Abstract
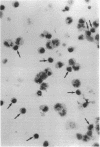
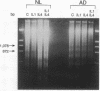
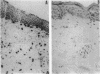
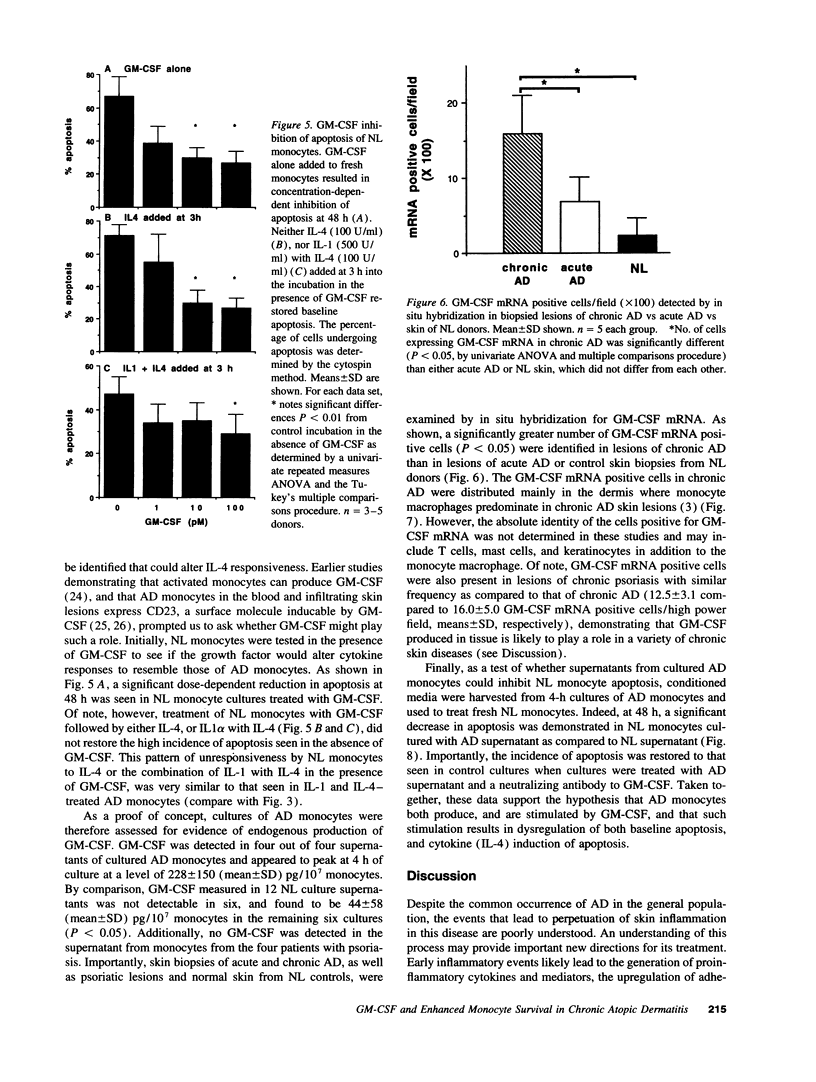
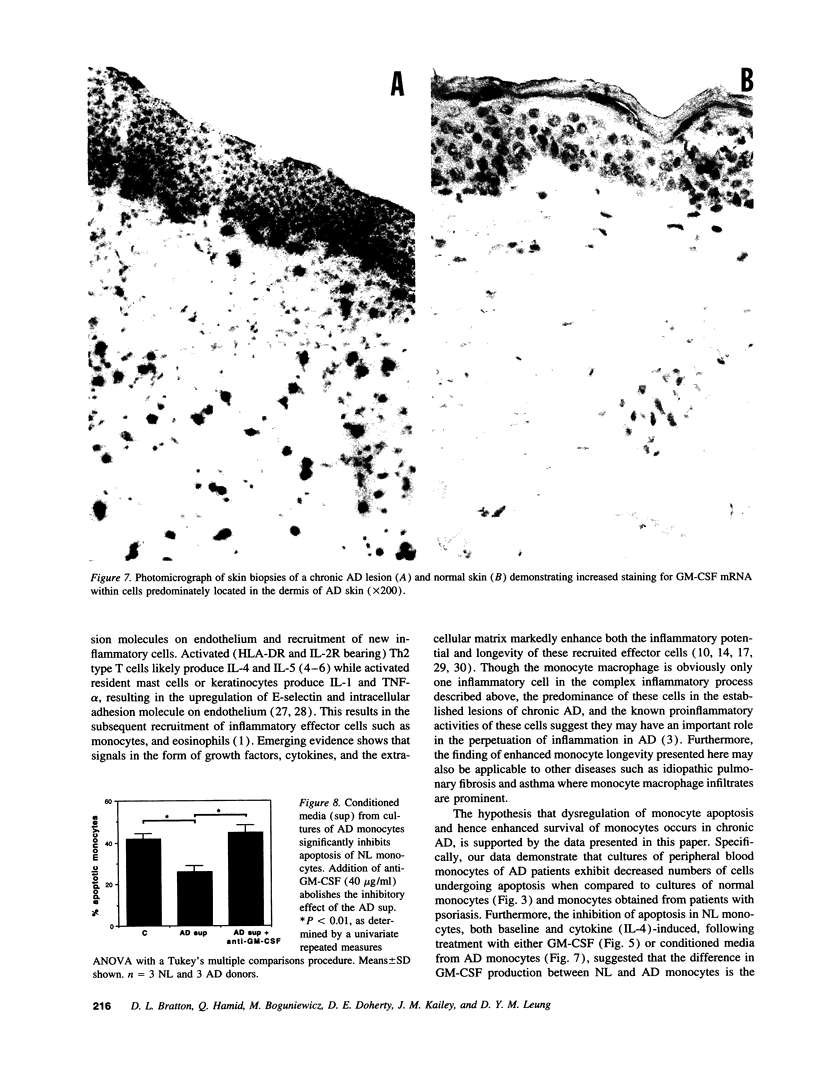
Evidence suggesting that prolonged effector cell survival may contribute to perpetuation of inflammation prompted us to ask whether monocyte macrophages, the predominate inflammatory cell in the lesion of chronic atopic dermatitis (AD), exhibit enhanced survival in AD. Cultures of peripheral blood monocytes from patients with chronic AD, psoriasis, and from normal (NL) donors were examined for morphologic features and DNA fragmentation characteristic of cells undergoing the process of apoptosis (programmed cell death). Cultures of AD monocytes exhibited a significantly lower incidence of apoptosis than did cultures of NL monocytes (45 vs 68%, P < 0.01), or psoriatic monocytes (45 vs 80%, P < 0.01). Furthermore, AD monocytes were unresponsive to both IL-1, an inhibitor of apoptosis, and IL-4, an enhancer of apoptosis, in comparison to cultured NL monocytes. Of note, GM-CSF in a concentration-dependent fashion, decreased the incidence of apoptosis in NL monocyte cultures and rendered them unresponsive to these cytokines. These findings suggested that GM-CSF may enhance monocyte survival in AD. In support of this hypothesis, AD monocyte cultures produced fivefold more GM-CSF than did cultures of NL monocytes or psoriatic monocytes (P < 0.05). Additionally, there was a significantly greater number of GM-CSF mRNA expressing cells detected by in situ hybridization in biopsies of lesions of chronic AD than in acute AD or NL skin (P < 0.05). Finally, NL monocytes incubated with supernatants obtained from monocytes of AD patients exhibited significant inhibition of apoptosis, an effect that could be ablated by a neutralizing antibody to GM-CSF. Taken together, these data strongly suggest that increased production of GM-CSF by cells from patients with AD inhibits monocyte apoptosis and may contribute to the chronicity of this inflammatory disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., Moqbel R., Walsh G. M., Kay A. B., Wardlaw A. J. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993 Mar 1;177(3):839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel P. L., Kuijper P. H., Rihs S., Betz S., Warringa R. A., Koenderman L. Eosinophil migration in atopic dermatitis. I: Increased migratory responses to N-formyl-methionyl-leucyl-phenylalanine, neutrophil-activating factor, platelet-activating factor, and platelet factor 4. J Invest Dermatol. 1993 Feb;100(2):137–142. doi: 10.1111/1523-1747.ep12462781. [DOI] [PubMed] [Google Scholar]
- Burke L. A., Hallsworth M. P., Litchfield T. M., Davidson R., Lee T. H. Identification of the major activity derived from cultured human peripheral blood mononuclear cells, which enhances eosinophil viability, as granulocyte macrophage colony-stimulating factor (GM-CSF). J Allergy Clin Immunol. 1991 Aug;88(2):226–235. doi: 10.1016/0091-6749(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J. G., Dower S. K., Sims J. E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993 Jul 23;261(5120):472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992 Oct 15;80(8):2012–2020. [PubMed] [Google Scholar]
- Doherty D. E., Haslett C., Tonnesen M. G., Henson P. M. Human monocyte adherence: a primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol. 1987 Mar 15;138(6):1762–1771. [PubMed] [Google Scholar]
- Groopman J. E., Molina J. M., Scadden D. T. Hematopoietic growth factors. Biology and clinical applications. N Engl J Med. 1989 Nov 23;321(21):1449–1459. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q., Wharton J., Terenghi G., Hassall C. J., Aimi J., Taylor K. M., Nakazato H., Dixon J. E., Burnstock G., Polak J. M. Localization of atrial natriuretic peptide mRNA and immunoreactivity in the rat heart and human atrial appendage. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6760–6764. doi: 10.1073/pnas.84.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci (Lond) 1992 Dec;83(6):639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- Howell C. J., Pujol J. L., Crea A. E., Davidson R., Gearing A. J., Godard P., Lee T. H. Identification of an alveolar macrophage-derived activity in bronchial asthma that enhances leukotriene C4 generation by human eosinophils stimulated by ionophore A23187 as a granulocyte-macrophage colony-stimulating factor. Am Rev Respir Dis. 1989 Nov;140(5):1340–1347. doi: 10.1164/ajrccm/140.5.1340. [DOI] [PubMed] [Google Scholar]
- Jujo K., Renz H., Abe J., Gelfand E. W., Leung D. Y. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):323–331. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Leiferman K. M., Ackerman S. J., Sampson H. A., Haugen H. S., Venencie P. Y., Gleich G. J. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985 Aug 1;313(5):282–285. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Bhan A. K., Schneeberger E. E., Geha R. S. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol. 1983 Jan;71(1 Pt 1):47–56. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- Leung D. Y. Immunopathology of atopic dermatitis. Springer Semin Immunopathol. 1992;13(3-4):427–440. doi: 10.1007/BF00200539. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Pober J. S., Cotran R. S. Expression of endothelial-leukocyte adhesion molecule-1 in elicited late phase allergic reactions. J Clin Invest. 1991 May;87(5):1805–1809. doi: 10.1172/JCI115201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. Y., Schneeberger E. E., Siraganian R. P., Geha R. S., Bhan A. K. The presence of IgE on macrophages and dendritic cells infiltrating into the skin lesion of atopic dermatitis. Clin Immunol Immunopathol. 1987 Mar;42(3):328–337. doi: 10.1016/0090-1229(87)90021-3. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Robertson B., Wahl S. M. IL-4 enhances programmed cell death (apoptosis) in stimulated human monocytes. J Immunol. 1992 Mar 15;148(6):1812–1816. [PubMed] [Google Scholar]
- Mangan D. F., Wahl S. M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991 Nov 15;147(10):3408–3412. [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Mihm M. C., Jr, Soter N. A., Dvorak H. F., Austen K. F. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol. 1976 Sep;67(3):305–312. doi: 10.1111/1523-1747.ep12514346. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Ezekowitz R. A., Leung D. Y. Monocytes from patients with atopic dermatitis are primed for superoxide production. J Allergy Clin Immunol. 1992 Feb;89(2):545–551. doi: 10.1016/0091-6749(92)90321-r. [DOI] [PubMed] [Google Scholar]
- Renz H., Jujo K., Bradley K. L., Domenico J., Gelfand E. W., Leung D. Y. Enhanced IL-4 production and IL-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J Invest Dermatol. 1992 Oct;99(4):403–408. doi: 10.1111/1523-1747.ep12616114. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Rousset F., Robert J., Andary M., Bonnin J. P., Souillet G., Chrétien I., Brière F., Pène J., de Vries J. E. Shifts in interleukin-4 and interferon-gamma production by T cells of patients with elevated serum IgE levels and the modulatory effects of these lymphokines on spontaneous IgE synthesis. J Allergy Clin Immunol. 1991 Jan;87(1 Pt 1):58–69. doi: 10.1016/0091-6749(91)90213-8. [DOI] [PubMed] [Google Scholar]
- Roux-Lombard P., Cruchaud A., Dayer J. M. Effect of interferon-gamma and 1 alpha,25-dihydroxyvitamin D3 on superoxide anion, prostaglandins E2, and mononuclear cell factor production by U937 cells. Cell Immunol. 1986 Feb;97(2):286–296. doi: 10.1016/0008-8749(86)90399-0. [DOI] [PubMed] [Google Scholar]
- Sager N., Feldmann A., Schilling G., Kreitsch P., Neumann C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J Allergy Clin Immunol. 1992 Apr;89(4):801–810. doi: 10.1016/0091-6749(92)90434-4. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Lee B. W., Woodland N., Geha R. S., Leung D. Y. Human recombinant interleukin 4 induces Fc epsilon R2/CD23 on normal human monocytes. J Exp Med. 1988 Apr 1;167(4):1406–1416. doi: 10.1084/jem.167.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider S., Saurat J. H., Röcken M., Hauser C. Evidence suggesting involvement of interleukin-4 (IL-4) production in spontaneous in vitro IgE synthesis in patients with atopic dermatitis. J Allergy Clin Immunol. 1991 Jun;87(6):1088–1095. doi: 10.1016/0091-6749(91)92154-s. [DOI] [PubMed] [Google Scholar]
- Williams J., Johnson S., Mascali J. J., Smith H., Rosenwasser L. J., Borish L. Regulation of low affinity IgE receptor (CD23) expression on mononuclear phagocytes in normal and asthmatic subjects. J Immunol. 1992 Oct 15;149(8):2823–2829. [PubMed] [Google Scholar]
- van Reijsen F. C., Bruijnzeel-Koomen C. A., Kalthoff F. S., Maggi E., Romagnani S., Westland J. K., Mudde G. C. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992 Aug;90(2):184–193. doi: 10.1016/0091-6749(92)90070-i. [DOI] [PubMed] [Google Scholar]