Abstract
Objective
To investigate fetal brain development of growth-restricted fetuses by using Auditory Evoked Responses (AER) recorded using the non-invasive magnetoencephalographic technique.
Methods
Serial fetal recordings starting at 27 weeks of gestation were conducted on a fetal magnetoencephalographic device (fMEG) specially designed for obstetrical assessment. Fifteen normotrophic fetuses were compared to 14 hypotrophic fetuses. After birth, 10 of the hypotrophic fetuses were diagnosed with asymmetric growth restriction, four were classified as symmetrically small for gestational age.
Results
Fetal AER latencies in both groups showed an average developmental decrease of 12.74 ms/week (p=0.0035). Hypotrophs had longer age-adjusted latencies compared to normotrophs, with a difference of 73.5 ms (p=0.034). The subgroup of symmetrically growth-restricted fetuses showed the longest latencies for age, with a difference from the normotrophs of 120.0 ms (p=0.045).
Conclusions
The results indicate that biomagnetically recorded AER can be used to monitor functional brain development in growth-restricted fetuses.
Keywords: Fetal magnetoencephalography, Auditory evoked response, IUGR, Fetal brain development
Introduction
The maturation of the growing brain in utero is highly vulnerable to oxygen deprivation, which, in turn, can lead to ischaemic brain defects. The detection and surveillance of fetuses under risk of hypoxia during gestation is thus important to prevent neurological damage. Intrauterine growth restriction (IUGR) is associated with increased risk of ischaemic cerebral injury through intrauterine hypoxia.1-3 The term ”intrauterine growth restriction” often is used synonymously with small-for-gestational-age (SGA). IUGR connotes an intrauterine pathophysiologic process resulting in restricted growth, mostly due to insufficient placental transfer of nutrients and oxygen. This leads to a higher risk of peri- and neonatal morbidity and mortality and impaired neurological long-term development.4,5 However, SGA is a more general description of infants with weights below the tenth percentile for gestational age, estimates of which can be calculated during gestation from ultrasound measurements. There is a considerable overlap of the two terms, but their distinction is important – not all SGA findings reflect growth restriction. Some may simply reflect normal biological diversity, in which fetuses that grow less do so because of their genetic determination.
To help resolve this dilemma, the ponderal index (PI) 6, a measure of the height-to-mass relationship in the newborn, is used as an indicator of wasting during gestation and is associated with fetal morbidity and mortality. It is a more sensitive method to diagnose an asymmetric (a.k.a. “disproportionate” or “head-sparing”) growth pattern than the weight percentile alone. Symmetrically growth-restricted infants have a normal PI and are only detected by weight. However, the growth pattern is also influenced by etiology as well as the time of onset and duration of growth restriction. Moreover, the PI only becomes valid as an assessment tool in the last trimester during the period of greatest fetal weight gain. It is here the difference between symmetrical and asymmetrical growth becomes evident. Yet, fetal distress and developmental harm can occur earlier. Finally, the PI is assessed upon delivery, after any damage from IUGR has already occurred.
The detection of actual growth-restricted fetuses remains challenging. There is currently no satisfactory means to assign the diagnosis of IUGR while it is ongoing or to monitor fetal neurological development directly and non-invasively under the condition of growth restriction. Reliable assessment tools are needed to detect fetal well-being and neurological development, before damage occurs, to weigh the risks of fetal distress in utero versus prematurity, and determine the best timing of delivery. Biophysical measurements, such as ultrasound biometry, doppler velocimetry, and fetal heart tones, are currently conducted to monitor and assess fetuses suspected of having IUGR.7,8 But these measures lack the direct insight into the neurofunctional development of the fetus.
The magnetoencephalography (MEG) technique uses superconducting quantum interference devices (SQUID) to non-invasively records magnetic signals corresponding to electrical currents in neuronal tissue. In contrast to electric currents, magnetic signals are not distorted by the different layers of biological tissue. Because MEG has the capacity to directly record non-distorted magnetic signals in a non-invasive manner, it is uniquely suited to the study of the magnetic fields generated in the human fetal brain in utero 9,10 The first MEG system specially designed for fetal studies called SARA (SQUID Array for Reproductive Assessment) has been operational since 2000 at UAMS. 11 Using the MEG technique it has been shown that evoked brain responses to sound or light can be recorded from fetuses at least as early as 28 weeks. 12-16 Further, studies on low-risk pregnancies have shown that response latencies decreased with increasing gestational age (GA) thus indicating that fMEG may provide an insight into fetal brain development.16-18
The purpose of this study was to investigate whether impaired fetal growth affected the development of auditory evoked responses (AER) and if fMEG could be a helpful tool to assess functional fetal brain development.
Methods
Subjects
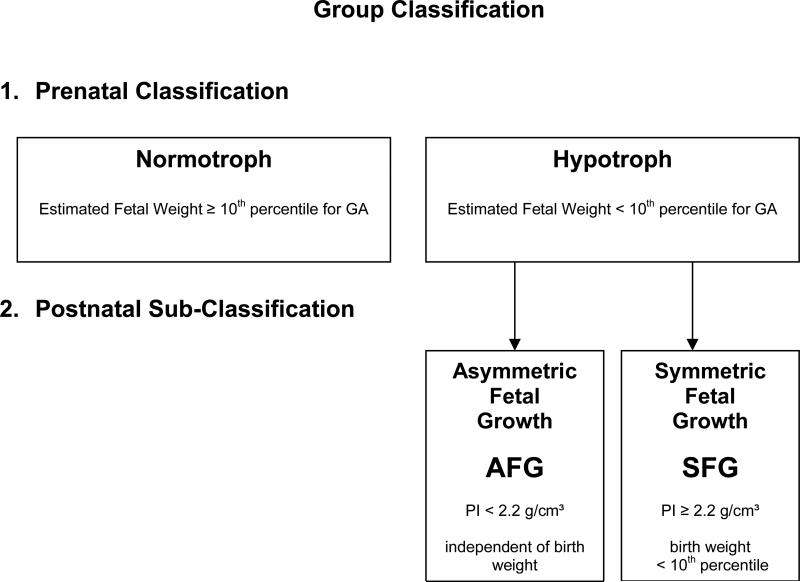
This study was based on 73 recordings on 29 pregnant mothers carrying singleton fetuses starting at 27 weeks of gestation, recruited from the obstetrical clinic of the University of Arkansas for Medical Sciences. Chromosomal abnormalities, fetal infections, and stillbirths were excluded. Out of the 29 mothers, 15 had uncomplicated pregnancies of adequately growing fetuses and were recruited as control subjects. The other 14 pregnant women had fetuses that were suspicious for hypotrophy, defined as having estimated weights below the 10th percentile for gestational age (GA) by ultrasound measurement according to the method of Hadlock.19 The prenatally classified group of hypotrophic fetuses was sub-classified after birth depending on their growth pattern by ponderal index (PI), defined as the birthweight in grams divided by the cube of length in centimeters. An index below 2.2 g/cm3 indicated asymmetric fetal growth (AGF). An index above 2.2 g/cm3 but with a neonatal weight below the tenth percentile for GA classified the infant as symmetric fetal growth (SFG). Figure 1 shows the group classifications.
Figure 1. Group Classification of the fetuses.
Prenatal group classification into normotrophic and hypotrophic by sonographic weight estimate for gestational age. Postnatal subclassification of the hypotrophic fetuses into symmetric and asymmetric growth pattern by ponderal index.
The resistance index (RI) of the umbilical artery conducted at UAMS and the neonatal outcome (weight, pH of the umbilical artery, APGAR score after 1 and 5 minutes after birth) were collected.
The mothers were asked to participate for developmental follow-up recordings. The study was approved by the Institutional Review Board of the hospital. A consent from the mother or their legal representative was received prior to the study.
Preparation
Ultrasound examination (GE Voluson 730) was performed prior to the fMEG recording to examine the general fetal position, head localization, and biometric measurements to estimate the fetal weight. Afterwards, the fMEG study was performed in a magnetic shielded room (Vacuumschmelze Hanau, Germany) using the SARA device. The sensor array consists of 151 SQUIDs specifically designed to noninvasively monitor the maternal-fetal physiology by recording abdominally generated biomagnetic fields. The mothers were asked to sit comfortably, and lean forward against the smooth surface of the concave sensor array. Once the mother was seated, the fetal head localization was confirmed with a portable ultrasound device (GE Logio a 100 MP). Four coils fixed on elastic belts were positioned around the maternal abdomen to indicate the fetal head location with respect to the sensor array and to detect maternal movement during the recording. After the study, the fetal head position was checked again.
Stimulation and Recording
The fetuses were acoustically stimulated with an external speaker transmitting repeated tone bursts of 120 dB via a plastic tube to a distally attached soft balloon that was placed over the maternal abdomen. The stimulation was presented in 500-ms-long tone bursts of 500 Hz frequent stimuli (80%) and 700 Hz rare stimuli (20%) in a random sequence to reduce possible habituation. The interstimulus interval was 2 s +/- 0.5 s. The recording sessions ranged from six to eight minutes. Further details are described in the publication of Holst et al.16
Signal analysis
The recordings were filtered with a high-pass filter of 0.5 Hz. After the removal of maternal and fetal heart signals using orthogonal projection 20,21, the data was divided into single trials depending on the trigger generated at the onset of the auditory stimulus. The single trials included 800 ms pre-stimulus and 800 ms post-stimulus intervals. Automatic threshold detection was applied to remove artifacts (MEG amplitude single channel threshold 2 pT). The resulting single trials were averaged, and a low-pass filter of 10 Hz was applied. The signals were visually analyzed to detect prominent peaks with latencies between 70 to 700 ms clearly deviating from the prestimulus baseline in either a positive or negative direction. To classify a peak as AER, the amplitude had to be greater than 10 femtoTesla (fT) in at least six channels of the sensor array. Orthogonal projection procedure can lead to a redistribution of the brain signal away from the head 20; therefore, channel selection was not mainly dependent on localization to the fetal head position. However, at least one of the selected channels had to be close to the area of the head coil to classify the detected peak as a fetal auditory evoked response. We determined the latency of the first fetal evoked brain response and included information when further response peaks were visible.
For further comparison of the AER latencies, they were divided into three groups according to a component classification of EEG studies on term- and preterm-born babies 22,23 :
Primary complex (Na - N1): < 150 ms
Early secondary complex (P2 - N2): 150 – 300 ms
Late secondary complex (> P3): > 300 ms
Statistical analysis
The prenatal classified study groups consisted of normotrophic and hypotrophic fetuses. After birth, the hypotrophic subjects were subdivided depending on their ponderal index and weight.
The relationship between latency and GA was analyzed using a mixed-models ANCOVA framework 24, in which the prenatal group was the class variable, GA was the continuous covariate, and Subject was the random effect for modeling the clustering among prenatal repeated measures. Different regression models were fit to the data using a mixed-models framework to adjust for the clustering among multiple measures over time from the same subject. The simplest ANCOVA model examined fit the fetal classifications with two linear regression lines having different intercepts but a common slope. More complex ANCOVA models included (1) a quadratic regression term to model a leveling off of the latency trend at later GA, and (2) a GA-by-group interaction to model differential maturation rates between groups. These more complex terms were retained in the final ANCOVA model only if statistically significant. The final ANCOVA model was used to characterize differences between the two fetal groups and also re-fit to the data divided according to birth outcome. Group differences of the detection rate were tested initially by Fisher's Exact Test and followed-up with logistic regression to adjust for GA and the dependency among repeated measures. Differences concerning the number of peaks detected were analyzed through the Cochran-Armitage Trend Test, and the Mantel-Haenszel Test for Linear Association was used to test for latency shifts between AER component ranges. The Wilcoxon Rank-Sum (WRS) test was used to examine amplitude differences between fetal groups. Effects with p< 0.05 were accepted as statistically significant despite multiple comparisons to not inflate the Type II error rate in this small-scale observational study.
Results
The study was based on 73 fetal MEG recordings on 29 pregnant women. Eighteen of the 29 mothers came more than once (up to six times) for fMEG measurements. Four measurements were excluded due to technical issues. Fifteen fetuses showing normal growth were compared to 14 fetuses showing hypotrophy during gestation. Ten of the latter were retrospectively confirmed as asymmetrically growth restricted, while the other four were classified as symmetrically small for gestational age. All symmetrically small infants had abnormal Doppler findings and were delivered preterm via C-section. Out of the asymmetrically grown fetuses, two (20%) had normal Doppler measurements, and three (30%) were delivered at term. Six were born via C-section, forceps were necessary for one, and three were spontaneous vaginal deliveries. Clinical data of the two subdivided groups of hypotrophic infants are shown in Table Ia (Doppler Findings) and Ib (Neonatal Outcome). APGAR scores and cord artery pH had similar medians, although the AFG group had a broader range for both. The median birth weight was 160 g lower in the SFG group.
Table Ia.
Doppler of Hypotrophic Fetuses subdivided according Growth Symmetry (determined postnatal by PI)
| Asymmetric Fetal Growth | Symmetric Fetal Growth | ||
|---|---|---|---|
| RI | Index | RI | Index |
| 0.5455 | 1 | 0.7283 | 2 |
| 0.7222 | 2 | 1 | 4 |
| 1 | 3 | 1 | 3 |
| 0.7297 | 2 | 0.7561 | 2 |
| 0.8158 | 2 | ||
| 0.6970 | 2 | ||
| 0.8780 | 2 | ||
| 1 | 4 | ||
| 0.8611 | 2 | ||
| 0.6255 | 1 | ||
Index: 1=normal, 2=elevated for GA, 3=no diastolic flow, 4=reverse flow
Table Ib.
Neonatal Outcome of the two groups of Hypotrophic Fetuses
| Asymmetric Fetal Growth | Symmetric Fetal Growth | |||
|---|---|---|---|---|
| median | range | median | range | |
| GA at birth (wk) | 34 | 27 - 39 | 32 | 31 - 36 |
| Weight (g) | 1427 | 549-2620 | 1267 | 886-1980 |
| pHumbilical artery | 7.3 | 7.2 - 7.36 | 7.28 | 7.2 - 7.36 |
| APGAR (1') | 7 | 2-9 | 8 | 6-8 |
| APGAR (5') | 8 | 5-9 | 9 | 8-9 |
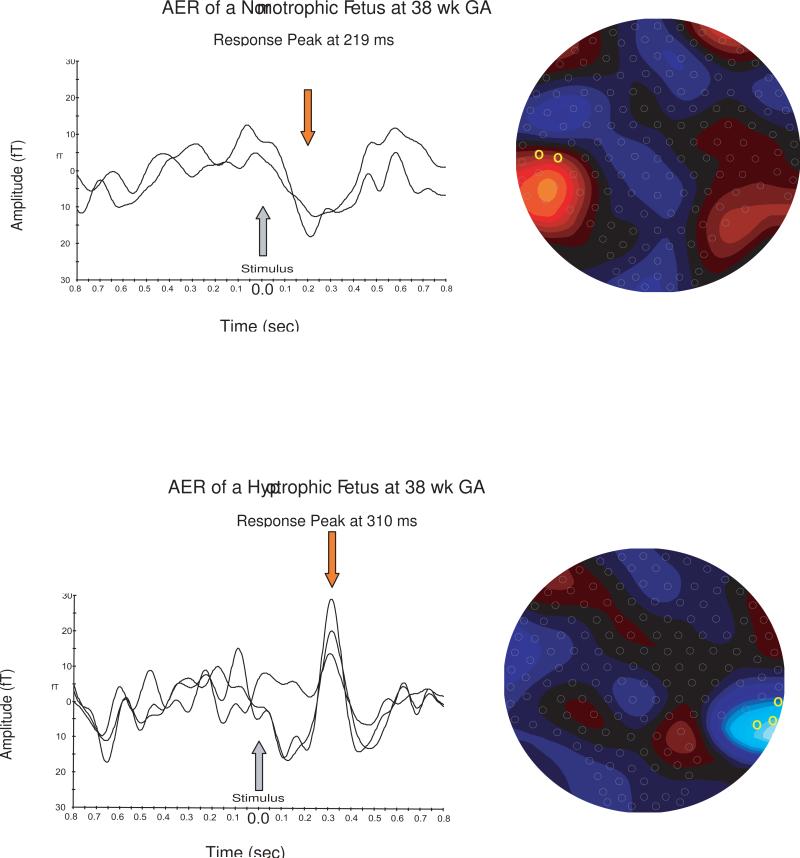
Figure 2 shows representative AER of a normotrophic and a hypotrophic fetus fetus at 38 weeks GA.
Figure 2. AER of a normotrophic and a hypotrophic fetus at 38 week of gestation.
The graphs on the left side show the channels with the clearest from the baseline deviating response peaks for each fetus. The location of the chosen channels and the dipole of the evoked magnetic field in the sensor array are plotted on the right side.
AER detection rates for normotrophic versus hypotrophic fetuses were 40 (85%) of 47 versus 16 (73%) of 22, respectively (Fisher exact p = 0.32); logistic regression revealed no significant effect in either group (p=0.22) or GA (p=0.16) on detection rate. For number of peaks detected, normotrophs had a slightly higher average (1.38) compared to hypotrophs (1.05), but the difference was not statistically significant (Cochran-Armitage p=0.14). The amplitudes ranged from 10.8 – 96.4 fT. The hypotrophic fetuses tended to have smaller amplitudes than normotrophic fetuses, with medians (quartiles) of 19.6 (14.6 – 21.7) fT for hypotrophs versus 22.2 (17 – 43.1) fT for normotrophs (WRS p = 0.074). Table II describes the AER latencies measured between the onset of the stimulus to the earliest evoked responses.
Table 2.
Auditory Evoked Responses: Latencies, GA at Detection and Group Differences
| Prenatal Group Definition (based on estimated Fetal Weight) | |||||
|---|---|---|---|---|---|
| GA* | Mean† (SD) | 1. –3. Quartile | Range | Group Difference to Normotrophs | |
| Normotroph | 33w (27w-39w) |
293.2 (19) ms | 233.5-379.5 ms | 74-474ms | - |
| Hypotroph | 33w (27w-38w) |
366.7 (27) ms | 248-475 ms) | 186-560 ms | 73.48 ms (s. p=0.034) |
| Postnatal GroupDefinition: “Hypotroph” subdivided based on Growth Symmetry | |||||
|---|---|---|---|---|---|
| GA* | Mean† (SD) | 1.–3. Quartile | Range | Group Difference to controls | |
| Normotroph | 33w (27w-39w) |
293.2 (19) ms | 233.5-379.5 ms | 74-474ms | - |
| Asymmetric Growth | 37w (27w-38w) |
349.4 (32) ms | 238.5-475 ms | 186-560 ms | 57.6 ms (n.s.) |
| Symmetric Growth | 32w (31w-33w) |
413 (54) ms | 371-482 ms | 355-506 ms | 120 ms (s. p=0.045) |
GA, Gestational Age; w, week
GA of the subject at the AER recording, median and range
Latency estimate after adjusting for mean GA (33w)
The linear ANCOVA model fit the fetal classifications with two linear regression lines with different intercepts but a common slope. This model was augmented with (a) a quadratic term to model leveling off of trend at late GA, or (b) a GA-by-fetal-group interaction to model differential maturation rates between groups. The augmented ANCOVA models yielded insignificant evidence for a quadratic component (F=1.32, DFs=(1,28); p=0.26) and insignificant evidence for a GA-by-fetal-group interaction (F=0.51, DFs=(1,28); p=0.48). The final analysis model was thus the linear ANCOVA model. AER latencies showed a mean decrease (95% confidence interval [CI]) of 12.74 (4.55 – 20.93) ms per week (p=0.0035) under this final model, while the latencies in hypotrophic fetuses were estimated to be significantly longer at all GA by 73.5 ms compared to the control group (95% CI = 6.0-to-141.0 ms; p=0.034). Re-fitting the linear ANCOVA model to the data after reclassification by birth outcome yielded a mean (95% CI) decrease in latencies of 12.39 (4.13 – 20.65) ms/week (p=0.0047). Relative to normal-weight neonates, AFG showed a latency delay (95% CI) of 57.6 (–18.0-to-+133.2) ms (p=0.13), while SFG showed a latency delay (95% CI) of 120.0 (2.8 – 237.2) ms (p=0.045).
The latencies were also grouped in latency ranges according to AER component classifications of EEG studies. 22,23 We noted that the latencies of hypotrophic fetuses tended to be more in the range of the late secondary complex (69%) and less in the early secondary complex (31%). Latencies of the normotrophic fetuses were equal distributed in the range of the early and the late second complex (45%); 10% were in the primary complex range. The shift towards late secondary complex in hypotrophs compared to normotrophs was suggestive, but not statistically significant (Mantel-Haenszel p=0.07).
Discussion
Fetal MEG is a new and non-invasive tool to determine prenatal brain function and development. This study investigated auditory evoked responses in growth-restricted fetuses compared to fetuses of adequate growth. AER latencies of the controls were consistent with existing literature. Latencies of the hypotrophic fetuses were longer compared to longitudinal studies on low-risk pregnancies. 16-18 Detection rates of 70 to 75% were similar to those of studies done on the same device.12,16 A high detection rate is an important premise for its use as a reliable monitoring tool.
To our knowledge, this is the first longitudinal study exploring maturational changes of auditory cortex activity in IUGR fetuses. Previous developmental AER studies on low-risk pregnancies showed decreasing AER latencies during gestation, indicating maturational changes. 16-18.
Gross et al.25 investigated AER changes in growth-restricted fetuses after 36 weeks and registered significantly longer latencies in IUGR. In their cross-sectional investigation, they could not make any statements about development changes during this short-period late in gestation. In our study, the hypotrophic fetuses had longer latencies compared to the control group of normally growing fetuses. Furthermore, we found evidence for linearly decreasing latencies during gestation in both groups.
Besides response latency, we wanted to know if other AER characteristics could provide further insight into brain maturation. Schleussner et al. 26 found additional components after 31 weeks in normal fetuses and attributed this to auditory cortical maturation. Most of all latencies were found in the range of 145 – 305 ms and were called P2pm and N2pm components, according to a components classification known from EEG studies on term- and preterm- born babies.
In our study the AER latencies of normotrophic fetuses were in the same range. The latencies in hypotrophic fetuses were longer, and the evidence for a shift to another component class was suggestive (p=0.07) but not statistically significant. In contrast to Schleussner we could detect more than one peak already in early ages beginning at 28w (normotrophic) or 29w (hypotrophic). Although multiple peaks were more frequent in normal versus growth-inhibited fetuses, the difference between groups did not rise to the level of statistical significance.
AER latencies proved to be useful markers to track fetal neurodevelopmental changes between normal and growth restricted fetuses. Latencies shortened during gestation and longer latencies in growth-restricted fetuses were found. The variation between individuals showed that the interpretation of fetal brain function can not be based on single measurements so far. More work needs to be done to investigate the range of AER latency values for GA in healthy and compromised fetuses, and to clarify further influences on AER such as fetal behavioral state during the fMEG recording.
AER-amplitudes were comparable to findings of other studies. The signal strength is largely affected by the distance between the fetal head and the magnetic sensors. Because this distance differs between fetuses and there is no established method to correct for the distance effect the signal strength itself it is of little value for statistical analysis.
The diagnosis of IUGR is crucial. We used fetal hypotrophy as inclusion criteria and subdivided this group of infants after birth depending on their growth pattern by ponderal index to detect fetal emaciation. The Index becomes evident in the last trimester when the normal fetus gains most of its weight. In contrast the growth-restricted fetus gains more length over mass.
In result, we found that symmetrically growth-retarded fetuses had the longest latencies after adjusting for gestational age. The latency delay for SFG alone was statistically significant compared to normals, as was the latency delay for SFG pooled with AFG. The latency delay for AFG alone was not statistically significant.
All SFG fetuses showed abnormal placental blood flow as diagnosed by Doppler velocimetry, which eventually precipitated the medical intervention of early delivery. Knowing this clinical information of the SGA group, we cannot assume that the ponderal index distinguished SFG from AFG because of smallness by genetic determination compared to the ones who experienced growth restriction. Both groups obviously represented IUGR but with probably different onset and severity of growth restriction.
The fetal growth pattern is influenced by the etiology, time of onset, and duration of growth restriction.27 If fetal growth is impaired during the first or second trimester, it leads to symmetric growth restriction, as this is the period of cellular proliferation contributing similarly to length and mass gain – the PI would not be affected. The more common form of asymmetric growth restriction occurs if growth restriction happens in the last trimester, of time at which predominant mass gain occurs.
The latencies of the SFG group were the longest. The group size does not allow general statements but gives some interesting information for further work in this field: It is known that the vulnerability of neurons depends on the progress of myelinization, and the susceptibility to impairment varies during gestation.3 The SGA-group had obviously an early onset of severe placental insufficiency. It is possible that the AER-development of fetuses at early gestational age could be more affected than in fetuses later in gestation.
Our observational study showed that AER latencies were reliably detectable in growth-restricted fetuses and were longer compared to normally growing fetuses. This finding encourages further investigation in this field. The advantages of fMEG to assess fetal brain development directly and non-invasively from the 27th week of GA onwards, could improve the diagnosis of IUGR fetuses and the obstetrical management and neonatal outcome of fetuses at risk of hypoxia. In the future broader application in clinical research needs to clarify pathology of abnormal brain development in the fetal period and in a long-term view.
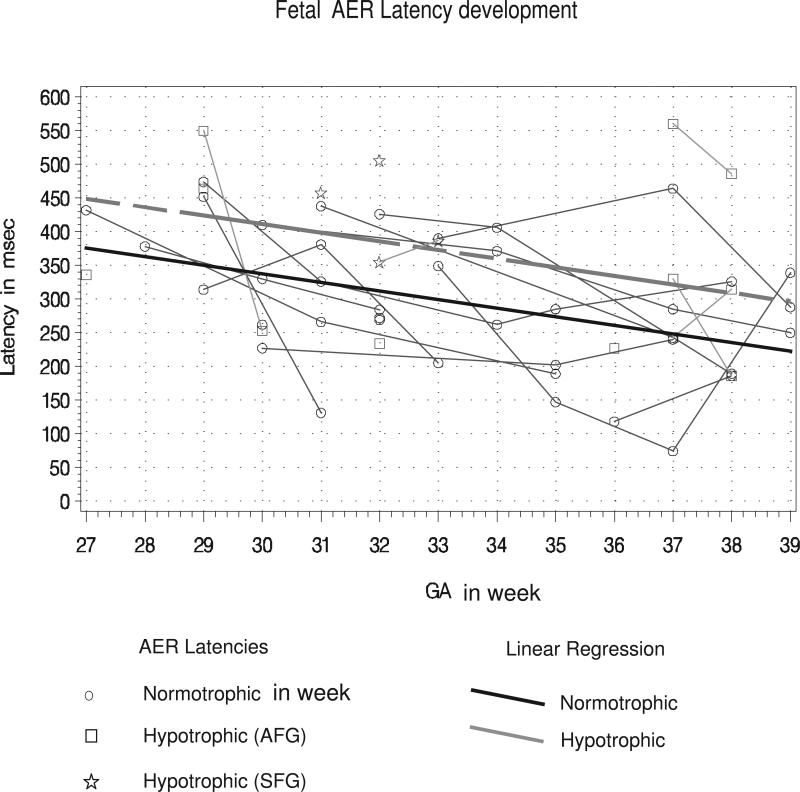
Figure 3. Fetal AER Latency Development over gestation.
Graph of the linear ANCOVA mixed model, along with the individual latency measurements over time in the normotrophic and hypotrophic fetuses. The regressions are presented for each group. Latencies measured at different times on the same fetus are connected by thin line segments represent latencies. The maturational latency decrease was 12.74 ms per week (p=0.0035). Latencies in hypotrophic fetuses were estimated to be 73.48 ms longer (p= 0.034) at all GA compared to the control group.
Acknowledgements
We thank Pam Murphy , R.N. at the SARA Research Center at UAMS, for her great help to conduct the MEG recordings and Harald Abele, MD, from the Department of Gynaecology and Obstetrics at the University of Tübingen for precious obstetrical counseling.
Funded by NINDS/ National Institute of Health Grant R01NS36277 and by the Deutsche Forschungsgemeinschaft (DFG) GZ: KI 1306/1-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation:
A longitudinal MEG-study showed Auditory Evoked Response (AER) latencies decreased during development but were longer in IUGR fetuses compared to controls.
References
- 1.Burke CJ, Tannenberg AE, Payton DJ. Ischaemic cerebral injury, intrauterine growth retardation, and placental infarction. Dev Med Child Neurol. 1997 Nov;39(11):726–30. doi: 10.1111/j.1469-8749.1997.tb07373.x. [DOI] [PubMed] [Google Scholar]
- 2.Gaffney G, Squier MV, Johnson A, Flavell V, Sellers S. Clinical associations of prenatal ischaemic white matter injury. Arch Dis Child Fetal Neonatal Ed. 1994 Mar;70(2):F101–6. doi: 10.1136/fn.70.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002 Jan 15;22(2):455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Wassenaer A. Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005 Mar;2(3):372–7. [PubMed] [Google Scholar]
- 5.McCarton CM, Wallace IF, Divon M, Vaughan HG., Jr Cognitive and neurologic development of the premature, small for gestational age infant through age 6: comparison by birth weight and gestational age. Pediatrics. 1996 Dec;98(6 Pt 1):1167–78. [PubMed] [Google Scholar]
- 6.Prasad SR, Mohan M, Kumar A, Kapani V. Ponderal index as a marker of intrauterine growth. Indian J Med Res. 1989 Dec;90:442–7. [PubMed] [Google Scholar]
- 7.Yoshida S, Unno N, Kagawa H, Shinozuka N, Kozuma S, Taketani Y. Prenatal detection of a high-risk group for intrauterine growth restriction based on sonographic fetal biometry. Int J Gynaecol Obstet. 2000 Mar;68(3):225–32. doi: 10.1016/s0020-7292(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 8.Baschat AA, Galan HL, Bhide A, Berg C, Kush ML, Oepkes D, Thilaganathan B, Gembruch U, Harman CR. Doppler and biophysical assessment in growth restricted fetuses: distribution of test results. Ultrasound Obstet Gynecol. 2006 Jan;27(1):41–7. doi: 10.1002/uog.2657. [DOI] [PubMed] [Google Scholar]
- 9.Blum T, Saling E, Bauer R. First magnetoencephalographic recording of the brain activity of the human fetus. Br J.Obstet.Gynaecol. 1985;92:1224–1229. doi: 10.1111/j.1471-0528.1985.tb04866.x. [DOI] [PubMed] [Google Scholar]
- 10.Wakai RT, Leuthold C, Martin CB. Fetal auditory evoked responses detected by magnetoencephalography. Am.J.Obstet.Gynaecol. 1996;174:1484–1486. doi: 10.1016/s0002-9378(96)70592-6. [DOI] [PubMed] [Google Scholar]
- 11.Eswaran H, Lowery CL, Robinson SE, Wilson JD, Cheyne D, Mc Kenzie D. Challenges of recording human fetal auditory-evoked response using magnetoencephalography. J Matern Fetal Med. 2000;9:303–307. doi: 10.1002/1520-6661(200009/10)9:5<303::AID-MFM10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Eswaran H, Preissl H, Wilson JD, Murphy P, Robinson SE, Rose D, Vrba J, Lowery CL. Short-Term Serial Magnetoencephalographic Recordings of Fetal auditory evoked responses. Neurosci Lett. 2002 Oct 11;331(2):128–32. doi: 10.1016/s0304-3940(02)00859-5. [DOI] [PubMed] [Google Scholar]
- 13.Draganova R, Eswaran H, Murphy P, Lowery C, Preissl H. Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Hum Dev. 2007 Mar;83(3):199–207. doi: 10.1016/j.earlhumdev.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Lowery CL, Eswaran H, Murphy P, Preissl H. Fetal magnetoencephalography. Semin Fetal Neonatal Med. 2006 Dec;11(6):430–6. doi: 10.1016/j.siny.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Preissl H, Lowery CL, Eswaran H. Fetal magnetoencephalography: viewing the developing brain in utero. Int Rev Neurobiol. 2005;68:1–23. doi: 10.1016/S0074-7742(05)68001-4. [DOI] [PubMed] [Google Scholar]
- 16.Holst M, Eswaran H, Lowery CL, Murphy P, Norton JD, Preissl H. Development of auditory evoked fields in human fetuses and newborns: A longitudinal MEG study. Clin Neurophysiol. 2005 Aug;116(8):1949–55. doi: 10.1016/j.clinph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Schleussner E, Schneider U. Developmental changes of auditory-evoked fields in fetuses. Exp Neurol. 2004 Nov;190(Suppl 1):S59–64. doi: 10.1016/j.expneurol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Lengle JM, Chen M, Wakai RT. Improved neuromagnetic detection of fetal and neonatal auditory evoked responses. Clin Neurophysiol. 2001 May;112(5):785–92. doi: 10.1016/s1388-2457(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 19.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991 Oct;181(1):129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 20.Vrba J, Robinson SE, McCubbin J, Lowery CL, Eswaran H, Wilson JD, Murphy P, Preissl H. Fetal MEG redistribution by projection operators. IEEE Trans Biomed Eng. 2004 Jul;51(7):1207–18. doi: 10.1109/TBME.2004.827265. [DOI] [PubMed] [Google Scholar]
- 21.McCubbin J, Robinson SE, Cropp R, Moiseev A, Vrba J, Murphy P, Preissl H, Eswaran H. Optimal reduction of MCG in fetal MEG recordings. IEEE Trans Biomed Eng. 2006 Aug;53(8):1720–4. doi: 10.1109/TBME.2006.876619. [DOI] [PubMed] [Google Scholar]
- 22.Rotteveel JJ, de Graaf R, Stegeman DF, Colon EJ, Visco YM. The maturation of the central auditory conduction in preterm infants until three months post term. V. The auditory cortical response (ACR). Hear Res. 1987;27(1):95–110. doi: 10.1016/0378-5955(87)90029-3. [DOI] [PubMed] [Google Scholar]
- 23.Pasman JW, Rotteveel JJ, de Graaf R, Maassen B, Notermans SL. Detectability of auditory evoked response components in preterm infants. Early Hum Dev. 1991 Aug-Sep;26(2):129–41. doi: 10.1016/0378-3782(91)90017-w. [DOI] [PubMed] [Google Scholar]
- 24.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Publishing; Cary, NC: 1996. ISBN-10 number - 1555447791. [Google Scholar]
- 25.Gross W, Kahler C, Koch K, Nowak H, Michels M, Seewald HJ. Acoustically evoked brain magnetic activity in normal and growth retarded fetuses during the third trimester of pregnancy] Z Geburtshilfe Neonatol. 1999 Mar-Apr;203(2):69–72. [PubMed] [Google Scholar]
- 26.Schleussner E, Schneider U, Kausch S, Kahler C, Haueisen J, Seewald HJ. Fetal magnetoencephalography: a non-invasive method for the assessment of fetal neuronal maturation. BJOG. 2001 Dec;108(12):1291–4. doi: 10.1111/j.1471-0528.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 27.Hankins G, editor. ACOG Task Force on Neonatal encephalopathy and cerebral palsy. Washington DC: 2003. Intrauterine Growth Restriction and Neonatal Encephalopathy. pp. 43–48. [Google Scholar]