Abstract
A series of peptidyl α-ketoamides with the general structure Cbz-L-Leu-D,L-AA-CONH-R were synthesized and evaluated as inhibitors for the cysteine proteases calpain I, calpain II and cathepsin B. Nucleobases, methylpiperazine and dimethylaminoalkyl groups were incorporated into the primed region of the inhibitors to generate compounds that potentially cross the blood-brain barrier. Two of these compounds (Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl and Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) have been shown to have useful concentrations in the brain in animals. The best inhibitor for calpain I was Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl (Ki = 23 nM) and the best inhibitor for calpain II was Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl (Ki = 68 nM). Based on the crystal structure obtained with heterocyclic peptidyl α-ketoamides, we have improved inhibitor potency by introducing a small hydrophobic group on the adenine ring. These inhibitors have good potential to be used in the treatment of neurodegenerative diseases.
Introduction
Calpains are cysteine proteases that require calcium for activation. They belong to Clan CA of cysteine proteases together with cruzain, rhodesain, papain and cathepsins. There are multiple isoforms of calpain that are both ubiquitous and tissue specific. Calpain I (µ-calpain) and calpain II (m-calpain) are the two major calpain isoforms that are widely distributed in mammalian cells. These two isoforms are very similar and differ in the calcium concentration that they require to become activated. Calpain I is activated by micromolar concentrations of Ca+2 whereas calpain II is activated by millimolar concentrations of Ca+2. Calpains are involved in a variety of calcium-regulated biological processes, such as cell proliferation and differentiation, apoptosis, membrane fusion, signal transduction and platelet activation. Enhanced calpain activity has been observed in a number of diseases including ischemic1, 2 and traumatic3, 4 brain injury, cancer,5–7 muscular dystrophy,8, 9 cataracts,10 strokes11 and neurological disorders like Alzheimer’s,12, 13 Huntington’s14 and Parkinson’s15, 16 diseases and multiple sclerosis.17, 18 Involvement of calpains in a wide variety of biological processes and diseases makes them important targets for the development of inhibitors. There are several reviews on the roles of calpains in diseases.19–26
Synthetic calpain inhibitors can be divided into two groups: peptidic inhibitors and non-peptidic inhibitors. Peptidic inhibitors can further be divided into two groups: reversible inhibitors and irreversible inhibitors. Peptidyl aldehydes,27–35 α-ketoacids,36, 37 α-ketoesters,36 α-ketoamides,36, 38–40 α-diketones41 and α-keto phosphorus42 are examples of reversible peptidyl inhibitors whereas peptidyl epoxysuccinates,43–45 vinyl sulfones,46 acyloxymethyl ketones,47 diazomethyl ketones,48 and chloromethyl ketones49 are examples of irreversible peptidyl inhibitors of calpain. Reversible inhibitors of calpain are favored over the irreversible inhibitors for drug development since there are many isoforms of calpains and nonspecific inhibition of these isoforms can cause severe side effects. Calpain inhibitors have been reviewed.50–52
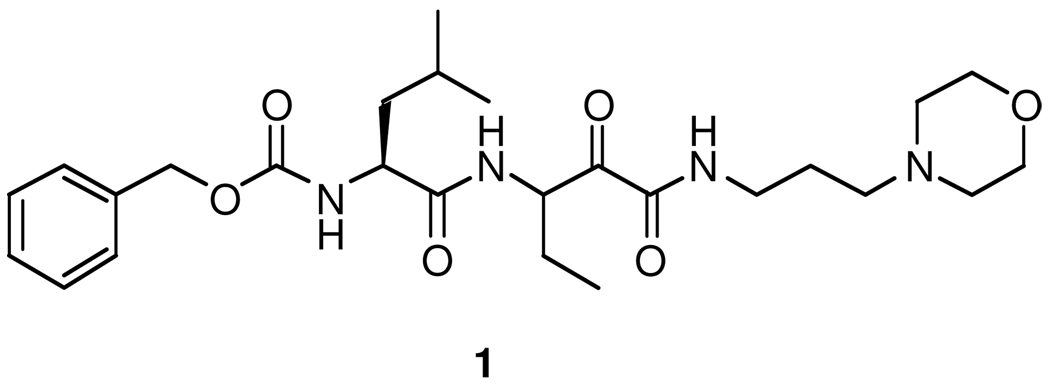
Synthetic calpain inhibitors protect against neuronal loss and improve neurological function in animal models of Alzheimer’s disease,53 traumatic brain injury,54 chronic progressive experimental autoimmune encephalomyelitis,55 cerebral ischemia,56 optic nerve degeneration,57 spinal cord injury,58, 59 and Taxol-induced sensory neuropathy.60 In addition, calpain inhibitors are effective for the treatment of cataracts61 and have antimalarial activity.62 The neuroprotective effects of calpain inhibitors are well established, but their use in treatment of human diseases is challenged by their inability to cross the blood-brain barrier (BBB). The BBB is a structural and physiological barrier that restricts the passage of various chemical substances into the central nervous system (CNS).63 The “protective” function of the BBB is also a major obstacle to the delivery of pharmacologic agents to the CNS for the treatment of neurological disorders. Our lead compound, Cbz-Leu-D,L-Abu-CONH-(CH2)3-morpholine (AK295, 1, Figure 1), is a reversible peptidyl α-ketoamide calpain inhibitor that is neuroprotective in models of head trauma,54 focal brain ischaemia64 and axonal degeneration caused by axotomy or exposure to vincristine65 and paclitaxel.60 The data document the potential for AK295 to be a potentially effective compound for the treatment of human disease, but the development of 1 as a drug may be hampered by its inability to cross the BBB. In order to design new analogs of 1 that may cross the BBB, we replaced the morpholine ring with structural features that could be recognized by the intrinsic BBB transport systems.
Figure 1.
Structure of Cbz-Leu-D,L-Abu-CONH-(CH2)3-morpholinyl (1).
Here we describe new calpain inhibitors that contain nucleobases, methylpiperazine, and dimethylaminoalkyl moieties in the primed region of the inhibitor. We hypothesized that these compounds could be recognized by BBB transport systems in the brain and thus would penetrate into the brain and spinal cord to inhibit calpain activation during the progression of neurological diseases.
Chemistry
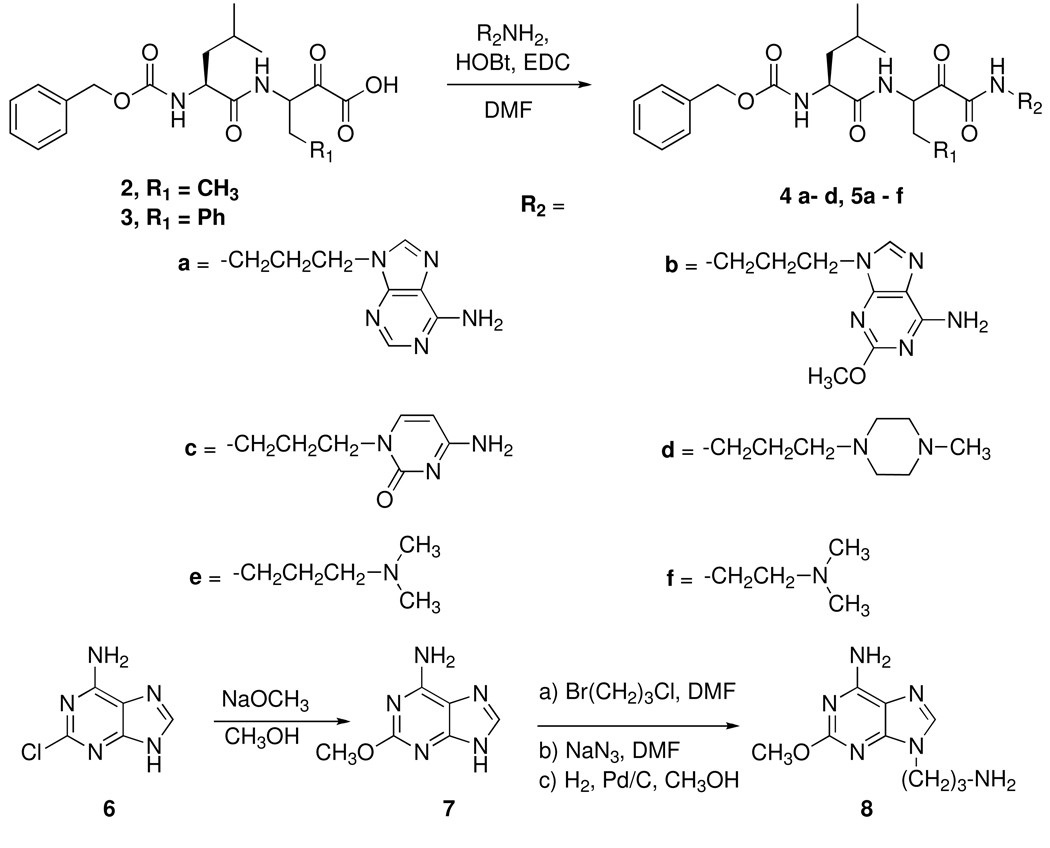
We previously reported synthetic methods for the preparation of peptidyl α-ketoamides.36, 38 The α-ketoesters Cbz-Leu-D,L-Abu-CO2Et and Cbz-Leu-D,L-Phe-CO2Et were prepared by a two step Dakin-West reaction from the corresponding dipeptide acids Cbz-Leu-Abu-OH and Cbz-Leu-Phe-OH. The dipeptide acids were reacted with ethyl oxalyl chloride in the presence of pyridine and 4-dimethylaminopyridine (DMAP) to form peptidyl α-enol esters. The peptidyl α-enol esters were then converted to peptidyl α-ketoesters by reacting with triethylamine. The peptidyl α-ketoacids were obtained by the hydrolysis of the peptidyl α-ketoesters with 1 M NaOH under standard deblocking conditions to give 2 and 3 (Figure 2).
Figure 2.
Synthesis of the peptidyl α-ketoamides.
Some of the P' amines such as N,N-dimethylpropane-1,3-diamine and N,N-dimethylethane-1,2-diamine were commercially available. Other amines such as 9-(3-aminopropyl)adenine66 and 9-(3-aminopropyl)-2-methoxyadenine were synthesized in three steps using the procedure described by Woollins and coworkers.66 Reaction of 2-chloroadenine (6) with sodium methoxide gave 2-methoxyadenine (7).67 Adenine and 2-methoxyadenine (7) were reacted with 1-bromo-3-chloropropane to add the linker by a single alkylation reaction. The chloro group on the linker was then reacted with sodium azide to obtain the corresponding azide derivatives. Catalytic reduction of the azide in the presence of palladium activated on carbon and hydrogen gas gave the precursor amines 9-(3-aminopropyl)adenine and 9-(3-aminopropyl)-2-methoxyadenine (8). For the synthesis of 1-(3-aminopropyl)cytosine,68 N-acetylcytosine was reacted with 1-bromo-3-chloropropane and then with sodium azide to form 1-(3-azidopropyl)-N-acetylcytosine. The acetyl group was deblocked with the ammonia and then catalytic reduction of azide to the amine was completed in the presence of palladium activated on carbon and hydrogen gas. For the synthesis of 1-(3-aminopropyl)-4-methylpiperazine,69 N-methylpiperazine was reacted with N-(3-bromopropyl)phthalimide and the corresponding amine was obtained after reacting N-(3-(4-methylpiperazin-1-yl)propyl)phthalimide with hydrazine monohydrate.
The target peptidyl α-ketoamides were obtained in low yields by coupling the appropriate α-ketoacid (2, 3) and the appropriate amine (R2NH2) using N-hydroxybenzotriazole (HOBt) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (Figure 2).
Results and Discussion
Synthetic Design
We designed a series of new peptidyl α-ketoamides extending to the primed region as calpain inhibitors. Peptidyl α-ketoamides inactivate the cysteine proteases by forming a reversible hemithioketal adduct with the active site cysteine residue that resembles the transition-state for peptide bond hydrolysis. This intermediate is quite stable and thus low Ki values have been observed with peptidyl α- ketoamide transition-state inhibitors. Peptidyl α-ketoamides have been extensively studied by our group36, 38 and other investigators.70–73 Our previous work has shown that extension of the inhibitors to the primed region increased the potency of the inhibitors and N-monosubstituted peptidyl α-ketoamides were more potent than the corresponding N,N-disubstituted peptidyl α-ketoamides.38 It has also been observed that α-aminobutyric acid (Abu), phenylalanine (Phe) or norvaline (Nva) in the P1 position and leucine in the P2 position was preferred.74 To generate compounds capable of crossing the BBB, we designed peptidyl α-ketoamides with nucleobases, methylpiperazine, and dimethylaminoalkyl structures in the primed region.
Charged and polar compounds such as nucleotides and choline, which are essential for the brain, do not cross the BBB and there are multiple transport systems to facilitate the delivery of these compounds to the brain.75 Choline, a positively charged molecule, has a critical role in the CNS as a precursor to the neurotransmitter acetylcholine but does not cross the BBB and its uptake into the brain is dependent upon carrier-mediated transport. Several BBB choline transporters such as the high affinity choline transporter (CHT) and the vesicular acetylcholine transporter (VAChT), are responsible for the transport of choline across the BBB.76 The choline transporters also deliver choline analogs such as N-n-octyl choline, N-n-decylnicotinium iodide, bis-pyridinium cyclophanes,77 and nicotine to the brain.78 Several cationic drugs such as verapamil, diphenhydramine, and donepezil are transported by or are competitive inhibitors of the choline transporters.79, 80 We synthesized several peptidyl α-ketoamides Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) (4d), Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl) (5d), Cbz-Leu-D,L-Phe-CONH-(CH2)3-N-(CH3)2 (5e), and Cbz-Leu-D,L-Phe-CONH-(CH2)2-N-(CH3)2 (5f) which contain N-methylpiperazine or dimethylaminoalkyl groups in the primed region. The methylpiperazine derivatives are similar to our lead structure 1 where there is a P’ morpholine ring, but not a methylated tertiary amine. The methylpiperazine ring has some features in common with donepezil (a nitrogen heterocycle), verapamil (a methyl tertiary amine), and nicotine (both). It was hypothesized that these structural features would provide sufficient recognition for some of the choline transporters to enable these compounds to penetrate the brain. The dimethylaminoalkyl moiety in ketoamides 5e and 5f is found in diphenhydramine and could also be recognized by the choline transporters. We planned to methylate these compounds to increase their resemblance to choline but abandoned that strategy when the dimethylaminoalkyl compounds proved to be difficult to purify and when the nucleobase derivatives proved to be more potent inhibitors.
Nucleosides, nucleotides and heterocyclic bases, the building blocks of RNA and DNA, are hydrophilic compounds and do not readily penetrate cell membranes by passive diffusion. Instead they are transported by several concentrative nucleoside transporters (CNT1, CNT2 and CNT3)81 which are specific for the transport of different heterocyclic bases and nucleotides. Several of the compounds (Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl (4a), Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin-3-yl (4c), Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl (5a), and Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl (5c)) which we synthesized have nucleobases such as adenine and cytosine in the primed region to facilitate their recognition by BBB nucleoside transporter systems. Several drugs, such as nucleoside reverse transcriptase inhibitors that are used in the treatment of HIV infection, are transported into the CNS by these nucleoside transporters, while many protease inhibitors are not effectively transported.82–86 Hence, attachment of structural features for recognition by brain transporter systems to calpain inhibitors appears to be a promising strategy for facilitating incorporation of these molecules into the brain. The effectiveness of this strategy for other tissue types has been demonstrated by Meier and coworkers who attached ketoamide calpain inhibitors to various muscle cell targeting capping groups to assist with accumulation of calpain inhibitors in muscle cells for the treatment of Duchenne Muscular Dystrophy and observed improved uptake of calpain inhibitors into muscle cells.87
Mechanism of Inhibition and Binding Mode
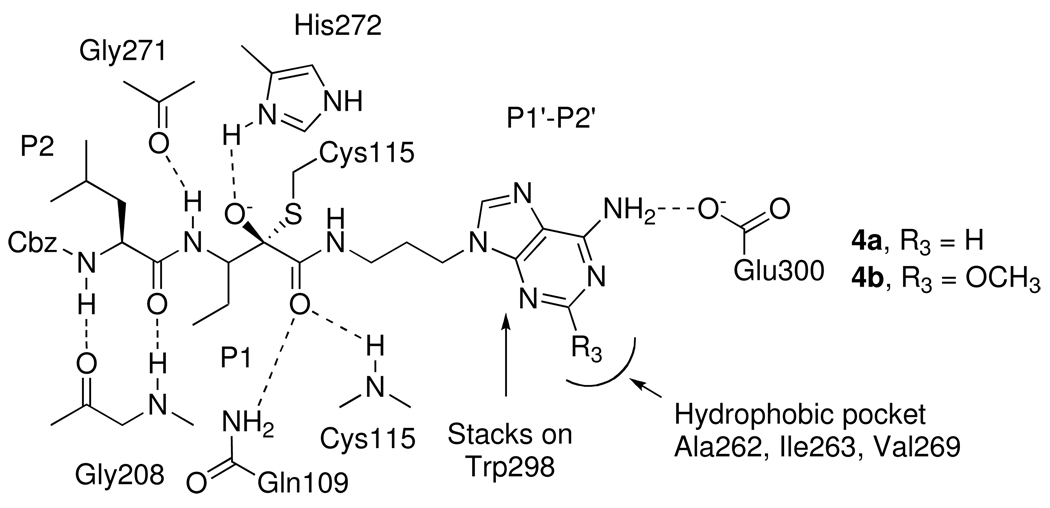
The mechanism of inhibition of calpain by α-ketoamides involves the formation of a reversible enzyme-inhibitor complex prior to attack of the active site cysteine residue (Cys115) on the keto carbonyl group of the α-ketoamides. This leads to the formation of a stable but reversible tetrahedral hemithioketal adduct (Figure 3) containing a hydrogen bond between the newly formed hydroxyl group of the tetrahedral adduct and the imidazole ring of His272.
Figure 3.
Proposed binding interactions of the peptidyl α-ketoamides 4a and 4b with the active site of calpain I.
Crystal structures of 4a (Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl) and 4d (Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) bound to the rat calpain I protease core (µI-II) have previously been reported by Campbell et al.88 Figure 3 shows a schematic drawing of the interaction of 4a with the active site of calpain. Important features in this structure are stacking of the adenine moiety of 4a against a tryptophan (Trp298) in the catalytic site of calpain I, formation of a hydrogen bond between the amino group of adenine and the side chain of Glu300, formation of two hydrogen bonds between the carbonyl oxygen of the carboxamide with Gln109 and Cys115, and formation of hydrogen bonds between the inhibitor backbone and Gly208, Gly271 (Figure 3). Neither the stacking interaction with Trp298 nor hydrogen bond formation with Glu300 was observed between the piperazinyl ring of compound 4d and the primed side region of the enzyme.
Interestingly, a hydrophobic pocket formed by Ala262, Ile263 and Val269 was observed in the crystal structure near the C2 carbon of the adenine moiety (Figure 3). To facilitate interactions with this hydrophobic pocket, we have synthesized several peptidyl α-ketoamides with a 2-methoxyadenine moiety in the primed region (Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl (4b) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl (5b).
The inhibitory potency of the new inhibitors toward calpain I, calpain II and cathepsin B are shown in Table 1.
Table 1.
Inhibition of Calpain I, Calpain II and Cathepsin B by Peptidyl α-Ketoamides.a
| Compound | Ki (µM) | ||||
|---|---|---|---|---|---|
| Cal I | Cal II | Cat B | Cal I/Cat B | ||
| 1 | Cbz-Leu-D,L-Abu-CONH-(CH2)3-morpholine38 | 0.150 ± 0.029 | 0.041 | 6.9 | 46 |
| 4a | Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl | 0.053 ± 0.001 | 0.070 ± 0.010 | 0.80 ± 0.15 | 15.1 |
| 5a | Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl | 0.055 ± 0.009 | 0.068 ± 0.006 | 1.75 ± 0.18 | 31.8 |
| 4b | Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl | 0.023± 0.006 | 0.077 ± 0.025 | 0.88 ± 0.02 | 38.3 |
| 5b | Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl | 0.041 ± 0.012 | 0.209 ± 0.029 | 2.34 ± 0 | 57.1 |
| 4c | Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin-3-yl | 0.165 ± 0.020 | 1.14 ± 0.06 | 0.75 ± 0.06 | 4.54 |
| 5c | Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl | 0.48 ± 0.06 | 0.438 ± 0.079 | 0.44 ± 0.03 | 0.92 |
| 4d | Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) | 0.640 ± 0.137 | 0.286 ± 0.074 | 1.42 ± 0.34 | 2.22 |
| 5d | Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl) | 1.37 ± 0.126 | 6.36 ± 1.06 | 111 ± 17 | 81.0 |
| 5e | Cbz-Leu-D,L-Phe-CONH-(CH2)3-N-(CH3)2 | 0.226 ± 0.036 | 0.844 ± 0.317 | 75.5 ± 6.8 | 334 |
| 5f | Cbz-Leu-D,L-Phe-CONH-(CH2)2-N-(CH3)2 | 0.711 ± 0.159 | 3.52 ± 0.54 | 475 ± 66 | 668 |
Calpain assays were performed in 50 mM Tris HCl, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% CHAPS, pH 7.5, 10 mM DTT, 5 mM CaCl2 and < 5% DMSO. Calpain I from porcine erythrocytes and calpain II from porcine kidney were used in the assays. The human liver cathepsin B assays were performed in 0.1 M NaHPO4, 1.25 mM EDTA, 0.01% Brij, pH 6.0 buffer and < 5% DMSO.
Calpain I Inhibition
As expected from the crystal structure, inhibitors with adenine (4a, 5a) and 2-methoxyadenine (4b, 5b) in the primed region were 3–7 fold more potent than our lead compound 1 (Ki = 150 nM). The best calpain I inhibitors were Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl (4b) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl (5b) with Ki values of 23 and 41 nM, respectively while compounds Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenine-9-yl (4a) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenine-9-yl (5a) were slightly less potent than the 2-methoxyadenine derivatives but still have Ki values of 53 and 55 nM, respectively. The increased potency of the 2-methoxyadenine derivatives confirmed that the 2-methoxy group in these compounds is probably interacting with the hydrophobic pocket (Ala262, Ile263, and Val269) in the primed region (Figure 3). Compounds 4c (Ki = 165 nM) and 5c (Ki = 480 nM) with cytosine in the primed side (Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin-3-yl, Ki = 165 nM; Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl, Ki = 480 nM) were 3- to 8-fold less potent than the adenine derivatives. However, the cytosine compounds are still very potent inhibitors since they can also form a hydrogen bond with Glu300 and can stack on Trp298, although not as well as the adenine or 2-methoxyadenine derivatives. Among the compounds with nucleobases in the primed region, Abu in the P1 position is slightly favored over Phe.
Compounds containing 4-methylpiperazine (4d, Ki = 640 nM and 5d, Ki = 1.37 µM) or dimethylamino alkyl groups (5e, Ki = 226 nM; and 5f, Ki = 711 nM) in the primed side were less potent than 1 and compounds with nucleobases in the primed side, but are still reasonable inhibitors of calpain I. The decreased potency is probably due to the lack of the stacking interactions with Trp298 and the hydrogen bond with Glu300. Changing the amino acid in the P1 position from an Abu to a Phe resulted in a 100-fold increase in potency in compound 5e while decreasing the alkyl spacer by one methylene group in compound 5f resulted in a 3-fold decrease in potency.
Calpain II Inhibition
In general, the inhibitors were more inhibitory toward calpain I but the order of reactivity of calpain II is similar to that of calpain I. Compounds with nucleobases (4a, 4b, 5a and 5b) in the primed region were more potent than those with dimethylaminoalkyl groups (5e – f). Compounds Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl (4a) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl (5a) were the most potent inhibitors of calpain II with Ki values of 70 nM and 68 nM; respectively. Introduction of the methoxy group to the C2 carbon of adenine did not significantly change the potency for Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl (4b) (Ki = 77 nM) but resulted in a 3-fold decrease in potency for Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl (5b (Ki = 209 nM). The cytosine derivatives Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin3-yl (4c) (Ki = 1.14 µM) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl (5c) (Ki = 438 nM) were less potent than the adenine and 2-methoxyadenine derivatives.
The 4-methylpiperazine derivatives Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) (4d) (Ki = 286 nM) and Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl) (5d) (Ki = 6.36 µM) were less potent than the adenine derivatives 4a and 5a. The dimethylamino alkyl analogs 5e and 5f have Ki values of 25.9 µM, 844 nM and 3.52 µM, respectively. Again, replacing Abu with Phe in the P1 position resulted in a 30-fold increase in potency for compound 5e and decreasing the alkyl spacer length by one methylene group decreased the potency 4-fold for Cbz-Leu-D,L-Phe-CONH-(CH2)2-N-(CH3)2 (5f).
Cathepsin B Inhibition and Selectivity
In order to determine the selectivity of the new inhibitors, we measured inhibitory potency with cathepsin B. In general, the peptidyl α-ketoamides displayed lower affinity towards cathepsin B. The most selective calpain I inhibitors among the ones with nucleobases in the primed region were the 2-methoxyadenine derivatives. The inhibitor 4b (Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl) with a Ki value of 23 nM for calpain I is 3- and 38-fold poorer with calpain II and cathepsin B, respectively. The Ki of 5b (Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl) is 41 nM, which makes it 5- and 57-fold more potent on calpain I than on calpain II and cathepsin B. The small hydrophobic pocket observed in the active site of calpain I is not present in cathepsin B, thus both selectivity and increased potency toward calpain I have been obtained by the introduction of a small hydrophobic group on to the adenine ring to interact with the pocket.
Except for 4d (Ki =1.42 µM), most of the inhibitors with 4-methylpiperazine and dimethylaminoalkyl substituents in the primed region were poor inhibitors of cathepsin B (Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl) (5d, Ki = 111 µM), Cbz-Leu-D,L-Phe-CONH-(CH2)3-N-(CH3)2 (5e, Ki = 75.5 µM), Cbz-Leu-D,L-Phe-CONH-(CH2)2-N-(CH3)2 and (5f, Ki = 475 µM) expect Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl) with a Ki value of 1.42 µM). The most selective calpain I inhibitors among those with N-methylpiperazine and dimethylaminoalkyl substituents in the primed region were Cbz-Leu-D,L-Phe-CONH-(CH2)3-N-(CH3)2 (5e) and Cbz-Leu-D,L-Phe-CONH-(CH2)2-N-(CH3)2 (5f). Inhibitor 5e (Ki = 0.226 µM towards calpain I) is 3- and 334-fold less potent on calpain II and cathepsin B, respectively, while inhibitor 5f (Ki = 0.711 µM towards calpain I) is 5- and 668-fold less potent on calpain II and cathepsin B, respectively. The calpain inhibitors 5e and 5f, although less potent than several other inhibitors, have increased specificity for calpain and may be more useful in biological experiments.
Overall, cathepsin B was inhibited by the new peptidyl α-ketoamides, but good selectivity was obtained for calpain I and calpain II in α-ketoamides 5d, 5e, and 5f. The cytosine derivative 5c was equally potent with calpain I, calpain II, and cathepsin B.
Brain Permeability
In our initial studies demonstrating axonal protection in the animal model of peripheral neuropathy,60 1 was delivered continuously from a subcutaneous mini diffusion pump at a dose of 1 at 24 mg/kg/day. These studies showed that 1 (Figure 1) is an effective calpain inhibitor in vivo and we therefore choose this dose to use in our animal studies. Liquid chromatography tandem mass spectrometric (LC-MS/MS) experiments were performed in order to determine the concentration of 1 in the brain, heart, kidney, liver, spinal cord, serum, peripheral (sciatic) nerve, and spleen of mice dosed with 24 mg 1/kg body weight via subcutaneous (sample cohort (N) = 2 mice), intravenous (N = 3 mice), or oral (N = 2 mice) administration and sacrificed after 1 (sc), 1 (iv), or 4 (oral) hours, respectively (Table 2). The inhibitor 1 could not be detected in the brain, but was present in the liver, heart, kidney, and spleen at levels > 0.5 µg/gram of tissue after a single subcutaneous or intravenous dose, indicating that the bioavailability of 1 via subcutaneous or oral administration was good, although the inhibitor passed through the excretory system without penetrating the BBB.
Table 2.
Concentrations of 1 in mouse serum and tissue samples.a
| (µg/g tissue) |
||||
|---|---|---|---|---|
| Compound | Tissue | Subcutaneous | Intravenous | Oral |
| (N = 2; 1 hour) | (N = 3; 1 hour) | (N = 2; 4 hours) | ||
| 1 | Brain | ND | ND | ND |
| Heart | 0.541 ± 0.008 | 0.561 ± 0.036 | NQ | |
| Kidney | 0.936 ± 0.068 | 1.816 ± 0.161 | NQ | |
| Liver | 1.550 ± 0.068 | 0.943 ± 0.052 | NQ | |
| Spinal cord | ND | ND | ND | |
| Serum | 0.895 ± 0.196 | NQ | NQ | |
| Peripheral nerve | NQ | 0.333 ± 0.009b | NQ | |
| Spleen | 1.380 ± 0.174 | 1.316 ± 0.067 | NQ | |
Concentration of 1 detected in the brain, heart, kidney, liver, spinal cord, serum, peripheral nerve, or spleen obtained from mice dosed with the inhibitor 1 at 24 mg/kg body weight. The mice received the drug by subcutaneous (N = 2, mice sacrificed after 1 hour), intravenous (N = 3, mice sacrificed after 1 hour) or oral (N = 2, mice sacrificed after 4 hours) administration. A calibration curve was measure at varying doses of 1 in mouse plasma. The quantitation limit was 0.004 mg/mL of plasma and the detection limit was 0.001 mg/mL (1 ng/mL) of plasma. No calibration curves were determined with individual tissues and the quantitation limit is likely higher in tissue. The errors in the measurements were determined using multiple HPLC sample injections from the individual animals. ND = not detected, NQ = not quantifiable.
The value listed for the sciatic nerve with 1 was from three injections of a sample from one mouse dosed intravenously. The other two mice were NQ.
Two of the newly synthesized compounds (4a and 4d), which were designed to cross the BBB, were also analyzed in the brain of mice. For these studies, inhibitors (24 mg/kg body weight) were subcutaneously administered to mice sacrificed after 1 (N = 3),2 (N = 3), 4 (N = 3), or 8 (N = 3) hours (Table 3). The adenine compound 4a, was only detected in quantifiable levels in one mouse out of twelve. This mouse at the eight hour time point had a concentration of 4a of 1.17 ± 0.01 µg/g tissue. The N-methylpiperazine derivative 4d could be detected in the three mice at the 1 hr time point, but could not be quantitated sufficient accuracy. After two hours, 4d could be detected and quantitated in one mouse at a concentration of 1.14 ± 0.02 µg/g tissue. The concentrations observed for 4a and 4d are approximately 2 µM which is several fold higher than the Ki values for inhibition of calpain I and II, and thus, this is likely a therapeutically useful concentration.
Table 3.
Concentration of Calpain Inhibitors in the Mouse Brain After Subcutaneous Administration.a
| (µg/g tissue) |
||||
|---|---|---|---|---|
| Compound | (N = 3; 1 hour) | (N = 3; 2 hour) | (N = 3; 4 hour) | (N = 3; 8 hour) |
| 4a | ND | ND | ND | 1.17 ± 0.01b |
| 4d | NQc | 1.14 ± 0.02b | ND | ND |
Concentration of 4a and 4d detected in the brain obtained from mice dosed with the inhibitors at 24 mg/kg body weight. The mice received the drug by subcutaneous administration and were sacrificed after 1, 2, 4, and 4 hours. A calibration curve was measures at varying doses of 4a and 4d in mouse plasma. The quantitation limit was 0.16 µg/mL of plasma for 4a and was 0.23 µg /mL plasma for 4d. No calibration curves were determined brain tissue. ND = not detected, NQ = not quantifiable.
The value listed is three injections of a sample from one mouse and this group is N = 1. The two other mice were NQ.
The inhibitor was detected in all three mice.
The detection of the two compounds in the brains of several animals, but not all the animals, is certainly encouraging. However this result only show that the compounds are incorporated into the brain. This could result from either passive diffusion or active transport by one of the BBB barrier transporters. Future experiments should involve in vitro studies with various BBB transporters to determine if the compounds are indeed interacting with the transporters or are simply entering the brain by passive diffusion. It also will be necessary to develop a more sensitive and routine analytical method for 4a and 4d in tissue samples in order to test our hypothesis further and to validate our design strategy. The current analytical method was optimized for 1 and didn’t allow us to detect and quantitate 4a and 4d in the majority of animals dosed.
Conclusions
We have shown that peptidyl α-ketoamides with the general structure of Cbz-L-Leu-D,L-AA-CONH-R, where R is a heterocyclic base, are effective inhibitors of the cysteine proteases, calpain I and II. It has been observed that Abu, which is a small hydrophobic residue, is slightly favored over the large hydrophobic residue Phe in the P1 position. It was observed that nucleobases were favored over dimethylaminoalkyl or methyl piperazine substituents in the primed region due to stacking interactions of the nucleobases with a Trp residue near the active site. Our hypothesis that introduction of a hydrophobic group on the adenine ring would facilitate interactions with the hydrophobic pocket observed in the crystal structure resulting in increased potency was verified experimentally for the new calpain inhibitors 4b and 5b with a 2-methoxyadenine group. In an effort to further extend permeability across the BBB to other structures, peptidyl α-ketoamides containing cytosine in the primed side were also synthesized, but were less effective than the adenine derivatives.
Although our lead compound 1 was found in the peripheral nerve and other tissue, it was not detected in the brain. However, two compounds, 4a and 4d, have been detected at therapeutically useful concentrations in the brain of some mice after subcutaneous administration. Increased levels of calpain activity have been observed in a number of neurodegenerative diseases with brain involvement including Alzheimer’s, Huntington’s and Parkinson’s diseases and multiple sclerosis. Development of selective calpain inhibitors that can cross the BBB is required for the treatment of these diseases. Here, we have shown that peptidyl α-ketoamide calpain inhibitors potentially can be designed to cross the BBB and thus may be useful in the treatment of a variety of neurodegenerative diseases.
Experimental
Material and Methods
Materials were obtained from Acros, Bachem Bioscience Inc., or Sigma Aldrich and used without further purification. The structures and purity of each target compound were confirmed by TLC, 1H NMR, MS, HPLC analysis and/or elemental analysis. TLC was performed on Sorbent Technologies (250 µm) silica gel plates. The 1H NMR spectra were obtained on a Varian Mercury 400 MHz spectrometer. Chemical shifts are reported in ppm relative to an internal standard (trimethylsilane). Electrospray ionization (ESI), fast-atom-bombardment (FAB) and high-resolution mass spectrometry (HRMS) were obtained using Micromass Quattro LC and VG Analytical 70-SE instruments. The purity of compounds 5a, 4b,5b, 4c, and 5c after purification was determined by elemental analysis and was higher than 95%. The elemental composition for each of these compounds is given in the experimental section for that compound. Elemental analyses were carried out by Atlantic Microlab Inc., Norcross, GA. The purity of 5d, 5e, and 5f were determined by HPLC. The analysis was run on a Beckman Coulter HPLC running 32Karat V4.0 software. The Alltech/Applied Science C18 column used was 250 mm by 4.6 mm and packed with 5 micron Sperisorb ODS 2. The column was eluted with an isocratic mixture of 60% 0.1% TFA in acetonitrile and 40% 0.1% TFA in water. Detection was at 220 and 254 nm, and the area percent was measured using the 32Karat software in duplicate HPLC runs. Compound 5d was 95–99% pure, 5e was 89–91% pure, and 5f was 96–99% pure. The synthesis of compounds 1, 4a, and 4d has previously been reported.36, 38, 88
Animal Studies
All experiments involving animals were approved by the Emory University Institutional Animal Care and Use Committee. Animals were 7-week old female C57BL/6 mice. Each experiment was done with cohorts of 2 – 3 animals per time point and dosed with 24 mg inhibitor/kg body weight, administered subcutaneously (N = 2 for 1, N = 3 for 4a and 4d), intravenously (N = 3 for 1), or orally via oral gavage (N = 2 for 1). For compound 1, mice dosed subcutaneously, intravenously, or orally were sacrificed after 1, 1, or 4 hours, respectively. For compounds 4a and 4d, mice (N = 3) were sacrificed 1, 2, 4, or 8 hours after the dose was administered. At the designated time, each animal was perfused with buffered saline at 37 °C to clear all blood vessels and was sacrificed. Serum and extracted tissue samples were frozen in liquid nitrogen and stored at −80 °C until analyzed by LC-MS/MS.
LC-MS/MS Assays
For the measurement of plasma pharmacokinetics, tissue distribution, and permeability into the nervous system of 1, 4a, and 4d, a LC-MS/MS assay was developed using another of our calpain inhibitor compounds, Cbz-Leu-Nva-CONH-(CH2)3-morpholine (ZLAK74) as an internal standard. The internal standard was added to serum or tissues early in the sample preparation procedure to compensate for incomplete analyte extraction. All drug standards and tissues were stored at −80 °C until needed. Control tissues from undosed mice were used to prepare calibration standards with concentrations of 0.10, 0.30, 0.50, and 1.00 µM for inhibitor 1. The calibration standards for 4a and 4d where prepared at identical concentration in mouse plasma. Sample preparation for the spiked control tissues (or plasma) and the sample tissues was identical and based on a protocol reported by Guo and coworkers.89 First, the tissues were homogenized with water in a 5% (weight/volume) ratio (i.e. 5 g tissue / mL water) and 100 µL aliquots of each homogenate were pipetted into centrifuge tubes and spiked with 1.00 µM of Cbz-Leu-Nva-CONH-(CH2)3-morpholine as an internal standard. The mixture was sonicated for 10 minutes to ensure homogeneity in the sample and 300 µL of 99.9:0.1 v/v acetonitrile:formic acid were added to precipitate proteins. The sample was briefly vortexed and sonicated for 15 minutes followed by centrifugation at 13,000 g for 30 minutes. The supernatant was removed and evaporated at 45 °C for 3 hours and the residue was reconstituted with 100 µL 30:69.9:0.1 v/v acetonitrile:water:formic acid. The final solution was filtered using a 0.45 µm syringe filter (Acrodisc, Pall) and analyzed by LC-MS/MS.
All measurements were carried out in an Agilent HPLC 1100 system coupled to a ThermoFinnigan LCQ Deca XP+ ion trap mass spectrometer using an ESI source operated in positive ion mode. For experiments with compound 1, an analytical Zorbax Extend® C18 column (1.0 × 150 mm, 5 µm particles, 80 Å pore size, Agilent) was used with the following binary gradient program: 0.0 min – 30% B, 0.5 min – 30% B, 0.7 min – 85% B, 2.5 min – 85% B, 2.70 min – 30% B, 5.5 min – 30% B. An analytical Symmetry Shield® reverse-phase C18 column (1.0 × 150 mm, 3.5 µm particles, 100Å pore size; Waters) preceded by a Zorbax® RX-C18 guard column (4.6 mm × 12.5 mm, 5.0 µm particles, 2 µm pore size; Agilent) was used for experiments with compounds 4a and 4d. For separations using this column, the binary solvent gradient program used was: 0.0 min – 30% B, 0.5 min – 30% B, 0.7 min – 100% B, 4.5 min – 100% B, 5.0 min – 30% B, 6.0 min – 30% B. The mobile phases used for all separations were: A = 0.1 % formic acid in water and B = 0.1 % formic acid in acetonitrile. The flow rate was 80 µL min–1, with an injection volume of 15 µL.
The mass spectrometer was operated in multiple reaction monitoring mode using the following precursor → fragment transitions: 1: m/z 505.2 → 443.2, Cbz-Leu-Nva-CONH-(CH2)3-morpholine (internal standard): m/z 519.2 → 457.2, 4a : m/z 553.2 → 509.2, 4d: m/z 518.3 → 410.3 with the following settings: ESI needle voltage +4.0 kV; sheath gas 15 arbitrary units (~0.6 L min−1); capillary temperature 275 °C; capillary voltage 34V (1), 28V (4a), 18V (4d); capillary-skimmer voltage 25V (1), 55V (4a), 45V (4d). For all experiments, the mass analyzer was set with the automatic gain control on 1E+7 with 2 microscans, 400 µs max. injection time, 1.2 Da mass selection window and 34% normalized collision energy. After acquisition, peak areas for the chromatographic peaks present in the extracted ion chromatograms for the internal standard and the compound of interest were determined using the mass spectrometer software Xcalibur 2.0 (Thermo) with the Genesis peak detection algorithm (15 smoothing points, signal-to-noise ratio threshold = 3.0). The areas for the compound of interest and the internal standard were exported to Excel and the ratio of the peak area of the compound of interest/peak area of Cbz-Leu-Nva-CONH-(CH2)3-morpholine (internal standard) was calculated. The amount of compound present in each sample was determined by correlating the area ratio to a concentration using the calibration curves obtained during each experiment.
Calpain I and Calpain II Assays
The fluorogenic substrate Suc-Leu-Tyr-AMC was obtained from Bachem. Calpain I from porcine erythrocytes and calpain II from porcine kidney were purchased from Calbiochem. The fluorescence was monitored using a Tecan Spectrafluor microplate reader. AMC was used as the calibration standard and the calibration curve was plotted against RFU for different concentrations of AMC within the range of 5-0.08 µM. Inhibitor stock solutions were prepared in DMSO and kept at 4 °C prior to use. Calpain assays were performed in 50 mM Tris HCl, 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% CHAPS, pH 7.5, 10 mM DTT, 5 mM CaCl2 and three different substrate (Suc-Leu-Tyr-AMC) concentrations (0.8, 0.4, 0.2 µM). A 10 µL aliquot of DMSO (control) or inhibitor solution in DMSO (DMSO content < 5%) was added to 200 µL buffer. The reaction was initiated by adding a 2 µL aliquot of enzyme (with a final concentration of 10 nM) to the well. The reaction was monitored by the release of 7-amino-4-methylcoumarin (λex = 360 nm, λem = 465 nm). The total volume in the reaction well was 212 µL and controls were run every hour. Velocities were determined at room temperature (RT) at five or more concentrations of inhibitor and at three fixed concentrations of substrate. A plot of 1/v versus [I] gave intersecting lines with a correlation coefficient of ≥ 0.95. Ki values were determined by Dixon plots.90
Cathepsin B Assay
The fluorogenic substrate Cbz-Arg-Arg-AMC was obtained from Bachem. Cathepsin B from human liver was purchased from Calbiochem. The fluorescence was monitored and calibrated using the method reported for calpain I and II above. Inhibitor stock solutions were prepared in DMSO and kept at 4 °C prior to use. The cathepsin B assay was performed in 0.1 M NaHPO4, 1.25 mM EDTA, 0.01% Brij, pH 6.0 buffer and three different substrate (Cbz-Arg-Arg-AMC) concentrations (0.5, 0.2, 0.1 µM). A 10 µL aliquot of DMSO (control) or inhibitor solution in DMSO (DMSO content < 5%) was added to 200 µL buffer. The reaction was initiated by adding 5 µL of activated enzyme (with a final concentration of 0.4 µM) to the well. The enzyme was activated by the addition of cathepsin B kinetic buffer (267 µL) and 0.1 M DTT (3 µL) to the enzyme stock solution (30 µL). The reaction was monitored by the release of 7-amino-4-methylcoumarin (λex = 360 nm, λem = 465 nm). The total volume in the reaction well was 215 µL and controls were run every hour. Velocities and Ki values were determined using the aforementioned method for calpain I and II.
Statistical Analysis
The data in Table 1 for calpain I, calpain II, and cathepsin B were analyzed using the VassarStats website for statistical computation. This website was developed by Professor Richard Lowry. Specifically a one-way ANOVA with a post-hoc Tukey HSD (honestly significant differences) test was performed. Further details and the comparisons are given in the Supporting Information section. In general, inhibition constants for calpain I, calpain II, and or cathepsin B that differed by less than a factor of approximately two were considered to be non-significant in this analysis. In general, differences in potency between calpain I and II for each compound were non-significant with three exceptions (4b, 5b, and 4c), while the differences between calpain I and cathepsin B, and between calpain II and cathepsin II were always significant with one exception (5c).
General Procedure for the Synthesis of Dipeptide α-Ketoesters
The synthesis of the dipeptide acids Cbz-Leu-Abu-OH and Cbz-Leu-Phe-OH are given in the Supporting Material. The dipeptide acid (1 eq) was dissolved in dry THF and 4-dimethylaminopyridine (0.05 eq), pyridine (3 eq), and ethyl oxalyl chloride (2.1 eq) were added sequentially. The resulting mixture was stirred at reflux temperature for 4 hours. After removing the heat source, 1 M HCl (50 mL) was added to the brown solution. The mixture was extracted with ethyl acetate (2 × 100 mL). The combined extract was washed with 100 mL of saturated NaCl, dried over MgSO4 overnight, and filtered. Ethyl acetate was removed from the filtrate to give a mixture of products containing the dipeptide enol ester. The mixture of products was dissolved in 20 mL of absolute ethanol and stirred in an ice bath. Triethylamine (1 eq) was added and the mixture was stirred for 1 hour at RT. Solvent was removed from the final mixture using a rotary evaporator. The crude oil was subjected to column chromatography to give the dipeptidyl α-ketoester. These compounds have previously been reported using a synthetic scheme with more steps.36, 38
Cbz-Leu-D,L-Abu-COOEt, light yellow oil, 76% yield. 1H NMR (CDCl3): 0.86–0.93 (m, 9H, 2 × Leu-CH3 and Abu-CH3), 1.26–1.37 (m, 4H, CH3 and Leu-CH), 1.49–1.98 (m, 4H, Abu-CH2, Leu-CH2 and CH3), 4.23–4.38 (m, 2H, 2 × α-H), 4.42–4.46 (m, 2H, CH2), 5.03–5.13 (m, 2H, Cbz), 5.67–5.73 (m, 1H, NH), 7.21–7.32 (m, 6H, Ph and NH). HRMS (FAB) Calcd. for C21H31N2O6: 407.2182. Observed m/z 407.2178 ([M+H]+).
Cbz-Leu-D,L-Phe-COOEt, light yellow oil, 68% yield. 1H NMR (CDCl3): 0.79–0.90 (m, 6H, 2 × Leu-CH3), 1.22–1.61 (m, 6H, Leu-CH2, CH3 and Leu-CH), 2.93–3.07 (m, 1H, CH), 3.19–3.28 (m, 1H, CH), 4.14–4.33 (m, 4H, CH2 and 2 × α-H), 5.08 (d, 2H, Cbz), 5.23–5.33 (m, 1H, NH), 6.77–6.84 (m, 1H, NH), 7.12–7.29 (m, 5H, Ph), 7.33 (s, 5H, Ph). HRMS (FAB) Calcd. for C26H33N2O6: 469.2339. Observed m/z 469.2337 ([M+H]+).
General Procedure for the Synthesis of Dipeptidyl α-Ketoacids
Dipeptidyl α-ketoesters (1 eq) were dissolved in ethanol and 1 M NaOH solution (1.1 eq) was added in portions while stirring in an ice bath. The resulting mixture was stirred at RT for an hour and extracted with anhydrous ether (4 × 30 mL). The aqueous layer was acidified to pH 4 with 2 M HCl in an ice bath and extracted with diethyl ether (Et2O, 2 × 50 mL). The combined ether extract was washed with saturated NaCl, dried over MgSO4 overnight, and filtered. Ether was removed from the filtrate by evaporation and the product was dried under reduced pressure. These compounds have previously been reported using a synthetic scheme with more steps.36, 38
Cbz-Leu-D,L-Abu-COOH (2), pale yellow hygroscopic flakes, 96% yield. 1H NMR (CDCl3): 0.91 (d, 9H, Abu-CH3 and 2 × Leu-CH3), 1.47–1.75 (m, 5H, Leu-CH2, Abu-CH2, Leu-CH), 4.13–4.35 (m, 2H, 2 × α-H), 5.04–5.13 (m, 3H, Cbz and NH), 7.32 (s, 5H, Ph), 8.35–8.41 (d, 1H, NH). HRMS (FAB) Calcd. for C19H27N2O6: 379.1869. Observed m/z 379.1870 ([M+H]+).
Cbz-Leu-D,L-Phe-COOH (3), pale yellow hygroscopic flakes, 89% yield. 1H NMR (CDCl3): 0.77–0.86 (m, 6H, 2 × Leu-CH3), 1.09–1.56 (m, 3H, Leu-CH2 and Leu-CH), 2.49–2.51 (m, 1H, CH), 2.75–2.91 (m, 1H, CH), 4.01–4.08 (m, 2H, 2 × α-H), 4.89–5.06 (m, 3H, Cbz and NH), 7.18–7.40 (m, 10H, 2 × Ph), 8.49 (t, 1H, NH). HRMS (FAB) Calcd. for C24H29N2O6: 441.2026. Observed m/z 441.2025 ([M+H]+).
Synthesis of 9-(3-aminopropyl)adenine66
The synthesis has been previously reported and experimental details are given in Supporting Information.
Synthesis of 9-(3-Aminopropyl)-2-methoxyadenine
A mixture of 2-chloroadenine (1 eq), sodium methoxide (7.5 eq) in anhydrous methanol (50 mL) was sealed in pressure vessel. The reaction mixture was held at an internal temperature of 100 °C for 24 hours before cooling to RT. Once cooled, the pressure vessel was opened and the suspension was diluted with water (50 mL). The resulting solution was evaporated under reduced pressure to give a final volume of 70 mL; water (30 mL) was added to this solution to give a final volume of 100 mL. The solution was transferred to a 3-neck flask equipped with a stirrer, thermometer and pH meter. The solution was heated to 60 °C (internal temperature) and 50% aq. HCl was added to adjust the pH to 9.5. The resulting suspension was stirred at 60 °C for 1 hour, cooled slowly to RT and stirred for 16 hours. The suspension was filtered and the filter cake was washed with water (10 mL) and methanol (2 × 10 mL). The solid was dried under vacuum to give 2-methoxyadenine in 70% yield. 1H NMR (DMSO-d6): 3.76 (s, 3H, OCH3), 7.12 (s, 2H, NH2), 7.86 (s, 1H, CH).
A mixture of 2-methoxyadenine (1 eq), 1-bromo-3-chloropropane (4.3 eq), and potassium carbonate (2.35) in DMF (200 mL) was stirred at RT under argon for 4 days, filtered, and evaporated to dryness. The crude product was purified by column chromatography and gave 9-(3-chloropropyl)-2-methoxyadenine in 66% yield. MS (ESI) m/z 241.9 ([M+H]+).
A mixture of 9-(3-chloropropyl)-2-methoxyadenine (1 eq) and sodium azide (3 eq) in DMF was stirred at 80 °C for 24 hours, cooled to RT, and filtered. The crude product was purified by column chromatography to give 9-(3-azidopropyl)-2-methoxyadenine as a white crystalline solid in 74% yield. 1H NMR (DMSO-d6): 1.99–2.06 (m, 2H, CH2), 3.33–3.37 (m, 2H, CH2), 3.80 (s, 3H, OCH3), 4.10 (t, 2H, CH2), 7.21 (s, 2H, NH2), 7.92 (s, 1H, CH). MS (ESI) m/z 249.0 ([M+H]+).
A mixture of 9-(3-azidopropyl)-2-methoxyadenine and 5 % palladium on carbon in MeOH was reacted with hydrogen gas at RT for 20 hours. The catalyst was removed by filtration, the solvent removed to give 9-(3-aminopropyl)-2-methoxyadenine as a white solid in 75% yield. 1H NMR (DMSO-d6): 1.82–1.85 (m, 2H, CH2), 2.46–2.49 (m, 2H, CH2), 3.03 (s, 2H, NH2), 3.79 (s, 3H, OCH3), 4.09 (t, 2H, CH2), 7.20 (s, 2H, NH2), 7.92 (s, 1H, CH). MS (ESI) m/z 223.2 ([M+H]+).
Synthesis of 1-(3-Aminopropyl)cytosine68
The synthesis has been previously reported and experimental details are given in Supporting Information.
Synthesis of 1-(3-Aminopropyl)-4-methylpiperazine69
The synthesis has been previously reported and experimental details are given in Supporting Information.
General Procedure for the Synthesis of Target Peptide α-Ketoamides by the HOBt and EDC Coupling Method
HOBt (1.5 eq), was added to a stirred solution of the dipeptidyl α-ketoacid (1.5 eq) in DMF at −10 °C, followed by addition of the heterocyclic amine (1 eq) and EDC (1.5 eq). The mixture was allowed to react for 16 h at RT. DMF was evaporated, and the residue was redissolved in EtOAc. The organic layer was washed with 2% citric acid, saturated NaHCO3, and saturated NaCl before being dried over MgSO4 and concentrated. Column chromatography on silica gel was used to purify the peptidyl α-ketoamides.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(6-amino-9H-purin-9-yl)propyl)-2-oxopentanamide (4a, Cbz-Leu-D,L-Abu-CONH-(CH2)3-adenin-9-yl)
This ketoamide has previously been reported88 and characterization data is shown in the Supporting Information.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(6-amino-9H-purin-9-yl)propyl)-2-oxophenylbutanamide (5a, Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl)
The ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)3-adenin-9-yl was obtained from 9-(3-aminopropyl)adenine and the ketoacid Cbz-Leu-D,L-Phe-COOH using the EDC/HOBt coupling method, purified by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent, then recrystallized from EtOAc/hexane to give a white solid (21% yield). 1H NMR (DMSO-d6): 0.69–0.86 (m, 9H, 2 × Leu-CH3 and Abu-CH3), 1.15–1.36 (m, 5H, 2 × CH2 and CH), 1.92–2.00 (m, 2H, CH2), 3.04–3.15 (m, 4H, 2 × CH2), 4.07–4.14 (m, 2H, CH2 and 2 × α-H), 4.95–5.01 (m, 2H, Cbz), 5.18 (s, 1H, NH), 7.12–7.40 (m, 12H, 2 × Ph and NH2), 8.08–8.14 (m, 2H, 2 × Adenine-CH), 8.33–8.39 (d, 1H, NH), 8.83–8.89 (t, 1H, NH). HRMS (FAB) Calcd. for C32H39N8O5: 615.3043. Observed m/z 615.3094 ([M+H]+). Anal. (C32H38N8O5·0.75H2O) C, H, N.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(6-amino-2-methoxy-9H-purin-9-yl)propyl)-2-oxopentanamide (4b, Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl)
The ketoamide product Cbz-Leu-D,L-Abu-CONH-(CH2)3-2-methoxyadenin-9-yl was obtained from 9-(3-aminopropyl)-2-methoxyadenine and the ketoacid Cbz-Leu-D,L-Abu-COOH using the EDC/HOBt coupling method, purified by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent, then recrystallized from EtOAc/hexane to give a yellowish white solid (26% yield). 1H NMR (DMSO-d6): 0.72–0.93 (m, 9H, 2 × CH3 of Leu and CH3 of Abu), 1.37–1.60 (m, 4H, CH2 of Leu and CH2 of Abu), 1.76 (m, 1H, CH of Leu), 1.93–1.97 (m, 2H, CH2), 3.09 (m, 2H, CH2), 3.78 (s, 3H, OCH3), 3.98–4.14 (m, 3H, CH2 and α-H), 4.84 (m, 1H, α-H), 4.99 (s, 2H, Cbz), 7.20 (s, 2H, NH2), 7.28–7.40 (m, 6H, Ph and NH), 7.93 (s, 1H, CH of adenine), 8.24–8.31 (m, 1H, NH), 8.77 (m, 1H, NH). HRMS (FAB) Calcd. for C28H41N6O6: 583.2993. Observed m/z 583.2900 ([M+H]+). Anal. (C28H39N8O6·0.55H2O) C, H, N.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(6-amino-2-methoxy-9H-purin-9-yl)propyl)-2-oxophenylbutanamide (5b, Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl)
The ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)3-2-methoxyadenin-9-yl was obtained from 9-(3-aminopropyl)-2-methoxyadenine and the ketoacid Cbz-Leu-D,L-Phe-COOH using the EDC/HOBt coupling method, purified by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent, then recrystallized from EtOAc/hexane to give a yellow solid (24% yield). 1H NMR (DMSO-d6): 0.73–0.75 (d, 6H, 2 × CH3 of Leu), 1.11–1.35 (m, 4H, CH2 Leu and CH2 of Phe), 1.54 (m, 1H CH of Leu), 1.95–1.98 (m, 2H, CH2), 3.09–3.12 (m, 2H, CH2), 3.77 (s, 3H, OCH3), 4.03 (m, 3H, CH2 and α-H), 4.97 (s, 2H, Cbz), 5.17 (m, 1H, α-H), 7.20–7.33 (m, 12H, 2 × Ph and NH2), 7.93 (d, 1H, CH of adenine), 8.38 (d, 1H, NH), 8.84 (m, 1H, NH). HRMS (FAB) Calcd. for C33H41N6O6: 645.3149. Observed m/z 645.3067 ([M+H]+). Anal. (C33H40N8O6·0.25H2O) C, H, N.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(4-amino-2-oxopyrimidin-1(2H)-yl)propyl))-2-oxopentanamide (4c, Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin-3-yl)
The ketoamide product Cbz-Leu-D,L-Abu-CONH-(CH2)3-cytosin-3-yl was obtained from 1-(3-aminopropyl)cytosine and the ketoacid Cbz-Leu-D,L-Abu-COOH using the EDC/HOBt coupling method, purified by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent, then recrystallized from EtOAc/hexane to give a yellowish white solid (11% yield). 1H NMR (DMSO-d6): 0.72–0.95 (m, 9H, 2 × CH3 of Leu and CH3 of Abu), 1.41–1.73 (m, 5H, CH2 of Leu, CH2 of Abu and CH of Leu), 2.13–2.19 (m, 2H, CH2), 3.07 (m, 2H, CH2), 3.56 (m, 1H, α-H), 3.93–4.10 (m, 3H, α-H and CH2), 4.99 (s, 2H, Cbz), 5.60 (d, 1H, CH of cytosine), 6.97 (d, 2H, NH2), 7.32–7.44 (m, 6H, Ph and NH), 7.68 (d, 1H, CH of cytosine), 8.22–8.28 (m, 1H, NH), 8.73 (m, 1H, NH). HRMS (FAB) Calcd. for C26H37N6O6: 529.2775. Observed m/z 529.2781 ([M+H]+). Anal. (C26H36N6O6·1EtOAc) C, H, N.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(4-amino-2-oxopyrimidin-1(2H)-yl)propyl))-2-oxophenylbutanamide (5c, Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl)
The ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)3-cytosin-3-yl was obtained from 1-(3-aminopropyl)cytosine and the ketoacid Cbz-Leu-D,L-Phe-COOH using the EDC/HOBt coupling method, purified by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent, then recrystallized from EtOAc/hexane to give a yellow solid (12% yield). 1H NMR (DMSO-d6): 0.74–0.76 (d, 6H, 2 × CH3 of Leu), 1.11–1.37 (m, 4H, CH2 Leu and CH2 of Phe), 1.59 (m, 1H CH of Leu), 2.11–2.15 (m, 2H, CH2), 3.01–3.10 (m, 2H, CH2), 3.82–4.03 (m, 3H, α-H and CH2), 4.99 (s, 2H, Cbz), 5.18 (m, 1H, NH), 5.61 (d, 1H, CH of cytosine), 6.97 (d, 2H, NH2), 7.13–7.57 (m, 11H, 2 × Ph and CH of cytosine), 8.36 (d, 1H, NH), 8.80 (m, 1H, NH). HRMS (FAB) Calcd. for C31H39N6O6: 591.2886. Observed m/z 591.2852 ([M+H]+). Anal. (C31H38N6O6·1EtOAc) C, H, N.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(4-methylpiperazin-1-yl)propyl)-2-oxopentanamide (4d, Cbz-Leu-D,L-Abu-CONH-(CH2)3-(4-methylpiperazin-1-yl)
This ketoamide has previously been reported88 and characterization data is shown in the Supporting Information.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(4-methylpiperazin-1-yl)propyl)-2-oxophenylbutanamide (5d, Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl))
The dipeptide ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)3-(4-methylpiperazin-1-yl was obtained from Cbz-Leu-D,L-Phe-COOH and 1-methyl-4-(3-aminopropyl)piperazine using the EDC/HOBt coupling method and purified twice by column chromatography on silica gel with 85:15 CH2Cl2:MeOH as the eluent to give a yellow semi-solid in 10% yield. 1H NMR (CDCl3): 0.81 (m, 6H, CH3 of Leu), 1.40–1.60 (m, 5H, CH2 and CH), 2.28 (s, 6H, CH3), 3.00 (m, 2H, CH2), 3.20 (m, 2H, CH2), 4.05 (m, 2H, CH2), 4.50 (b, 1H, α-H), 5.02 (m, 3H, Cbz and α-H), 6.70 (b, 1H, NH), 7.05–7.30 (m, 7H, Ph and NH). The purity was 95–99% by HPLC using either 220 or 254 nm detection.
3-(Benzyloxycarbonyl-L-leucylamino)-N-(3-(dimethylamino)propyl)-2-oxophenylbutanamide (5e, Cbz-Leu-D,L-Phe-CONH-(CH2)3-N(CH3)2)
The dipeptide ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)3-N(CH3)2 was obtained from Cbz-Leu-D,L-Phe-COOH and (CH3)2N(CH2)3NH2 using the EDC/HOBt coupling method. Purification by column chromatography twice on silica gel with 80:20 CH2Cl2:MeOH as the eluent provided a yellow semi-solid, in 10% yield. 1H NMR (CDCl3): 0.84 (m, 6H, CH3 of Leu), 1.50–1.80 (m, 5H, CH2 and CH), 2.12 and 2.19 (d, 6H, CH3), 3.00 (m, 2H, CH2), 3.20 (m, 2H, CH2), 4.15 (m, 2H, CH2), 4.50 (b, 1H, α-H), 5.10 (m, 3H, Cbz and α-H), 6.82 (b, 1H, NH), 7.05–7.30 (m, 6H, Ph and NH), 7.40 (b, 1H, NH). The purity was 89–91% by HPLC using either 220 or 254 nm detection. The impurity is likely the dipeptide Cbz-Leu-Phe-NH-(CH2)3-N(CH3)2. HRMS (FAB) for C29H41N4O5: m/z 525.3077 ([M+H]+).
3-(Benzyloxycarbonyl-L-leucylamino)-N-(2-(dimethylamino)ethyl)-2-oxopentanamide (5f, Cbz-Leu-D,L-Phe-CONH-(CH2)2-N(CH3)2)
The dipeptide ketoamide product Cbz-Leu-D,L-Phe-CONH-(CH2)2-N(CH3)2 was obtained from Cbz-Leu-D,L-Phe-COOH and (CH3)2N(CH2)2NH2 using the EDC/HOBt coupling method. Purification twice by column chromatography on silica gel with 80:20 CH2Cl2:MeOH as the eluent gave a yellow semi-solid in 7% yield. 1H NMR (CDCl3): 0.85 (m, 6H, CH3 of Leu), 1.50–1.70 (m, 3H, CH2 and CH), 2.46 (s, 6H, CH3), 3.00 (m, 2H, CH2), 4.20 (m, 2H, CH2), 4.31 (m, 2H, CH2), 4.90 (b, 1H, α-H), 5.00 (m, 3H, Cbz and α-H), 6.20 (b, 1H, NH), 7.00–7.30 (m, 7H, Ph and NH). The purity was 96–99% by HPLC using either 220 or 254 nm detection. HRMS (FAB) for C28H39N4O5: m/z 511.3099 ([M+H]+).
Supplementary Material
Acknowledgement
This publication was made possible by Grant Number R21 NS053801 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Abbreviations
- Abu
4-aminobutyric acid
- AMC
7-amino-4-methylcoumarin
- BBB
blood brain barrier
- Brij
polyoxyethylene lauryl ether
- Bzl
benzyl, CH2Ph
- Cbz
benzyloxycarbonyl
- CDCl3
deuterated chloroform
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CHT
high affinity choline transporter
- CNS
central nervous system
- CNT
concentrative nucleoside transporters
- DCC
dicyclohexylcarbodiimide
- DMAP
4-dimethylaminopyridine
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- DMSO-d6
deuterated dimethylsulfoxide
- DTT
dithiothreitol
- EDC
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethyleneglycoltetraacetic acid
- Et2O
diethyl ether
- EtOAc
ethyl acetate
- EtOH
ethanol
- HOBt
N-hydroxybenzotriazole
- iBCF
isobutyl chloroformate
- LC-MS
liquid chromatography-mass spectrometry
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MeOH
methanol
- Nva
norvaline
- NMM
N-methylmorpholine
- OBzl
benzyloxy
- Phe
phenylalanine
- RT
room temperature
- TLC
thin layer chromatography
- Tris
tris(hydroxymethyl)aminomethane
- RFU
relative fluorescence units
- VAChT
vesicular acetylcholine transporter
Footnotes
Supporting Information Available: Synthesis of precursor dipeptides, amines, and characterization of previously reported peptidyl ketoamides. Statistical analysis of the results in Table 1 using a one-way ANOVA with a post-hoc Tukey HSD (honestly significant differences) test. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical Role of Calpain I in Mitochondrial Release of Apoptosis-Inducing Factor in Ischemic Neuronal Injury. Journal of Neuroscience. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stys PK, Jiang Q. Calpain-Dependent Neurofilament Breakdown in Anoxic and Ischemic Rat Central Axons. Neuroscience Letters. 2002;328:150–154. doi: 10.1016/s0304-3940(02)00469-x. [DOI] [PubMed] [Google Scholar]
- 3.Kampfl A, Posmantur R, Nixon R, Grynspan F, Zhao X, Liu SJ, Newcomb JK, Clifton GL, Hayes RL. Mu-Calpain Activation and Calpain-Mediated Cytoskeletal Proteolysis Following Traumatic Brain Injury. J. Neurochem. 1996;67:1575–1583. doi: 10.1046/j.1471-4159.1996.67041575.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu MC, Akle V, Zheng W, Kitlen J, O'Steen B, Larner SF, Dave JR, Tortella FC, Hayes RL, Wang KK. Extensive Degradation of Myelin Basic Protein Isoforms by Calpain Following Traumatic Brain Injury. J. Neurochem. 2006;98:700–712. doi: 10.1111/j.1471-4159.2006.03882.x. [DOI] [PubMed] [Google Scholar]
- 5.Mamoune A, Luo JH, Lauffenburger DA, Wells A. Calpain-2 as a Target for Limiting Prostate Cancer Invasion. Cancer Res. 2003;63:4632–4640. [PubMed] [Google Scholar]
- 6.Shiba E, Kim S, Fujitani M, Kambayashi JI, Kawamura I, Tsujimoto S, Shimomura K, Tanji Y, Taguchi T, Kimoto Y, Izukura M, Takai SI. Possible Involvement of Calpain in the Growth of Estrogen Receptor Positive Breast Cancer Cells. Anticancer Res. 1996;16:773–777. [PubMed] [Google Scholar]
- 7.Yang H, Murthy S, Sarkar FH, Sheng S, Reddy GP, Dou QP. Calpain-Mediated Androgen Receptor Breakdown in Apoptotic Prostate Cancer Cells. Journal of Cellular Physiology. 2008;217:569–576. doi: 10.1002/jcp.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumamoto T, Ueyama H, Watanabe S, Yoshioka K, Miike T, Goll DE, Ando M, Tsuda T. Immunohistochemical Study of Calpain and Its Endogenous Inhibitor in the Skeletal Muscle of Muscular Dystrophy. Acta Neuropathology. 1995;89:399–403. doi: 10.1007/BF00307642. [DOI] [PubMed] [Google Scholar]
- 9.Shanmuga Sundaram J, Mohana Rao V, Meena AK, Anandaraj MP. Altered Expression, Intracellular Distribution and Activity of Lymphocyte Calpain II in Duchenne Muscular Dystrophy. Clinica Chimica Acta. 2006;373:82–87. doi: 10.1016/j.cca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Tang YJ, Liu XY, Zoltoski RK, Novak LA, Herrera RA, Richard I, Kuszak JR, Kumar NM. Age-Related Cataracts in α3Cx46-Knockout Mice Are Dependent on a Calpain 3 Isoform. Invest Ophth Vis Sci. 2007;48:2685–2694. doi: 10.1167/iovs.06-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun M, Zhao Y, Gu Y, Xu C. Inhibition of nNOS Reduces Ischemic Cell Death through Down-Regulating Calpain and Caspase-3 after Experimental Stroke. Neurochemistry International. 2008;54:339–346. doi: 10.1016/j.neuint.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Di Rosa G, Odrijin T, Nixon RA, Arancio O. Calpain Inhibitors: A Treatment for Alzheimer's Disease. Journal of Molecular Neuroscience. 2002;19:135–141. doi: 10.1007/s12031-002-0024-4. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji T, Shimohama S, Kimura J, Shimizu K. M-Calpain (Calcium-Activated Neutral Proteinase) in Alzheimer's Disease Brains. Neuroscience Letters. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- 14.Gafni J, Ellerby LM. Calpain Activation in Huntington's Disease. Journal of Neuroscience. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvira D, Ferrer I, Gutierrez-Cuesta J, Garcia-Castro B, Pallas M, Camins A. Activation of the Calpain/Cdk5/P25 Pathway in the Girus Cinguli in Parkinson's Disease. Parkinsonism & Related Disorders. 2008;14:309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Samantaray S, Ray SK, Banik NL. Calpain as a Potential Therapeutic Target in Parkinson's Disease. CNS & Neurological Disorders - Drug Targets. 2008;7:305–312. doi: 10.2174/187152708784936680. [DOI] [PubMed] [Google Scholar]
- 17.Das A, Guyton MK, Butler JT, Ray SK, Banik NL. Activation of Calpain and Caspase Pathways in Demyelination and Neurodegeneration in Animal Model of Multiple Sclerosis. CNS & Neurological Disorders - Drug Targets. 2008;7:313–320. doi: 10.2174/187152708784936699. [DOI] [PubMed] [Google Scholar]
- 18.Shields DC, Schaecher KE, Saido TC, Banik NL. A Putative Mechanism of Demyelination in Multiple Sclerosis by a Proteolytic Enzyme, Calpain. Proceedings of the National Academy of Sciences USA. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zatz M, Starling A. Calpains and Disease. N. Engl. J. Med. 2005;352:2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 20.Ray SK. Currently Evaluated Calpain and Caspase Inhibitors for Neuroprotection in Experimental Brain Ischemia. Curr Med Chem. 2006;13:3425–3440. doi: 10.2174/092986706779010342. [DOI] [PubMed] [Google Scholar]
- 21.Carragher NO. Calpain Inhibition: A Therapeutic Strategy Targeting Multiple Disease States. Curr. Pharm. Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- 22.Bertipaglia I, Carafoli E. Calpains and Human Disease. Subcell. Biochem. 2007;45:29–53. doi: 10.1007/978-1-4020-6191-2_2. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Banik NL, Ray SK. Mechanism of Apoptosis with the Involvement of Calpain and Caspase Cascades in Human Malignant Neuroblastoma Sh-Sy5y Cells Exposed to Flavonoids. Int. J. Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- 24.Das A, Guyton MK, Butler JT, Ray SK, Banik NL. Activation of Calpain and Caspase Pathways in Demyelination and Neurodegeneration in Animal Model of Multiple Sclerosis. CNS & Neurol. Disord. - Drug Targets. 2008;7:313–320. doi: 10.2174/187152708784936699. [DOI] [PubMed] [Google Scholar]
- 25.Samantaray S, Ray SK, Banik NL. Calpain as a Potential Therapeutic Target in Parkinson's Disease. CNS Neurol Disord Drug Targets. 2008;7:305–312. doi: 10.2174/187152708784936680. [DOI] [PubMed] [Google Scholar]
- 26.Vosler PS, Brennan CS, Chen J. Calpain-Mediated Signaling Mechanisms in Neuronal Injury and Neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donkor IO, Korukonda R, Huang TL, LeCour L. Peptidyl Aldehyde Inhibitors of Calpain Incorporating P-2-Proline Mimetics. Bioorg. Med. Chem. Lett. 2003;13:783–784. doi: 10.1016/s0960-894x(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 28.Fukiage C, Azuma M, Nakamura Y, Tamada Y, Nakamura M, Shearer TR. SJA6017, a Newly Synthesized Peptide Aldehyde Inhibitor of Calpain: Amelioration of Cataract in Cultured Rat Lenses. Biochim. Biophys. Acta-Mol. Basis. Dis. 1997;1361:304–312. doi: 10.1016/s0925-4439(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal M, Messina PA, Freed B, Das M, Chatterjee S, Tripathy R, Tao M, Josef KA, Dembofsky B, Dunn D, Griffith E, Siman R, Senadhi SE, Biazzo W, BozyczkoCoyne D, Meyer SL, Ator MA, Bihovsky R. Subsite Requirements for Peptide Aldehyde Inhibitors of Human Calpain I. Bioorg. Med. Chem. Lett. 1997;7:539–544. [Google Scholar]
- 30.Abell AD, Jones MA, Coxon JM, Morton JD, Aitken SG, McNabb SB, Lee HY, Mehrtens JM, Alexander NA, Stuart BG, Neffe AT, Bickerstaffe R. Molecular Modeling, Synthesis, and Biological Evaluation of Macrocyclic Calpain Inhibitors. Angew. Chem., Int. Ed. 2009;48:1455–1458. doi: 10.1002/anie.200805014. [DOI] [PubMed] [Google Scholar]
- 31.Jones MA, Morton JD, Coxon JM, McNabb SB, Lee HY, Aitken SG, Mehrtens JM, Robertson LJ, Neffe AT, Miyamoto S, Bickerstaffe R, Gately K, Wood JM, Abell AD. Synthesis, Biological Evaluation and Molecular Modelling of N-Heterocyclic Dipeptide Aldehydes as Selective Calpain Inhibitors. Bioorg. Med. Chem. 2008;16:6911–6923. doi: 10.1016/j.bmc.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 32.Jones SA, Jones MA, McNabb SB, Aitken SG, Coxon JM, Abell AD. N-Heterocyclic Dipeptide Aldehyde Calpain Inhibitors. Protein Pept. Lett. 2009;16:1466–1472. doi: 10.2174/092986609789839296. [DOI] [PubMed] [Google Scholar]
- 33.Donkor IO, Korukonda R. Synthesis and Calpain Inhibitory Activity of Peptidomimetic Compounds with Constrained Amino Acids at the P2 Position. Bioorg. Med. Chem. Lett. 2008;18:4806–4808. doi: 10.1016/j.bmcl.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abell AD, Jones MA, Neffe AT, Aitken SG, Cain TP, Payne RJ, McNabb SB, Coxon JM, Stuart BG, Pearson D, Lee HY, Morton JD. Investigation into the P3 Binding Domain of M-Calpain Using Photoswitchable Diazo- and Triazene-Dipeptide Aldehydes: New Anticataract Agents. J. Med. Chem. 2007;50:2916–2920. doi: 10.1021/jm061455n. [DOI] [PubMed] [Google Scholar]
- 35.Inoue J, Nakamura M, Cui YS, Sakai Y, Sakai O, Hill JR, Wang KK, Yuen PW. Structure-Activity Relationship Study and Drug Profile of N-(4-Fluorophenylsulfonyl)-L-Valyl-L-Leucinal (SJA6017) as a Potent Calpain Inhibitor. J Med Chem. 2003;46:868–871. doi: 10.1021/jm0201924. [DOI] [PubMed] [Google Scholar]
- 36.Li ZZ, Patil GS, Golubski ZE, Hori H, Tehrani K, Foreman JE, Eveleth DD, Bartus RT, Powers JC. Peptide Alpha-Keto Ester, Alpha-Keto Amide, and Alpha-Keto Acid Inhibitors of Calpains and Other Cysteine Proteases. J. Med. Chem. 1993;36:3472–3480. doi: 10.1021/jm00074a031. [DOI] [PubMed] [Google Scholar]
- 37.Donkor IO, Assefa H, Liu J. Structural Basis for the Potent Calpain Inhibitory Activity of Peptidyl Alpha-Ketoacids. J. Med. Chem. 2008;51:4346–4350. doi: 10.1021/jm800182c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li ZZ, OrtegaVilain AC, Patil GS, Chu DL, Foreman JE, Eveleth DD, Powers JC. Novel Peptidyl Alpha-Keto Amide Inhibitors of Calpains and Other Cysteine Proteases. J. Med. Chem. 1996;39:4089–4098. doi: 10.1021/jm950541c. [DOI] [PubMed] [Google Scholar]
- 39.Lee KY, Lee KS, Jin C, Lee YS. Design and Synthesis of Calpain Inhibitory 6-Pyridone 2-Carboxamide Derivatives. Eur. J. Med. Chem. 2009;44:1331–1334. doi: 10.1016/j.ejmech.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Nam DH, Lee KS, Kim SH, Kim SM, Jung SY, Chung SH, Kim HJ, Kim ND, Jin C, Lee YS. Design and Synthesis of 4-Quinolinone 2-Carboxamides as Calpain Inhibitors. Bioorg. Med. Chem. Lett. 2008;18:205–209. doi: 10.1016/j.bmcl.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 41.Angelastro MR, Mehdi S, Burkhart JP, Peet NP, Bey P. Alpha-Diketone and Alpha-Keto Ester Derivatives of N-Protected Amino-Acids and Peptides as Novel Inhibitors of Cysteine and Serine Proteinases. J. Med. Chem. 1990;33:11–13. doi: 10.1021/jm00163a002. [DOI] [PubMed] [Google Scholar]
- 42.Tao M, Bihovsky R, Wells GJ, Mallamo JP. Novel Peptidyl Phosphorus Derivatives as Inhibitors of Human Calpain I. J. Med. Chem. 1998;41:3912–3916. doi: 10.1021/jm980325e. [DOI] [PubMed] [Google Scholar]
- 43.Barrett AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, Tamai M, Hanada K. L-Trans-Epoxysuccinyl-Leucylamido(4-Guanidino)Butane (E-64) and Its Analogs as Inhibitors of Cysteine Proteinases Including Cathepsins B, H and L. Biochemical J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanada K, Tamai M, Ohmura S, Sawada J, Seki T, Tanaka I. Structure and Synthesis of E-64, a New Thiol Protease Inhibitor. Agr. Biol. Chem. Tokyo. 1978;42:529–536. [Google Scholar]
- 45.Hanada K, Tamai M, Yamagishi M, Ohmura S, Sawada J, Tanaka I. Isolation and Characterization of E-64, a New Thiol Protease Inhibitor. Agr. Biol. Chem. Tokyo. 1978;42:523–528. [Google Scholar]
- 46.Palmer JT, Rasnick D, Klaus JL, Bromme D. Vinyl Sulfones as Mechanism-Based Cysteine Protease Inhibitors. J. Med. Chem. 1995;38:3193–3196. doi: 10.1021/jm00017a002. [DOI] [PubMed] [Google Scholar]
- 47.Pliura DH, Bonaventura BJ, Smith RA, Coles PJ, Krantz A. Comparative Behaviour of Calpain and Cathepsin B toward Peptidyl Acyloxymethyl Ketones, Sulphonium Methyl Ketones and Other Potential Inhibitors of Cysteine Proteinases. Biochem. J. 1992;288(Pt 3):759–762. doi: 10.1042/bj2880759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris AL, Gregory JS, Maycock AL, Graybill TL, Osifo IK, Schmidt SJ, Dolle RE. Characterization of a Continuous Fluorogenic Assay for Calpain-I - Kinetic Evaluation of Peptide Aldehydes, Halomethyl Ketones and (Acyloxy)Methyl Ketones as Inhibitors of the Enzyme. Bioorg. Med. Chem. Lett. 1995;5:393–398. [Google Scholar]
- 49.Sasaki T, Kikuchi T, Fukui I, Murachi T. Inactivation of Calpain I and Calpain II by Specificity-Oriented Tripeptidyl Chloromethyl Ketones. J. Biochem. 1986;99:173–179. doi: 10.1093/oxfordjournals.jbchem.a135457. [DOI] [PubMed] [Google Scholar]
- 50.Neffe AT, Abell AD. Developments in the Design and Synthesis of Calpain Inhibitors. Curr Opin Drug Discov Devel. 2005;8:684–700. [PubMed] [Google Scholar]
- 51.Donkor IO. A Survey of Calpain Inhibitors. Curr. Med. Chem. 2000;7:1171–1188. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 52.Krauser JA, Powers JC. Calpain. In: Smith HJ, Simons C, editors. Proteinase and Peptidase Inhibition: Recent Potential Targets for Drug Development. New York: Taylor & Francis; 2002. pp. 127–153. [Google Scholar]
- 53.Higuchi M, Iwata N, Saido TC. Understanding Molecular Mechanisms of Proteolysis in Alzheimer's Disease: Progress toward Therapeutic Interventions. Biochim. Biophys. Acta. 2005;1751:60–67. doi: 10.1016/j.bbapap.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK. Calpain Inhibitor AK295 Attenuates Motor and Cognitive Deficits Following Experimental Brain Injury in the Rat. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3428–3433. doi: 10.1073/pnas.93.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. Prevention of Axonal Injury Using Calpain Inhibitor in Chronic Progressive Experimental Autoimmune Encephalomyelitis. Brain Res. 2008;1236:206–215. doi: 10.1016/j.brainres.2008.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koumura A, Nonaka Y, Hyakkoku K, Oka T, Shimazawa M, Hozumi I, Inuzuka T, Hara H. A Novel Calpain Inhibitor, ((1s)-1((((1s)-1-Benzyl-3-Cyclopropylamino-2,3-Di-Oxopropyl)Amino)Carbonyl )-3-Methylbutyl) Carbamic Acid 5-Methoxy-3-Oxapentyl Ester, Protects Neuronal Cells from Cerebral Ischemia-Induced Damage in Mice. Neuroscience. 2008;157:309–318. doi: 10.1016/j.neuroscience.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Araujo Couto L, Sampaio Narciso M, Hokoc JN, Blanco Martinez AM. Calpain Inhibitor 2 Prevents Axonal Degeneration of Opossum Optic Nerve Fibers. J. Neuroscience Res. 2004;77:410–419. doi: 10.1002/jnr.20170. [DOI] [PubMed] [Google Scholar]
- 58.Ray SK, Hogan EL, Banik NL. Calpain in the Pathophysiology of Spinal Cord Injury: Neuroprotection with Calpain Inhibitors. Brain Res. Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 59.Akdemir O, Ucankale M, Karaoglan A, Barut S, Sagmanligil A, Bilguvar K, Cirakoglu B, Sahan E, Colak A. Therapeutic Efficacy of SJA6017, a Calpain Inhibitor, in Rat Spinal Cord Injury. J. Clin. Neurosci. 2008;15:1130–1136. doi: 10.1016/j.jocn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Wang MS, Davis AA, Culver DG, Wang Q, Powers JC, Glass JD. Calpain Inhibition Protects against Taxol-Induced Sensory Neuropathy. Brain. 2004;127:671–679. doi: 10.1093/brain/awh078. [DOI] [PubMed] [Google Scholar]
- 61.Lee HY, Morton JD, Robertson LJ, McDermott JD, Bickerstaffe R, Abell AD, Jones MA, Mehrtens JM, Coxon JM. Evaluation of a Novel Calpain Inhibitor as a Treatment for Cataract. Clin. Experment. Ophthalmol. 2008;36:852–860. doi: 10.1111/j.1442-9071.2009.01925.x. [DOI] [PubMed] [Google Scholar]
- 62.Jung SY, Zheng B, Choi YY, Soh BY, Kim SY, Park KI, Park H. Antimalarial Effect of N-Acetyl-L-Leucyl-L-Leucyl-L-Norleucinal by the Inhibition of Plasmodium Falciparum Calpain. Arch. Pharm. Res. 2009;32:899–906. doi: 10.1007/s12272-009-1612-4. [DOI] [PubMed] [Google Scholar]
- 63.Czeisler BM, Janigro D. Reading and Writing the Blood-Brain Barrier: Relevance to Therapeutics. Recent Pat. CNS Drug Discov. 2006;1:157–173. doi: 10.2174/157488906777452712. [DOI] [PubMed] [Google Scholar]
- 64.Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, Akiyama A, Straub JA, Harbeson SL, Li Z, Powers J. Calpain Inhibitor Ak295 Protects Neurons from Focal Brain Ischemia - Effects of Postocclusion Intraarterial Administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- 65.Wang MS, Wu Y, Culver DG, Glass JD. Pathogenesis of Axonal Degeneration: Parallels between Wallerian Degeneration and Vincristine Neuropathy. J. Neuropath. Exp. Neurology. 2000;59:599–606. doi: 10.1093/jnen/59.7.599. [DOI] [PubMed] [Google Scholar]
- 66.Zhang QZ, Hua GX, Bhattacharyya P, Slawin AMZ, Woollins JD. Syntheses and Coordination Chemistry of Aminomethylphosphine Derivatives of Adenine. Eur. J. Inorg. Chem. 2003;2003:2426–2437. [Google Scholar]
- 67.Bergmann W, Stempien MF. Contributions to the Study of Marine Products .43. The Nucleosides of Sponges .5. The Synthesis of Spongosine. J. Org. Chem. 1957;22:1575–1577. [Google Scholar]
- 68.Summers WA, Lee JY, Burr JG. Synthesis of Fluorescent Labeled Derivatives of Aminopropylpyrimidines. J. Org. Chem. 1975;40:1559–1561. [Google Scholar]
- 69.Hou DR, Cheng HY, Wang EC. Efficient Syntheses of Oncinotine and Neooncinotine. J. Org. Chem. 2004;69:6094–6099. doi: 10.1021/jo049526i. [DOI] [PubMed] [Google Scholar]
- 70.Chatterjee S, Dunn D, Tao M, Wells G, Gu ZQ, Bihovsky R, Ator MA, Siman R, Mallamo JP. P-2-Achiral, P'-Extended Alpha-Ketoamide Inhibitors of Calpain I. Bioorg. Med. Chem. Lett. 1999;9:2371–2374. doi: 10.1016/s0960-894x(99)00392-3. [DOI] [PubMed] [Google Scholar]
- 71.Donkor IO, Han J, Zheng XZ. Design, Synthesis, Molecular Modeling Studies, and Calpain Inhibitory Activity of Novel Alpha-Ketoamides Incorporating Polar Residues at the P-1'-Position. J. Med. Chem. 2004;47:72–79. doi: 10.1021/jm0301336. [DOI] [PubMed] [Google Scholar]
- 72.Donkor IO, Zheng XZ, Miller DD. Synthesis and Calpain Inhibitory Activity of Alpha-Ketoamides with 2,3-Methanoleucine Stereoisomers at the P-2 Position. Bioorg. Med. Chem. Lett. 2000;10:2497–2500. doi: 10.1016/s0960-894x(00)00518-7. [DOI] [PubMed] [Google Scholar]
- 73.Lescop C, Herzner H, Siendt H, Bolliger R, Hennebohle M, Weyermann P, Briguet A, Courdier-Fruh I, Erb M, Foster M, Meier T, Magyar JP, von Sprecher A. Novel Cell-Penetrating Alpha-Keto-Amide Calpain Inhibitors as Potential Treatment for Muscular Dystrophy. Bioorg. Med. Chem. Lett. 2005;15:5176–5181. doi: 10.1016/j.bmcl.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 74.Cuerrier D, Moldoveanu T, Davies PL. Determination of Peptide Substrate Specificity for M-Calpain by a Peptide Library-Based Approach - the Importance of Prime Side Interactions. J. Biol. Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- 75.Dantzig AH, Hillgren KM, de Alwis DP. Drug Transporters and Their Role in Tissue Distribution. Ann. Reports of Med. Chem. 2004;39:279–291. [Google Scholar]
- 76.Sarter M, Parikh V. Choline Transporters, Cholinergic Transmission and Cognition. Nature Rev. Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Lockman PR, Mittapalli RK, Allen DD, Dwoskin LP, Crooks PA. Bis-Pyridinium Cyclophanes: Novel Ligands with High Affinity for the Blood-Brain Barrier Choline Transporter. Bioorg. Med. Chem. Lett. 2008;18:5622–5625. doi: 10.1016/j.bmcl.2008.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active Transport of High-Affinity Choline and Nicotine Analogs into the Central Nervous System by the Blood-Brain Barrier Choline Transporter. J. Pharm. Exp. Ther. 2003;304:1268–1274. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- 79.Kim MH, Maeng HJ, Yu KH, Lee KR, Tsuruo T, Kim DD, Shim CK, Chung SJ. Evidence of Carrier-Mediated Transport in the Penetration of Donepezil into the Rat Brain. J Pharm Sci. 2009;99:1548–1566. doi: 10.1002/jps.21895. [DOI] [PubMed] [Google Scholar]
- 80.Lee NY, Choi HM, Kang YS. Choline Transport Via Choline Transporter-Like Protein 1 in Conditionally Immortalized Rat Syncytiotrophoblast Cell Lines Tr-Tbt. Placenta. 2009;30:368–374. doi: 10.1016/j.placenta.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Kong W, Engel K, Wang J. Mammalian Nucleoside Transporters. Curr. Drug Metab. 2004;5:63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs JE, Jayabalan P, Thomas SA. Mechanisms by Which 2 ',3 '-Dideoxyinosine (Ddi) Crosses the Guinea-Pig CNS Barriers; Relevance to HIV Therapy. J. Neurochem. 2003;84:725–734. doi: 10.1046/j.1471-4159.2003.01560.x. [DOI] [PubMed] [Google Scholar]
- 83.Franke RM, Scherkenbach LA, Sparreboom A. Pharmacogenetics of the Organic Anion Transporting Polypeptide 1A2. Pharmacogenomics. 2009;10:339–344. doi: 10.2217/14622416.10.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varatharajan L, Thomas SA. The Transport of Anti-HIV Drugs across Blood-CNS Interfaces: Summary of Current Knowledge and Recommendations for Further Research. Antiviral Res. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, Azeem A, Jain N, Lalani JR, Khar RK, Ahmad FJ. CNS Drug Delivery Systems: Novel Approaches. Recent Pat. Drug Deliv. Formul. 2009;3:71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 86.Rao KS, Ghorpade A, Labhasetwar V. Targeting Anti-HIV Drugs to the CNS. Expert Opin. Drug Deliv. 2009;6:771–784. doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weyermann P, Herzner H, Lescop C, Siendt H, Bolliger R, Hennebohle M, Rummey C, Briguet A, Courdier-Fruh I, Erb M, Foster M, Magyar JP, von Sprecher A, Meier T. Synthesis and Evaluation of Calpain Inhibitors Carrying Muscle Cell Targeting Groups. Lett. Drug Des. Discov. 2006;3:152–158. [Google Scholar]
- 88.Qian J, Cuerrier D, Davies PL, Li ZZ, Powers JC, Campbell RL. Cocrystal Structures of Primed Side-Extending Alpha-Ketoamide Inhibitors Reveal Novel Calpain-Inhibitor Aromatic Interactions. J. Med. Chem. 2008;51:5264–5270. doi: 10.1021/jm800045t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo W, Johnson JL, Khan S, Ahmad A, Ahmad I. Paclitaxel Quantification in Mouse Plasma and Tissues Containing Liposome-Entrapped Paclitaxel by Liquid Chromatography-Tandem Mass Spectrometry: Application to a Pharmacokinetics Study. Anal. Biochem. 2005;336:213–220. doi: 10.1016/j.ab.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 90.Dixon M. Graphical Determination of Equilibrium Constants. Biochem. J. 1965;94:760–762. doi: 10.1042/bj0940760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.