CHF was ischemic in 54 patients (57%) and idiopathic in 40 patients (43%). Considering these two subgroups, LAVmax was greater in those with idiopathic rather than ischemic systolic CHF (13.5±5.0 mL/m3 versus 10.2±4.0 mL/m3; P=0.003). LASF was similar between the two subgroups (20.1±11.5 kdynes versus 19.5±10.2 kdynes, respectively; P not significant), which did not differ for any other clinical or echocardiographic variable, with the exception of LV end-diastolic and end-systolic volume (40±18 mL/m3 versus 31±12 mL/m3 [P=0.004], and 28±19 mL/m3 versus 20±9 mL/m3 [P=0.009], respectively) greater in the idiopathic than ischemic systolic CHF.
LA size and force in the total population
The mean LAVmax and LASF of the study population were 8.9±3.3 mL/m3 and 13.5±9.4 kdynes/cm2, respectively. Multiple linear regression analyses were performed to assess the covariates of these two variables in the total study population. The following variables were introduced in the models: age, body weight, body mass index, heart rate, systolic blood pressure, diastolic blood pressure, LV mass index, cardiac output, stroke volume, NYHA functional class, LV ejection fraction, end-diastolic volume, systolic CHF, AS, HCMP, midwall shortening and relative wall thickness. The independent predictors of LAVmax were LV end-diastolic volume, body mass index and age (Table 2). LASF was related to systolic CHF, AS, age, LV mass, HCMP, heart rate and stroke volume (Table 3).
TABLE 2.
Variables independently related to maximal left atrial volume in the study population (n=500): Multivariate linear regression model
| Variable | Standardized beta coefficients | P | Collinearity statistics (tolerance/VIF) |
|---|---|---|---|
| Left ventricular end-diastolic volume, mL/m3 | 0.50 | <0.001 | 0.997/1.003 |
| Body mass index, kg/m2 | 0.30 | <0.001 | 0.992/1.008 |
| Age, years | 0.21 | <0.001 | 0.995/1.005 |
| Final results for multivariate regression model (SEE 2.6, R2=0.35) | 0.60 | <0.001 |
SEE Standard error of estimation; VIF Variance inflation factor
TABLE 3.
Variables independently related to left atrial systolic force in the study population (n=500): Multivariate linear regression model
| Variable | Standardized beta coefficients | P | Collinearity statistics (tolerance/VIF) |
|---|---|---|---|
| Dilated cardiomyopathy (n=94) | 0.45 | <0.001 | 0.80/1.41 |
| Aortic stenosis (n=85) | 0.32 | <0.001 | 0 88/1.13 |
| Age, years | 0.26 | <0.001 | 0 83/1.21 |
| Left ventricular mass, g/m2 | 0.17 | <0.001 | 0.80/1.40 |
| Hypertrophic cardiomyopathy (n=40) | 0.14 | <0.001 | 0.83/1.20 |
| Heart rate, beats/min | 0.14 | <0.001 | 0.97/1.03 |
| Stroke volume, mL | 0.12 | 0.002 | 0.93/1.08 |
| Final results for multivariate regression model (SEE 6.8, R2=0.44) | 0.66 | <0.001 |
SEE Standard error of estimation; VIF Variance inflation factor
LA dilation and supernormal LASF
Patients without either LA dilation or supernormal LASF comprised 67% of the study sample; LA dilation without supernormal LASF was found in 8% of patients; supernormal LASF without LA dilation was found in 14%; and LA dilation associated with supernormal LASF was present in 11% of patients. The main characteristics of the study patients according to the presence of LA dilation and/or supernormal LASF, and the prevalence of these two conditions in the study subgroups, are shown in Table 4.
TABLE 4.
Main characteristics of the study patients according to the presence of left atrial (LA) dilation and/or supernormal systolic force
| No LA dilation and no supernormal LASF | LA dilation ‘alone’ | Supernormal LASF ‘alone’ | LA dilation and supernormal LASF | P | |
|---|---|---|---|---|---|
| Prevalence of patients, % | |||||
| Controls (n=100) | 95 | 2 | 2 | 1 | |
| Hypertension (n=181) | 80 | 9 | 7 | 4 | |
| Aortic stenosis (n=85) | 46 | 8 | 27 | 19 | |
| Hypertrophic CMP (n=40) | 53 | 11 | 20 | 15 | |
| Dilated CMP (n=94) | 30 | 13 | 29 | 28 | |
| Total (n=500) | 67 | 8 | 14 | 11 | |
| Age, years | 71±9 | 74±8 | 74±8 | 76±10 | *†‡ |
| Male sex, % | 43 | 49 | 45 | 51 | |
| Body mass index, kg/m2 | 25±4 | 27±4 | 26±4 | 27±4 | *†‡ |
| Heart rate, beats/min | 68±11 | 66±10 | 69±10 | 68±9 | |
| Systolic blood pressure, mmHg | 144±18 | 145±20 | 144±21 | 143±26 | |
| NYHA functional class (I–IV scale) | 1.1±0.4 | 1.5±0.8 | 1.7±0.7 | 1.9±0.8 | *†‡ |
| Relative wall thickness | 0.40±0.07 | 0.41±0.08 | 0.41±0.1 | 0.38±0.1 | |
| LV mass, g/m2.7 | 43±12 | 59±12 | 56±13 | 67±19 | *†‡ |
| Midwall fractional shortening, % | 16.5±3.0 | 15.2±3.3 | 15.3±3.4 | 13.8±3.3 | *†‡ |
| CESS, mmHg/mm | 135±41 | 145±60 | 130±44 | 138±38 | |
| Peak LV end-systolic pressure, mmHg | 146±22 | 153±29 | 154±30 | 155±37 | *†‡ |
| LV end-diastolic volume, mL/m3 | 20.5±4.8 | 29.4±8.1 | 26.7±7.9 | 34.4±12.2 | *†‡ |
| LV ejection fraction, % | 62±9 | 55±17 | 53±14 | 50±15 | *†‡ |
| LV stroke volume, mL/beat | 70±17 | 80±20 | 79±22 | 80±20 | *†‡ |
| Peak E wave velocity mitral flow, cm/s | 58±15 | 63±14 | 60±15 | 66±21 | |
| Peak A wave velocity mitral flow, cm/s | 69±14 | 59±14 | 92±12 | 93±19 | †‡ |
| Deceleration time of mitral flow, ms | 239±70 | 243±95 | 270±90 | 245±62 | † |
| Normal diastolic function, % | 76 | 32 | 21 | 15 | *†‡ |
| Mild diastolic dysfunction, % | 24 | 68 | 79 | 85 | *†‡ |
Data presented as mean ± SD unless otherwise indicated.
P<0.05 when comparing normal LA size/LA systolic force (LASF) with LA dilation ‘alone’;
P<0.05 when comparing normal LA size/LASF with LASF ‘alone’;
P<0.05 when comparing normal LA size/LASF with LA dilation and supernormal LASF. CESS Circumferential end-systolic stress; CMP Cardiomyopathy; LV Left ventricular; NYHA New York Heart Association
Multiple logistic regression analysis showed that the phenomenon of LA dilation was associated with higher LV mass (Exponent [Exp] β 1.07, 95% CI 1.05 to 1.09; P< 0.001), older age (Exp β 1.06, 95% CI 1.05 to 1.09; P<0.001), greater stroke volume (Exp β 1.02, 95% CI 1.01 to 1.04; P=0.006) and greater LV end-diastolic volume (Exp β 1.02, 95% CI 1.01 to 1.03; P=0.01).
The status of supernormal LASF was associated with AS (Exp β 6.21, 95% CI 3.11 to 12.39; P<0.001), systolic CHF (Exp β 5.52, 95% CI 2.56 to 11.92; P<0.001), higher LV mass (Exp β 1.06, 95% CI 1.03 to 1.08; P<0.001), older age (Exp β 1.06, 95% CI 1.03 to 1.10; P<0.001) and higher NYHA functional class (Exp β 2.20, 95% CI 1.30 to 3.70; P<0.001). The same variables predicted the condition of LA dilation associated with supernormal LASF (data not shown).
Comparison of the study groups
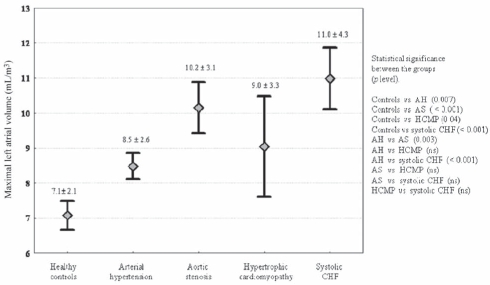
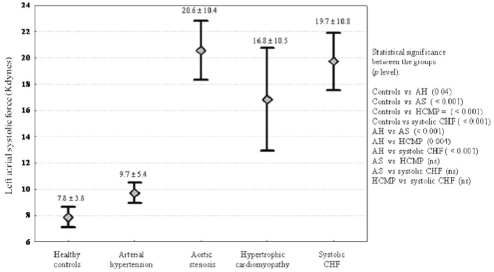
All study subgroups had significantly greater LAVmax and higher LASF than controls (Figures 1 and 2, respectively). The values of these two variables were markedly high in patients with systolic CHF. The differences among systolic CHF, AS and HCMP were not significant.
Figure 1).
Values of maximal left atrial volume (mL/m3) in the different subgroups of the study population. Mean ± SD and the statistical significance between the groups are shown. AH Arterial hypertension; AS Aortic stenosis; CHF Chronic heart failure; HCMP Hypertrophic cardiomyopathy; ns Not significant; vs Versus
Figure 2).
Values of left atrial systolic force (kdynes) in the different subgroups of the study population. Mean ± SD and the statistical significance between the groups are shown. AH Arterial hypertension; AS Aortic stenosis; CHF Chronic heart failure; HCMP Hypertrophic cardiomyopathy; ns Not significant; vs Versus
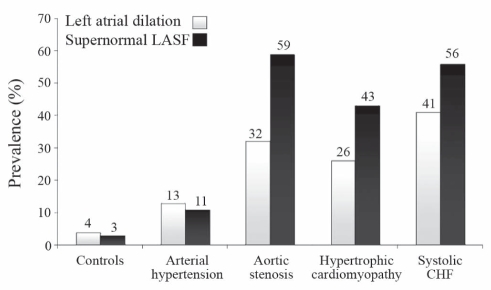
The prevalence of LA dilation and supernormal LASF was 10-fold and 19-fold higher in systolic CHF patients than in controls, respectively (Figure 3). The magnitude of these two phenomena was similar in systolic CHF, AS and HCMP patients, while it was significantly lower in HT patients.
Figure 3).
Distribution of the phenomena of left atrial dilation and supernormal left atrial systolic force (LASF) in the healthy controls and subgroups of patients with cardiac diseases. CHF Chronic heart failure
Correlates of LA size in systolic CHF
Variables related to LAVmax in controls, patients with systolic CHF and subjects with HT, AS and HCMP (considered together in the present analysis) are listed in Table 5. Higher LV mass and LV end-diastolic volume emerged as independent factors associated with higher LA volume in all groups. In patients with systolic CHF, even older age and higher PAWP were independent markers of higher LA volume.
TABLE 5.
Multivariate regression analysis: Independent predictors of maximal left atrial (LA) volume in controls, and in patients with dilated cardiomyopathy and other cardiac diseases
|
Healthy controls* (n=100) |
Dilated cardiomyopathy†(n=94) |
Aortic stenosis, HCMP, hypertension‡(n=306) |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Beta | P | Variable | Beta | P | Variable | Beta | P |
| Systolic blood pressure, mmHg | 0.24 | 0.008 | LV EDV, mL/m3 | 0.35 | 0.001 | LV mass, g/m2.7 | 0.31 | <0.001 |
| LV mass, g/m2.7 | 0.30 | <0.001 | LV mass, g/m2.7 | 0.25 | 0.003 | LV EDV, mL/m3 | 0.24 | <0.001 |
| LV EDV, mL/m3 | 0.22 | 0.02 | PAWP, mmHg | 0.24 | 0.005 | LA systolic force, kdynes | 0.21 | <0.001 |
| Age, years | 0.19 | 0.03 | Body mass index, kg/m2 | 0.10 | 0.04 | Age, years | 0.10 | 0.04 |
r=0.50, standard error of estimation (SEE) = 1.85, P<0.001;
r=0.69, SEE=3.3, P<0.001;
r=0.64, SEE=2.2, P<0.001. EDV End-diastolic volume; HCMP Hypertrophic cardiomyopathy; LV Left ventricular; PAWP Pulmonary artery wedge pressure
Correlates of LA systolic force in systolic CHF
In patients with systolic CHF, as well in those belonging to the other groups, LASF increased with age. LV geometry was independently associated with LASF in both systolic CHF patients and controls. Interestingly, concentric LV geometry was associated with higher LASF in controls, while eccentric LV geometry and cardiac output were associated with higher LASF in systolic CHF patients (Table 6). Fifty-three of 94 patients (56%) with systolic CHF had supernormal LASF. The variables associated with this condition were older age (Exp β 1.13, 95% CI 1.05 to 1.23; P=0.001) and higher systolic blood pressure (Exp β 1.07, 95% CI 1.02 to 1.13; P=0.01).
TABLE 6.
Multivariate regression analysis: Independent predictors of left atrial systolic force in controls, and in patients with dilated cardiomyopathy and other cardiac diseases
|
Healthy controls* (n=100) |
Dilated cardiomyopathy†(n=94) |
Aortic stenosis, HCMP, hypertension‡(n=306) |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Beta | P | Variable | Beta | P | Variable | Beta | P |
| Relative wall thickness | 0.30 | 0.002 | Age, years | 0.49 | 0.001 | Age, years | 0.18 | 0.001 |
| Age, years | 0.25 | 0.01 | Cardiac output, L/min/m2 | 0.33 | 0.003 | LV mass, g/m2.7 | 0.24 | <0.001 |
| Relative wall thickness | 0.21 | 0.005 | LV EDV, mL/m3 | 0.21 | 0.002 | |||
r=0.50, standard error of estimation (SEE) = 1.85, P<0.001;
r=0.59, SEE=9.1, P<0.001;
r=0.43, SEE=7.4, P<0.001. EDV End-diastolic volume; HCMP Hypertrophic cardiomyopathy; LV Left ventricular
DISCUSSION
With augmented stiffness or reduced compliance of the left ventricle, LA pressure increases to preserve LV filling (27). As a result, the Frank-Starling mechanism begins to operate in the left atrium, leading to chamber dilation and increased contractility, systolic force and work (1,5,28–30). In chronic pathological conditions, all of these changes progress according to the severity of LV diastolic function (1,3,31–34). From this perspective, it is not surprising that increased LA size and LASF have been proven to be strong predictors of adverse cardiovascular events in several recent publications including population-based cohort studies (35,36), clinical trials in CHF (37–39) and in HT patients (40,41).
Relationships between systolic CHF and LA size
The present study demonstrated that in patients with systolic CHF and stable hemodynamic conditions, the Frank-Starling mechanism in the left atrium is maximally used. Our analyses showed that the significant increase in LAVmax detected in systolic CHF patients was not due to the ‘systolic CHF entity’ per se, but mostly due to systolic CHF-related factors such as older age, higher body mass index and greater LV end-diastolic volume. Together with these conditions, many other factors such as higher LV mass, mitral regurgitation, LV diastolic dysfunction, reduction of LA compliance, LA myocardial fibrosis and/or ischemia, angiotensin II activation, atrial fibrillation and HT have been described as markers of LA remodelling and performance in humans coexisting with LV dilation (1,5,34,39,41). Excluding atrial fibrillation and mitral regurgitation, all other factors could be operating in our systolic CHF patients. Statistical analysis revealed that in our patients, LAVmax was mostly determined by LV end-diastolic volume and mass, PAWP and age. These results are consistent with those recently reported by Rossi et al (38) who identified the degree of LV dilation, diastolic dysfunction and the extent of mitral regurgitation as the variables independently related to LA volume in a large group of patients with systolic CHF. Our findings confirm that in these patients, LV wall stress and stiffness (identified through increased LV size and mass) mainly impact LA geometry. Differing from the results of Rossi et al (38), mitral regurgitation in our patients did not emerge as a factor associated with LA volume. This was clearly due to the exclusion of subjects with moderate-severe mitral regurgitation and those with high PAWP who may consequently experience a greater extension of LA volume.
Despite stable hemodynamic conditions, PAWP was positively related to LA volume in our patients. It is well known that increased LA volume usually reflects elevated PAWP in subjects without primary LA pathologies, or congenital heart or mitral valve disease. This result may indicate that among systolic CHF patients with normal PAWP (which is a mean value of LV filling pressure), some individuals have significant alterations to the active or passive phase of LV diastolic function, leading to a higher degree of LA deformation.
Systolic CHF and LA systolic force
Because of its thin wall, the main response of the left atrium to increased PAWP is dilation. However, under this condition, significant increase in systolic function has been documented in patients with HT (5,41), AS (1) and also with systolic CHF (7,8). In systolic CHF patients, it has been demonstrated that increased LA systolic function is closely associated with exercise performance (42,43). In our patients with normal PAWP, we found that LASF was markedly increased (with a magnitude similar to that measured in AS and HCMP). Etiology did not influence LASF, which was comparable in our patients with ischemic or idiopathic systolic CHF. This is an unexpected finding, considering that one decade ago Triposkiadis et al (44) demonstrated that LA systolic function is depressed in idiopathic and preserved in ischemic dilated cardiomyopathy, despite similar LA loading conditions. The authors, however, commented that such an index of LA myopathy may be related to the differences in the response to medical treatment and clinical outcome, which have changed considerably in the past few years. More recently, and in line with our results, the same investigators found that LA systolic function measured at rest in patients with systolic CHF receiving optimized pharmacological treatment for systolic CHF was similar in patients with idiopathic and ischemic etiology of cardiac disease, while a reduced LA systolic reserve could be documented in the former during dobutamine infusion (45). Improvement in the clinical and pharmacological management of patients with systolic CHF resulting in improvements in LA function (46) may explain these discrepancies.
In our study, we also documented that LASF was strongly influenced by systolic CHF, which represents its most important determinant. The probability of supernormal LASF was 5.5-fold higher in patients with systolic CHF than in those without systolic CHF. The increase in LASF in these patients is independent of significant contributions from age, cardiac output and LV eccentric geometry (expressed as relative wall thickness). The direct relationship between LASF and age has been previously described in patients with HT (5,41) and AS (1). Our results add to their findings by also demonstrating the same relationship in patients with systolic CHF. Furthermore, we documented a positive relationship between cardiac output and LV eccentric geometry (two raw indexes of volume overload) and LASF. Interestingly, in controls, concentric rather than eccentric LV geometry was associated with higher LA systolic function. We recently reported similar findings in patients with AS (1) and HT (4). In these three conditions, to avoid unfavourable changes in PAWP secondary to LV diastolic impairment, the Frank-Starling mechanism mainly operates by increasing LV and LA myocardial contractility. In systolic CHF patients with an inadequate LV systolic reserve, the failing hearts primarily increase chamber volumes in response to increased PAWP.
Limitations
We must call attention to some limitations of the present study. Our data do not allow accurate assessment of LA and LV filling pressures due to the noninvasive (echocardiographic) technique used. The mitral annulus excursion velocity measurements using tissue Doppler could have been useful for evaluating LA contractility; however, this parameter was not routinely measured in our patients. Finally, the estimation of mitral area (mandatory for the calculation of LASF) was not precise in some patients (particularly in those with systolic CHF) because the geometry of the mitral annulus was not circular.
CONCLUSIONS
Patients with systolic CHF and stable hemodynamic conditions have a marked increase in LA size and systolic force. The magnitude of these LA structural and functional changes is significantly higher than in patients with HT and similar to values observed in patients with HCMP and AS. Older age, higher PAWP and greater LV volumes largely explain the LA remodelling in systolic CHF patients. In our setting of community patients with different cardiac diseases, systolic CHF represented the most powerful stimulus for the left atrium in increasing LASF. The increase in LA volume was mainly related to indexes of volume load, not to systolic CHF per se.
REFERENCES
- 1.Cioffi G, Stefenelli C. Comparison of left ventricular geometry and left atrial size and function in patients with aortic stenosis versus those with pure aortic regurgitation. Am J Cardiol. 2002;90:601–6. doi: 10.1016/s0002-9149(02)02563-8. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda M, Matsuda Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin Cardiol. 1996;19:954–9. doi: 10.1002/clc.4960191211. [DOI] [PubMed] [Google Scholar]
- 3.Triposkiadis F, Pitsavos C, Boudoulas H, et al. Left atrial volume and function in valvular aortic stenosis. J Heart Valve Dis. 1993;2:104–13. [PubMed] [Google Scholar]
- 4.Cioffi G, Mureddu GF, Stefenelli C, de Simone G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22:1589–96. doi: 10.1097/01.hjh.0000125454.28861.76. [DOI] [PubMed] [Google Scholar]
- 5.Mureddu GF, Cioffi G, Stefenelli C, Boccanelli A. Relationships of the appropriateness of left ventricular mass to left atrial size and function in arterial hypertension. J Cardiovasc Med (Hagerstown) 2007;8:445–52. doi: 10.2459/01.JCM.0000269718.41059.62. [DOI] [PubMed] [Google Scholar]
- 6.Triposkiadis F, Harbas C, Sitafidis G, Skoularigis J, Demopoulos V, Kelepeshis G. Echocardiographic assessment of left atrial ejection force and kinetic energy in chronic heart failure. Int J Cardiovasc Imaging. 2008;24:15–22. doi: 10.1007/s10554-007-9219-7. [DOI] [PubMed] [Google Scholar]
- 7.Triposkiadis F, Harbas C, Kelepeshis G, et al. Left atrial remodeling in patients younger than 70 years with diastolic and systolic heart failure. J Am Soc Echocardiogr. 2007;20:177–85. doi: 10.1016/j.echo.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Shin MS, Fukuda S, Song JM, et al. Relationship between left atrial and left ventricular function in hypertrophic cardiomyopathy: A real-time 3-dimensional echocardiographic study. J Am Soc Echocardiogr. 2006;19:796–801. doi: 10.1016/j.echo.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Nakao T, Shimizu M, Sugihara N, Kita Y, Shimizu K, Takeda R. Preload dependency of left atrial pump function in hypertrophic cardiomyopathy. Jpn Circ J. 1993;57:47–54. doi: 10.1253/jcj.57.47. [DOI] [PubMed] [Google Scholar]
- 10.Dardas PS, Filippatos GS, Tsikaderis DD, et al. Noninvasive indexes of left atrial diastolic function in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2000;13:809–17. doi: 10.1067/mje.2000.105579. [DOI] [PubMed] [Google Scholar]
- 11.Rossebo AB, Pedersen TR, Allen C, et al. Design and baseline characteristics of the simvastatin and ezetimibe in aortic stenosis (SEAS) study. Am J Cardiol. 2007;99:970–3. doi: 10.1016/j.amjcard.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Peterson EE, Maron MS, Peterson JE. Prevalence of hypertrophic cardiomyopathy in an outpatient population referred for echocardiographic study. Am J Cardiol. 1994;73:577–80. doi: 10.1016/0002-9149(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 13.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: A study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–9. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 14.Cioffi G, Tarantini L, De Feo S, et al. Dilated versus nondilated cardiomyopathy in the elderly population treated with guideline-based medical therapy for systolic chronic heart failure. J Card Fail. 2004;10:481–9. doi: 10.1016/j.cardfail.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Sahn DJ, Demaria A, Kisslo J, Weyman A. The committee on M-Mode standardization on the American Society of Echocardiography: Recommendations regarding quantitation in M-Mode echocardiography. Results of a survey study of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 18.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 19.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodelling in arterial hypertension. J Am Coll Cardiol. 1992;19:1550–8. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall fractional shortening/end systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–51. doi: 10.1016/0735-1097(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 21.Miyatake K, Izumi S, Okamoto M, et al. Semiquantitative grading of severity of mitral regurgitation by real time two dimensional Doppler flow imaging technique. J Am Coll Cardiol. 1986;7:82–8. doi: 10.1016/s0735-1097(86)80263-7. [DOI] [PubMed] [Google Scholar]
- 22.Pozzoli M, Capomolla S, Pinna G, Cobelli F, Tavazzi L. Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure with and without mitral regurgitation. J Am Coll Cardiol. 1996;27:883–93. doi: 10.1016/0735-1097(95)00553-6. [DOI] [PubMed] [Google Scholar]
- 23.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 24.Gola A, Pozzoli M, Capomolla S, et al. Comparison of Doppler echocardiography with thermodilution for assessing cardiac output in advanced congestive heart failure. Am J Cardiol. 1996;78:708–12. doi: 10.1016/s0002-9149(96)00406-7. [DOI] [PubMed] [Google Scholar]
- 25.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–32. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 26.Manning WJ, Silverman DI, Katz SE, Douglas PS. Atrial ejection force: A noninvasive assessment of atrial systolic function. J Am Coll Cardiol. 1993;22:221–5. doi: 10.1016/0735-1097(93)90838-r. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg B, Chatterjee K, Parmley WW, Werner JA, Holly AN. The influence of left ventricular filling pressure on atrial contribution to cardiac output. Am Heart J. 1979;98:742–51. doi: 10.1016/0002-8703(79)90473-3. [DOI] [PubMed] [Google Scholar]
- 28.Braunwald E, Frahm CJ. Studies on Starling’s law of the heart. IV. Observations on the hemodynamic functions of the left atrium in man. Circulation. 1961;24:633. [Google Scholar]
- 29.Yamaguchi M, Arakawa M, Tanaka T, Takaya T, Nagano T, Hirakawa S. Study on left atrial contractile performance – participation of Frank-Starling mechanism. Jpn Circ J. 1987;51:1001–9. doi: 10.1253/jcj.51.1001. [DOI] [PubMed] [Google Scholar]
- 30.Stott DK, Marpole DGF, Bristow JD, Kloster FE, Griswold HE. The role of left atrial transport in aortic and mitral stenosis. Circulation. 1970;41:1031. doi: 10.1161/01.cir.41.6.1031. [DOI] [PubMed] [Google Scholar]
- 31.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 32.Simek CL, Feldman MD, Haber HL, Wu CC, Jayaweera AR, Kaul S. Relationship between left ventricular wall thickness and left atrial size: Comparison with other measures of diastolic function. J Am Soc Echocardiogr. 1995;8:37–47. doi: 10.1016/s0894-7317(05)80356-6. [DOI] [PubMed] [Google Scholar]
- 33.Douglas PS. The left atrium: A biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–7. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 34.Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: Physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–41. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 36.Barnes ME, Miyasaka Y, Seward JB, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–14. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 37.Quinones MA, Greenberg BH, Kopelen HA, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: Significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 2000;35:1237–44. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 38.Rossi A, Cicoira M, Florea VG, et al. Chronic heart failure with preserved left ventricular ejection fraction: Diagnostic and prognostic value of left atrial size. Int J Cardiol. 2006;110:386–92. doi: 10.1016/j.ijcard.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 39.Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425–30. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 40.Gerdts E, Wachtell K, Omvik P, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: Losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49:311–6. doi: 10.1161/01.HYP.0000254322.96189.85. [DOI] [PubMed] [Google Scholar]
- 41.Chinali M, de Simone G, Roman MJ, et al. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005;18:1570–6. doi: 10.1016/j.amjhyper.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Terzi S, Dayi SU, Akbulut T, et al. Value of left atrial function in predicting exercise capacity in heart failure with moderate to severe left ventricular systolic dysfunction. Int Heart J. 2005;46:123–31. doi: 10.1536/ihj.46.123. [DOI] [PubMed] [Google Scholar]
- 43.Triposkiadis F, Trikas A, Pitsavos C, Papadopoulos P, Toutouzas P. Relation of exercise capacity in dilated cardiomyopathy to left atrial size and systolic function. Am J Cardiol. 1992;70:825–7. doi: 10.1016/0002-9149(92)90572-g. [DOI] [PubMed] [Google Scholar]
- 44.Triposkiadis F, Moyssakis I, Hadjinikolaou L, et al. Left atrial systolic function is depressed in idiopathic and preserved in ischemic dilated cardiomyopathy. Eur J Clin Invest. 1999;29:905–12. doi: 10.1046/j.1365-2362.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 45.Moyssakis I, Papadopoulos DP, Kelepeshis G, Gialafos E, Votteas V, Triposkiadis F. Left atrial systolic reserve in idiopathic vs. ischaemic-dilated cardiomyopathy. Eur J Clin Invest. 2005;35:355–61. doi: 10.1111/j.1365-2362.2005.01505.x. [DOI] [PubMed] [Google Scholar]
- 46.Sevimli S, Yilmaz M, Gundogdu F, et al. Carvedilol therapy is associated with an improvement in left atrial appendage function in patients with congestive heart failure. Echocardiography. 2007;24:623–8. doi: 10.1111/j.1540-8175.2007.00440.x. [DOI] [PubMed] [Google Scholar]