Abstract
Depressive symptoms in alcohol-dependent individuals are well recognized and clinically relevant phenomena. The etiology has not been elucidated although it is clear that the depressive symptoms may be alcohol independent or alcohol-induced. In order to contribute to the understanding of the neurobiology of chronic ethanol use, we investigated the effects of chronic intermittent ethanol vapor exposure on behaviors in the forced swim test (FST) and neuropeptide Y (NPY) and corticotropin releasing factor (CRF) levels in specific brain regions. Adult male Wistar rats were subjected to intermittent ethanol vapor (14 hours on / 10 hours off) or air exposure for two weeks and were then tested at three time points corresponding to acute withdrawal (8–12 hours into withdrawal) and protracted withdrawal (30 and 60 days of withdrawal) in the FST. The behaviors that were measured in the five minute FST consisted of latency to immobility, swim time, immobility time and climbing time. The FST results showed that the vapor-exposed animals displayed depressive-like behaviors, for instance decreased latency to immobility in acute withdrawal and decreased latency to immobility, decreased swim time and increased immobility time in protracted withdrawal, with differences between air- and vapor-exposed animals becoming more pronounced over the 60 day withdrawal period. NPY levels in the frontal cortex of the vapor-exposed animals were decreased compared to the control animals and CRF levels in the amygdala were correlated with increased immobility time. Thus, extended ethanol vapor exposure produced long-lasting changes in FST behavior and NPY levels in the brain.
Keywords: Alcohol, CRF, Dependence, Depression, Forced Swim Test, NPY
Introduction
A significant proportion of alcohol-dependent individuals suffer from affective disorders such as depression (Regier et al., 1990; Grant and Harford, 1995; Kessler et al., 1997; Schuckit et al., 1997a; Schuckit et al., 1997b; Hasin and Grant, 2002; Gilder et al., 2004). However, the onset of major depression following alcohol dependence/abuse does not necessarily imply a causative relationship and the pathogenesis remains obscure. For those diagnosed with alcohol dependence, up to one-third have experienced major depression (Roy et al., 1991) and it has been identified that the depressive endophenotype can stem from different etiologies that may be alcohol independent or alcohol-induced (Schuckit et al., 1997a; Hasin and Grant, 2002). Furthermore, it has been shown that of those diagnosed with comorbid clinical depression and alcohol dependence, a substantial percentage had symptomology that was determined to be alcohol-induced (Schuckit et al., 1997b). It has been suggested that some individuals may use alcohol to “self-medicate” symptoms of depression (O'Sullivan, 1984; Khantzian, 1990; Markou et al., 1998).
In view of these clinical observations, and in conjunction with preclinical evidence that ligands with anti-depressant properties can reduce the motivation of chronic ethanol-exposed animals to self-administer alcohol during withdrawal (Walker and Koob, 2008), we decided to explore the neurobiological effects of chronic exposure to ethanol in Wistar rats. The need for further scientific study of alcohol-induced depression is highlighted by the observation that there are no current pharmacotherapies that target negative affective states for those with a history of alcoholism (Heilig and Koob, 2007).
In addition to monoamines, other classes of compounds, such as neuropeptides, play important roles in affective disorders. There is strong clinical as well as experimental support for the hypotheses that an increased expression of corticotropin releasing factor (CRF) and a reduced expression of neuropeptide Y (NPY) play a role in pathogenesis of depression and anxiety and, conversely, that one common mechanism of action of anti-depressive and anxiolytic treatment modalities is a decrease in CRF and an increase in NPY expression (Mathé et al., 1996; Nikisch and Mathé, 2008; Mathé et al., 2007; Nikisch et al., 2005; Husum and Mathé, 2002; Stenfors et al., 1989; Holmes et al., 2003; Madaan and Wilson, 2009; Paschos et al., 2009; Heilig et al., 1989; Redrobe et al., 2002; Redrobe, Dumont, and Quirion, 2002; Jimenez-Vasquez et al., 2007). Finally, some data suggest that alterations in NPY and CRF systems in response to chronic alcohol exposure could contribute to escalated ethanol intake (Sommer et al., 2008; Rassnick, Heinrichs, Britton, and Koob, 1993; Thorsell, Slawecki, and Ehlers, 2005; Gilpin et al., 2008; Funk et al., 2006; Funk et al., 2007; Valdez et al., 2002).
Cumulatively, these findings strongly indicate that ethanol-induced depressive-like behavior involves alterations in CRF and NPY systems in the brain. Consequently, the present experiment evaluated behavior of adult Wistar rats in the modified forced swim test (Lucki, 1997; Cryan et al., 2002) and subsequently levels of brain neuropeptides following long-term ethanol exposure. Performance in the FST was evaluated in adult Wistar rats during acute withdrawal and protracted abstinence following a two-week intermittent ethanol vapor exposure period. In addition, because of their involvement with negative affect and depressive like behaviors, NPY and CRF levels were measured in selected brain regions of the vapor- and air-exposed animals following their final protracted withdrawal test.
Materials and Methods
Subjects
Eighteen male Wistar rats (Charles River, Wilmington, MA) weighing approximately 250 g upon arrival were used in the present study. The animals were pair-housed within a temperature-controlled (21.5 °C) vivarium and maintained on a 12-hour light/dark cycle (lights on at 3 a.m.). Upon arrival, animals were handled daily for one week prior to the onset of the experiment. Ad libitum food and water were provided for the duration of the experiment. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Procedure
The subjects were divided into two groups that were subjected to either intermittent ethanol vapor or air exposure for two weeks (see below). Following chronic ethanol vapor or air exposure, the animals were tested in the FST at three different time points corresponding to acute withdrawal (8–12 hours into withdrawal) and protracted abstinence (30 and 60 days of withdrawal). The FST apparatus was a white plastic tub (diameter = 34 cm, height = 66 cm). The tub was filled to a level of 48 cm with 24±2° C water. Illumination at the surface of the water was approximately 25 lux. One day prior to the initial acute withdrawal test, rats were placed in the apparatus for 10 minutes but behavior was not recorded. On the day of the acute, 30 and 60 day withdrawal tests, behavior in the apparatus was assessed during a 5 minute test session. Each test session was videotaped and later analyzed by two researchers that were blind to the exposure conditions. The behaviors that were measured consisted of latency to immobility once being placed in the apparatus, swim time, immobility time and climbing time. Immobility time was defined as a lack of active swimming and floating to maintain the head above water with only minor paw movement. Climbing was defined by active attempts to climb the walls of the apparatus in order to escape. Inter-rater reliability was very high, with less than 10% deviation between scorers on all parameters that were evaluated.
Intermittent Ethanol Vapor Exposure
Ethanol vapor exposure has been shown to reliably allow for the titration of blood alcohol levels that are sufficient for inducing behavioral changes reflective of ethanol dependence-like symptoms (Roberts et al., 1996; Roberts et al., 2000; O'Dell et al., 2004; Walker and Koob, 2007; Walker and Koob, 2008). In this paradigm, blood alcohol levels can be titrated by the experimenter to fit established criterion and the animals show normal weight gain and are freely moving (Rogers et al., 1979). Standard rat cages were sealed and ethanol vapor or air was pumped through the chambers. Ethanol vapor was created by dripping 95% ethanol into 2000ml Erlenmeyer flasks that were maintained at 50°C due to a warming tray. Air (11L/min) was passed over the bottom of the flask so that when the ethanol hit the warm glass and was vaporized, the air carried it into the vapor chamber. Alteration of the ethanol vapor concentration was accomplished by modulating the air flow carrying the ethanol vapor into the chamber. Target blood alcohol levels (BALs) were 175–225 mg% across the two week exposure period and were determined by sampling blood collected from the tail (0.5 ml) twice a week. Following centrifugation, plasma alcohol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
Animals were subjected to intermittent vapor exposure (14 hours on / 10 hours off) over the course of two weeks; this paradigm has previously been shown to be effective at inducing motivational signs of ethanol dependence such as ethanol self-administration (O'Dell et al., 2004). Furthermore, when compared to chronic vapor exposure, intermittent ethanol vapor exposure has been shown to enhance ethanol self-administration during acute withdrawal (O'Dell et al., 2004).
Analysis of NPY and CRF levels
NPY and CRF levels were assessed in the amygdala (AMYG), frontal cortex (FCTX), hippocampus (HPC) and hypothalamus (HYPO). To ensure that the measurement of NPY and CRF levels reflected baseline changes (as opposed to swim stress-induced), brains were collected from animals fourteen days after the 60-day withdrawal FST. The animals were decapitated without anesthesia and the brain regions of interest were microdissected over ice according to the coordinates identified in Palkovits and Brownstein (1988) using a chilled metal rat brain matrix (Roboz Surgical Instruments Company, Inc., Gaithersburg, MD) to identify the appropriate slices from which tissue was collected. Tissue samples were immediately weighed, frozen using dry-ice and stored at −80 °C until they were assayed for NPY- and CRF-like immunoreactivity (NPY-LI and CRF-LI, respectively) as previously described (Husum and Mathé, 2002; Ehlers et al., 1998; Mathé et al., 1997; Stenfors et al., 1989)
Statistical Analysis
Performance in the FST was individually evaluated for each of the three test sessions (i.e., acute withdrawal, 30 and 60 days of protracted withdrawal) using a multivariate analysis of variance (ANOVA). The independent factor was level of vapor exposure (vapor-exposed or air-exposed) and the dependent measures were latency to immobility, swim time, immobility time and climbing time. To evaluate whether changes in behavior occurred within the FST over the course of the experiment, a two-way repeated measures ANOVA was conducted. The within- subject factors were latency to immobility, swim time, immobility time and climbing time over the three test sessions (i.e., acute, 30- and 60-day withdrawal) and the between-groups factor was condition (i.e., air- or vapor-exposed). NPY-LI and CRF-LI in the AMYG, FCTX, HPC and HYPO from the vapor- and air-exposed animals were individually evaluated using a between groups one-way ANOVA. In addition, correlations of NPY-LI and CRF-LI with depressive-like behavior were calculated.
Results
Data from the acute, 30 day and 60 day withdrawal FST were individually evaluated using a multivariate analysis of variance. During acute withdrawal (see Fig. 1), compared to air-exposed controls, the vapor-exposed animals had a significantly reduced latency to immobility (F (1, 16) = 5.722, p < 0.05). The initial protracted withdrawal test (30 days of withdrawal; see Fig. 1) showed that the vapor-exposed animals had not only a significantly reduced latency to immobility (F (1, 16) = 13.964, p < 0.01), but also a significantly reduced swim time (F (1, 16) = 5.155, p < 0.05) when compared to air-exposed controls. Lastly, in the 60 day protracted withdrawal test (see Fig. 1), the vapor-exposed animals showed significant reduction in latency to immobility (F (1, 16) = 5.083, p < 0.05) and swim time (F (1, 16) = 5.12.695, p < 0.01), but also significantly increased immobility time (F (1, 16) = 6.066, p < 0.05) when compared to control animals. Thus, the ethanol vapor-exposed animals displayed three behavioral signs indicative of an increased depressive-like state.
Figure 1.
Mean (± S.E.M.) performance in the modified forced swim test over the three withdrawal test sessions for (A) latency to immobility, (B) swim time, (C) immobility time and (D) climbing time (* = p < 0.05 when compared to air-exposed animals). Significant interactions in behavior (p < 0.05) were seen between the ethanol vapor-exposed and air-exposed animals over the three test sessions for the parameters of swim time and immobility time.
To address the question of whether the observed behaviors in the FST changed over time, a two-way repeated measures ANOVA was used to evaluate latency to immobility, swim time, immobility time and climbing time for the air- and vapor-exposed animals across the three different time points (i.e., acute, 30 and 60 days of withdrawal) that were utilized. As seen in Figure 1, the results of the two-way ANOVA showed that there was a significant within-subjects effect for latency to immobility (F (2, 32) = 14.963, p < 0.001) and a significant between-groups effect of condition (F (1, 16) = 11.98, p < 0.01), which reflected that although there were group differences, time to immobility changed over time (i.e., decreased) in a comparable fashion for both groups, but they remained different from each other. When evaluating swim time (see Fig. 1), a between-subjects effect of condition (F (1, 16) = 13.387, p < 0.01) and a Swim Time × Condition interaction (F (2, 32) = 4.028, p < 0.05) were found. This indicates that not only did the air- and vapor-exposed animals express different behaviors from each other, but that those behaviors changed over time differently for each group. For immobility time (see Fig. 1), the two-way ANOVA showed significant within-subject (F (2, 32) = 3.454, p < 0.05) and between-subjects (F (1, 16) = 7.861, p < 0.05) main effects, as well as, a significant Immobility Time × Condition interaction (F (2, 32) = 3.453, p < 0.05). This illustrates that immobility time was different for air- and vapor-exposed animals and that the behavior changed over time differently for each group (i.e., increased for vapor-exposed and decreased for air-exposed). Lastly, the two-way ANOVA identified a within-subjects effect (F (2, 32) = 13.141, p < 0.001) for climbing time (see Fig. 1), but no significant between-subjects effects or interactions. This illustrates that that the air- and vapor-exposed animals showed comparable climbing behavior that changed over time in a similar fashion.
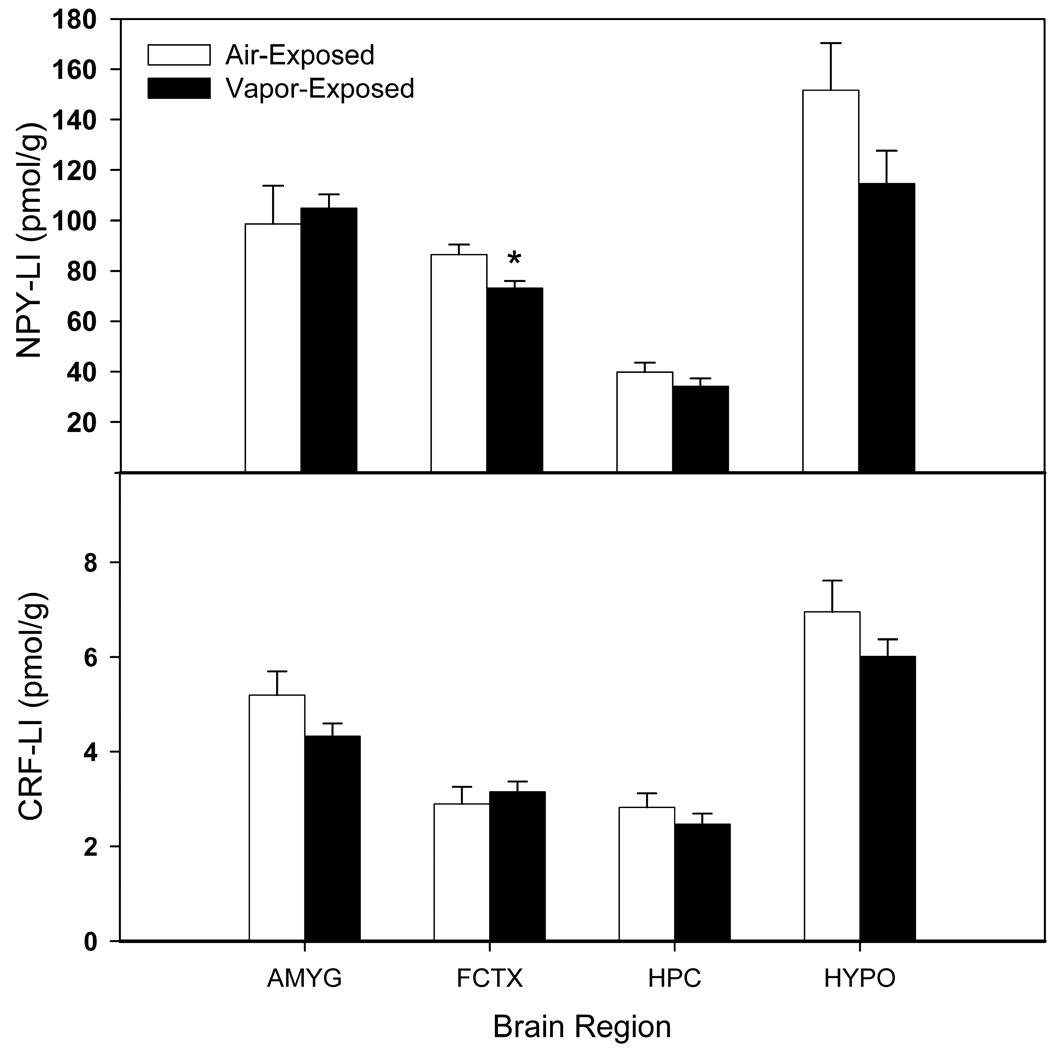
The levels of NPY- and CRF-LI in the AMYG, FCTX, HPC and HYPO are shown in Figure 2. The one-way ANOVA showed that NPY-LI was significantly decreased (F (1, 17) = 7.438, p < 0.05) in the FCTX of vapor-exposed when compared to air-exposed rats with no other brain regions showing significant differences in levels of NPY- or CRF-LI. However, when evaluating correlations of NPY- and CRF-LI in the AMYG, FCTX, HPC and HYPO with depressive-like behavior in the vapor-exposed animals and air-exposed animals, CRF-LI in the AMYG was shown to be positively correlated with immobility time (r = .627, p < 0.05) in the FST (at 60 days of withdrawal) following 74 days of protracted abstinence, whereas there were no significant associations between the depressive-like behaviors and immunoreactivity in the air-exposed controls. There were no significant correlations between depressive-like behaviors and NPY levels in the vapor- or air-exposed animals, although there was a trend towards a negative correlation between NPY-LI in the AMYG and immobility time in the air-exposed animals (r = −.60, p = 0.056).
Figure 2.
Mean (± S.E.M.) NPY- and CRF-LI (neuropeptide Y- and corticotropin releasing factor-like immunoreactivity) in the amygdala (AMYG), frontal cortex (FCTX), hippocampus (HPC) and hypothalamus (HYPO) in protracted abstinence after two weeks of chronic vapor exposure (* = p < 0.05 when compared to air-exposed control).
Discussion
In the present study, we confirmed that ethanol vapor-exposed animals showed differential behavior in the FST when compared to air-exposed controls during both acute and protracted withdrawal time periods. Specifically, different latencies to immobility were seen during acute withdrawal between air- and vapor-exposed animals. Furthermore, during protracted withdrawal, not only were differential latencies to immobility maintained, but dissociations between air- and vapor-exposed animals were also observed in swim time following 30 days of withdrawal and both swim time and immobility time after 60 days of withdrawal. Thus, the differences that were observed between air- and vapor-exposed animals became more apparent over the 60 day withdrawal period as evidenced by the expression of depressive-like behavior across multiple dependent variables. Furthermore, over the course of the experiment, air-exposed and vapor-exposed animals showed differences in swim time and immobility time that were distinct from each other. While swim time increased for air-exposed controls and decreased for vapor-exposed animals, immobility time increased for vapor-exposed animals, but showed no change over time for air-exposed animals. Thus, two indices of depressive-like behavior changed for the vapor-exposed group in a manner consistent with increased ‘depression’ over time. These data are also in accordance with previous evidence demonstrating that adolescent animals with a history of ethanol vapor exposure also showed decreased latencies to immobility during protracted withdrawal (Slawecki, Thorsell, and Ehlers, 2004).
The present data set lends support to the clinical data showing that a substantial number of individuals diagnosed with co-morbid depression and alcohol dependence have depressive symptomology that can be attributed to alcohol use itself (Schuckit et al., 1997a; Hasin and Grant, 2002). In humans, it has been shown that major depressive symptoms generally last for 2 – 4 weeks after abstinence from alcohol is initiated (Brown and Schuckit, 1988). However, individuals for whom the diagnosis of clinical depression was maintained following one-month of abstinence also reported a significantly greater incidence of withdrawal symptoms (Brown and Schuckit, 1988), perhaps indicative of greater severity of ethanol dependence prior to abstinence. In that study (Brown and Schuckit, 1988), depressive symptomology was only evaluated up to 4 weeks following abstinence, so further longitudinal data is unavailable. In the present study, depressive-like behavior that was observed in rats following ethanol exposure persisted for up to 8 weeks into abstinence. Although the temporal expression of depressive-like behaviors following chronic ethanol exposure appears to persist for a longer duration in Wistar rats when compared to humans, it should be noted that the ethanol vapor exposure paradigm utilized in the present study produces physiological withdrawal symptoms (Roberts et al., 1996). Thus, taken in context with the results of the study by Brown and Schuckit (1988), the current paradigm may be modeling a more ‘extreme’ form of dependence that translates into protracted depressive-like behaviors.
One point to consider in regards to the present study is that the two week vapor exposure period can induce excessive alcohol intake during acute withdrawal, but to observe protracted increases in ethanol self-administration, a more prolonged period is needed. It has been shown that seven weeks of exposure can induce protracted elevations in self-administration that persist for at least five to six weeks of abstinence (Rimondini et al., 2003). In the present study, the vapor exposure length was selected because it is one of the shortest intervals that has been sown to induce elevated alcohol self-administration(O'Dell et al., 2004), however, it would be ideal to identify a length of vapor exposure that induces protracted changes in negative affect that overlap with persistent increased levels of alcohol self-administration.
There are two possible interpretations of our data. The first would suggest that in alcohol-dependent subjects during withdrawal, depressive-like behaviors become more pronounced over time. However, as mentioned above, this interpretation is not consistent with observations in humans showing that depression associated with abstinence from alcohol in previously dependent individuals is maximal early in withdrawal and typically subsides within one month (Brown and Schuckit, 1988). The other, and more likely, interpretation is that chronic ethanol exposure has induced a neuroadaptive state that makes rats more susceptible to the depression-promoting effects of repeated stress. Considering that the FST has been used to induce stress responses (for example, see Land et al., 2008), this concept fits well with other existing data regarding repeated ethanol withdrawal or stress and sensitized anxiety-like behavior (Overstreet et al., 2002; Breese et al., 2004; Breese et al., 2005; Knapp et al., 2007). However, a critical factor when considering this interpretation is that the air-exposed rats do not show any alterations in depressive-like behavior, so it would appear as though repeated FST testing alone is not sufficient to induce a depressive-like phenotype and that exposure to chronic vapor exposure is necessary for the formation of depressive-like behaviors, as was observed in the present study. However, because the present study used a repeated-measures design, future between-group experiments would have to be conducted in vapor exposed animals to determine the precise contribution of stress versus vapor exposure.
NPY and CRF are associated with the reduction (Heilig et al., 1989; Stogner and Holmes, 2000; Redrobe et al., 2002) and production (Overstreet and Griebel, 2004; Chaki et al., 2004; Heinrichs et al., 1994), respectively of depressive- and anxiety-like behavior in preclinical models. Furthermore, data has been generated suggesting that alterations in NPY and CRF systems in response to chronic alcohol exposure could contribute to escalated ethanol intake (Sommer et al., 2008; Rassnick et al., 1993; Thorsell et al., 2005; Gilpin et al., 2008; Valdez et al., 2002; Funk et al., 2006; Funk et al., 2007). In the present study, when evaluating NPY and CRF levels in the brains of those animals that were tested at time points corresponding to acute, 30-day and 60-days of withdrawal, it was shown that, following protracted withdrawal, NPY-LI was decreased in the fontal cortex. It is important to recognize that in the present experiment, the neuropeptide levels were measured in brains that were extracted two weeks after the final behavioral test and thus, reflect baseline changes as opposed to swim stress-induced changes. NPY has been shown to have anti-depressant and anxiolytic effects (Heilig et al., 1989; Stogner and Holmes, 2000; Redrobe et al., 2002) and all so far tested anti-depressant treatments (e.g., electroconvulsive therapy, SSRIs and lithium) produce increases in NPY-LI immunoreactivity the frontal cortex and hippocampus of rats (Mathé et al., 1998; Stenfors et al., 1989; Stenfors et al., 1994; Mathé et al., 1997) and thus, the present data are consistent with the published literature and support the hypothesis of NPY being an endogenous modulator of emotionality (Mathé et al., 2007). In addition, the correlation of CRF-LI in the amygdala and immobility corresponds well with published reports of amygdalar CRF differences in genetically selected Flinders Sensitive Line rats used as a model of depression (Zambello et al., 2008), alterations in amygdalar CRF during withdrawal from alcohol in dependent rats (Rassnick et al., 1993; Zorrilla et al., 2001; Merlo et al., 1995) and escalated alcohol self-administration in dependent animals related to amygdalar CRF levels (Funk et al., 2006). Furthermore, the decreased levels of NPY and CRF-associated increases in immobility are consistent with a recognized trend that NPY levels are lower and CRF levels are enhanced in anxiety and depression and that following treatment, those profiles are reversed (Nikisch et al., 2005; Nikisch and Mathé, 2008).
In summary, intermittent ethanol vapor exposure produced depressive-like behaviors in male Wistar rats that persisted for at least 60 days into withdrawal. Furthermore, immobility time increased over the 60 day test period in a manner consistent with an increase in depressive-like symptoms that appeared to require previous ethanol exposure to be expressed. Lastly, NPY-LI was reduced in the frontal cortex of rats exhibiting depressive-like behavior in protracted withdrawal and CRF-LI in the AMYG was correlated with immobility time in those same rats. Therefore, this model should prove valuable for further characterization of chronic ethanol exposure-induced depressive-like symptoms from behavioral, neurochemical and neuropsychopharmacological perspectives, as well as, the development of therapies to treat certain aspects of alcoholism.
Acknowledgements
Support for this research was provided by a National Institute on Alcohol Abuse and Alcoholism grant awarded to CLE (AA006059 and AA014339) and by the Swedish Medical Research Council grant 10414 to AAM. We would like to thank Maury Cole for his technical assistance and Dr. David Gilder for his scholarly contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J. Stud. Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, et al. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol. Clin. Exp. Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder DA, Wall TL, Ehlers CL. Comorbidity of select anxiety and affective disorders with alcohol dependence in southwest California Indians. Alcohol. Clin. Exp. Res. 2004;28:1805–1813. doi: 10.1097/01.alc.0000148116.27875.b0. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF. Neuropeptide Y in the central nucleus of the amygdale suppresses dependence-induced increases in alcohol drinking. Pharmacol. Biochem. Behav. 2008;90:475–480. doi: 10.1016/j.pbb.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch. Gen. Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, et al. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 1994;11:179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol. Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathé AA. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacol. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Jimenez-Vasquez PA, Diaz-Cabiale Z, Caberlotto L, Bellido I, Overstreet D, Fuxe K, et al. Electroconvulsive stimuli selectively affect behavior and neuropeptide Y (NPY) and NPY Y(1) receptor gene expressions in hippocampus and hypothalamus of Flinders Sensitive Line rat model of depression. Eur. Neuropsychopharmacol. 2007;17:298–308. doi: 10.1016/j.euroneuro.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. Self-regulation and self-medication factors in alcoholism and the addictions. Similarities and differences. Recent Dev. Alcohol. 1990;8:255–271. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol. Clin. Exp. Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Madaan V, Wilson DR. Neuropeptides: relevance in treatment of depression and anxiety disorders. Drug News Perspect. 2009;22:319–324. doi: 10.1358/dnp.2009.22.6.1395255. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacol. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mathé AA, Gruber S, Jimenez PA, Theodorsson E, Stenfors C. Effects of electroconvulsive stimuli and MK-801 on neuropeptide Y, neurokinin A, and calcitonin gene-related peptide in rat brain. Neurochem. Res. 1997;22:629–636. doi: 10.1023/a:1022482322329. [DOI] [PubMed] [Google Scholar]
- Mathé AA, Husum H, El KA, Jimenez-Vasquez P, Gruber SH, Wortwein G, et al. Search for biological correlates of depression and mechanisms of action of antidepressant treatment modalities. Do neuropeptides play a role? Physiol. Behav. 2007;92:226–231. doi: 10.1016/j.physbeh.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Mathé AA, Jimenez PA, Theodorsson E, Stenfors C. Neuropeptide Y, neurokinin A and neurotensin in brain regions of Fawn Hooded "depressed", Wistar, and Sprague Dawley rats. Effects of electroconvulsive stimuli. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:529–546. doi: 10.1016/s0278-5846(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Mathé AA, Rudorfer MV, Stenfors C, Manji HK, Otter WC, Theodorsson E. Effects of electroconvulsive treatment on somatostatin, neuropeptide Y, endothelin and neurokinin A concentrations in cerebrospinal fluid of depressed patients. Depression. 1996;3:250–257. [Google Scholar]
- Merlo PE, Lorang M, Yeganeh M, Rodriguez dF, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikisch G, Agren H, Eap CB, Czernik A, Baumann P, Mathé AA. Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int. J. Neuropsychopharmacol. 2005;8:403–410. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathé AA. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur. Psychiatry. 2008;23:356–359. doi: 10.1016/j.eurpsy.2008.03.003. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O'Sullivan K. Depression and its treatment in alcoholics: a review. Can. J. Psychiatry. 1984;29:379–384. doi: 10.1177/070674378402900503. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur. J. Pharmacol. 2004;497:49–53. doi: 10.1016/j.ejphar.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol. Clin. Exp. Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and guide to microdissection of the rat brain. New York: Elsevier; 1988. [Google Scholar]
- Paschos KA, Veletza S, Chatzaki E. Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs. 2009;23:755–772. doi: 10.2165/11310830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotrophin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacol. 2002;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Quirion R. Neuropeptide Y (NPY) and depression: from animal studies to the human condition. Life Sci. 2002;71:2921–2937. doi: 10.1016/s0024-3205(02)02159-8. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J. Stud. Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol. Clin. Exp. Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacol. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav. Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Roy A, DeJong J, Lamparski D, George T, Linnoila M. Depression among alcoholics. Relationship to clinical and cerebrospinal fluid variables. Arch. Gen. Psychiatry. 1991;48:428–432. doi: 10.1001/archpsyc.1991.01810290040007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am. J. Psychiatry. 1997a;154:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Jr, Hesselbrock VM, Crowe RR, et al. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997b;92:1289–1304. [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann. N. Y. Acad. Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Mathé AA, Theodorsson E. Repeated electroconvulsive stimuli: changes in neuropeptide Y, neurotensin and tachykinin concentrations in time. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1994;18:201–209. doi: 10.1016/0278-5846(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Theodorsson E, Mathé AA. Effect of repeated electroconvulsive treatment on regional concentrations of tachykinins, neurotensin, vasoactive intestinal polypeptide, neuropeptide Y, and galanin in rat brain. J. Neurosci. Res. 1989;24:445–450. doi: 10.1002/jnr.490240315. [DOI] [PubMed] [Google Scholar]
- Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur. J. Pharmacol. 2000;387:R9–R10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav. Brain Res. 2005;161:133–140. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacol. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambello E, Jimenez-Vasquez PA, El KA, Mathé AA, Caberlotto L. Acute stress differentially affects corticotropin-releasing hormone mRNA expression in the central amygdala of the "depressed" flinders sensitive line and the control flinders resistant line rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:651–661. doi: 10.1016/j.pnpbp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]