Abstract
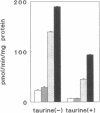
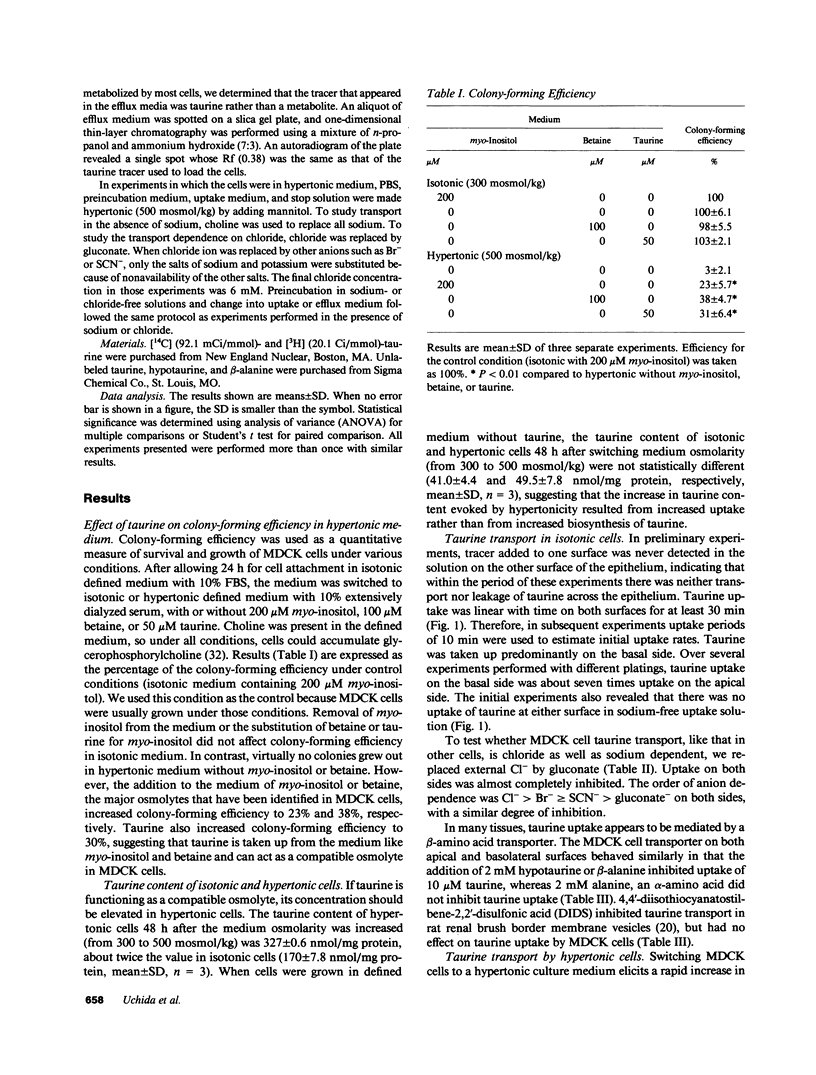
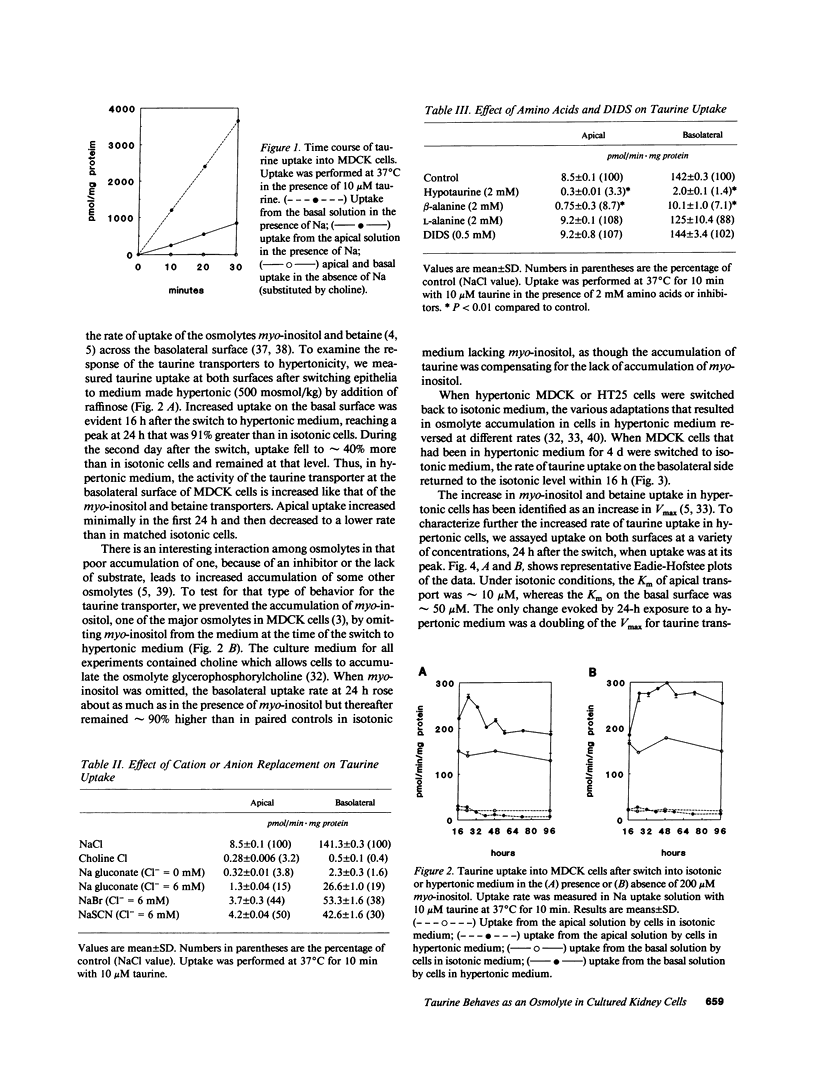
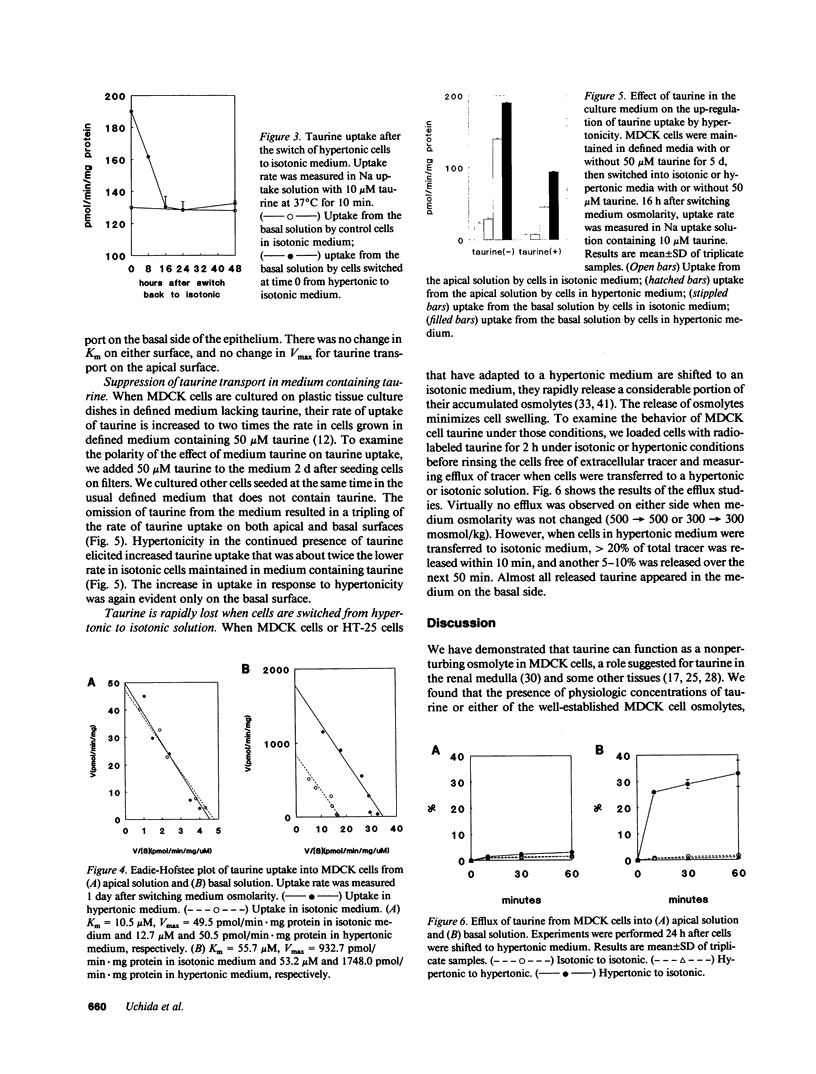
Using a clonal growth assay, we demonstrated that taurine, a nonperturbing osmolyte accumulated in kidney medulla, brain, and some other tissues of hypertonic experimental animals can function as a nonperturbing osmolyte in Madin-Darby canine kidney (MDCK) cells. The taurine content of hypertonic MDCK cells is twice that of isotonic MDCK cells (isotonic 160 nmol/mg protein; hypertonic 320 nmol/mg protein). Therefore we studied taurine transport in MDCK cells grown on porous supports and then studied the effect of hypertonicity which is known to elicit increased uptake of some other nonperturbing osmolytes by MDCK cells. Basal uptake exceeded apical uptake, with Km and Vmax of 56 microM and 933 pmol/min.mg protein on the basal surface and 10 microM and 50 pmol/min.mg protein on the apical surface. On both surfaces, virtually all taurine uptake was Na+ and Cl- dependent. 24 h after cells were shifted to hypertonic medium (500 mosmol/kg), taurine uptake doubled on the basolateral surface without change on the apical surface. The response to hypertonicity was the result of an increase in Vmax without change in Km. There was no change in taurine efflux when cells were shifted from isotonic to hypertonic medium. When cells adapted to hypertonic medium were shifted to isotonic medium, a large transient basolateral efflux of taurine occurred within 10 min. We conclude that taurine can function as a nonperturbing osmolyte in MDCK cells and that tonicity-regulated taurine transport is a basolateral function in MDCK cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S., Balaban R., Fales H. M., Yang Y. M., Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986 May 5;261(13):5872–5877. [PubMed] [Google Scholar]
- Barnard J. A., Thaxter S., Kikuchi K., Ghishan F. K. Taurine transport by rat intestine. Am J Physiol. 1988 Mar;254(3 Pt 1):G334–G338. doi: 10.1152/ajpgi.1988.254.3.G334. [DOI] [PubMed] [Google Scholar]
- Bucuvalas J. C., Goodrich A. L., Suchy F. J. Hepatic taurine transport: a Na+-dependent carrier on the basolateral plasma membrane. Am J Physiol. 1987 Sep;253(3 Pt 1):G351–G358. doi: 10.1152/ajpgi.1987.253.3.G351. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Gusowski N., Dabbagh S. Renal cortex taurine content regulates renal adaptive response to altered dietary intake of sulfur amino acids. J Clin Invest. 1985 Dec;76(6):2213–2221. doi: 10.1172/JCI112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. W. Taurine: its biological role and clinical implications. Adv Pediatr. 1985;32:1–42. [PubMed] [Google Scholar]
- Dantzler W. H., Silbernagl S. Renal tubular reabsorption of taurine, gamma-aminobutyric acid (GABA) and beta-alanine studied by continuous microperfusion. Pflugers Arch. 1976 Dec 28;367(2):123–128. doi: 10.1007/BF00585147. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Martin B., Murphy H. R., Uchida S., Murer H., Cowley B. D., Jr, Handler J. S., Burg M. B. Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J Biol Chem. 1989 Oct 5;264(28):16815–16821. [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B., Daughaday W. H. myo-Inositol transport in renal brush border vesicles and it inhibition by D-glucose. Am J Physiol. 1980 Aug;239(2):F113–F120. doi: 10.1152/ajprenal.1980.239.2.F113. [DOI] [PubMed] [Google Scholar]
- Huxtable R. J., Lippincott S. E. Relative contribution of diet and biosynthesis to the taurine content of the adult rat. Drug Nutr Interact. 1982;1(2):153–168. [PubMed] [Google Scholar]
- Jones D. P., Miller L. A., Chesney R. W. Adaptive regulation of taurine transport in two continuous renal epithelial cell lines. Kidney Int. 1990 Aug;38(2):219–226. doi: 10.1038/ki.1990.189. [DOI] [PubMed] [Google Scholar]
- Karl P. I., Fisher S. E. Taurine transport by microvillous membrane vesicles and the perfused cotyledon of the human placenta. Am J Physiol. 1990 Mar;258(3 Pt 1):C443–C451. doi: 10.1152/ajpcell.1990.258.3.C443. [DOI] [PubMed] [Google Scholar]
- King P. A., Beyenbach K. W., Goldstein L. Taurine transport by isolated flounder renal tubules. J Exp Zool. 1982 Oct 10;223(2):103–114. doi: 10.1002/jez.1402230202. [DOI] [PubMed] [Google Scholar]
- Kwon H. M., Yamauchi A., Uchida S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Renal Na-myo-inositol cotransporter mRNA expression in Xenopus oocytes: regulation by hypertonicity. Am J Physiol. 1991 Feb;260(2 Pt 2):F258–F263. doi: 10.1152/ajprenal.1991.260.2.F258. [DOI] [PubMed] [Google Scholar]
- Lien Y. H., Shapiro J. I., Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990 May;85(5):1427–1435. doi: 10.1172/JCI114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners B. A., Speth R. C., Bresolin N., Huxtable R. J., Yamamura H. I. Sodium-dependent, high-affinity taurine transport into rat brain synaptosomes. Fed Proc. 1980 Jul;39(9):2695–2700. [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Factors affecting the ratio of different organic osmolytes in renal medullary cells. Am J Physiol. 1990 Nov;259(5 Pt 2):F847–F858. doi: 10.1152/ajprenal.1990.259.5.F847. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Olson A. D., Burg M. B. Intracellular betaine substitutes for sorbitol in protecting renal medullary cells from hypertonicity. Am J Physiol. 1991 Apr;260(4 Pt 2):F494–F497. doi: 10.1152/ajprenal.1991.260.4.F494. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Balaban R. S., Burg M. B. Survey of osmolytes in renal cell lines. Am J Physiol. 1988 Aug;255(2 Pt 1):C181–C191. doi: 10.1152/ajpcell.1988.255.2.C181. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Burg M. B. Osmoregulation of glycerophosphorylcholine content of mammalian renal cells. Am J Physiol. 1989 Oct;257(4 Pt 1):C795–C801. doi: 10.1152/ajpcell.1989.257.4.C795. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Burg M. B. Osmoregulatory fluxes of myo-inositol and betaine in renal cells. Am J Physiol. 1989 Nov;257(5 Pt 1):C964–C970. doi: 10.1152/ajpcell.1989.257.5.C964. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulation of betaine transport in mammalian renal medullary cells. Am J Physiol. 1990 Apr;258(4 Pt 2):F1061–F1067. doi: 10.1152/ajprenal.1990.258.4.F1061. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Turner R. J., Burg M. B. Osmoregulatory changes in myo-inositol transport by renal cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):6002–6006. doi: 10.1073/pnas.86.15.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini C. A., Fini C., Cantelmi M. G., Floridi A. Assessment of taurine in plasma and urine by anion-exchange high-performance liquid chromatography with pre-column derivatization. J Chromatogr. 1987 Dec 25;423:292–296. doi: 10.1016/0378-4347(87)80353-5. [DOI] [PubMed] [Google Scholar]
- Schröck H., Forster R. P., Goldstein L. Renal handling of taurine in marine fish. Am J Physiol. 1982 Jan;242(1):R64–R69. doi: 10.1152/ajpregu.1982.242.1.R64. [DOI] [PubMed] [Google Scholar]
- Siebens A. W., Spring K. R. A novel sorbitol transport mechanism in cultured renal papillary epithelial cells. Am J Physiol. 1989 Dec;257(6 Pt 2):F937–F946. doi: 10.1152/ajprenal.1989.257.6.F937. [DOI] [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtman H., Barbour R., Sturman J. A., Finberg L. Taurine and osmoregulation: taurine is a cerebral osmoprotective molecule in chronic hypernatremic dehydration. Pediatr Res. 1988 Jan;23(1):35–39. doi: 10.1203/00006450-198801000-00008. [DOI] [PubMed] [Google Scholar]
- Turner R. J. beta-Amino acid transport across the renal brush-border membrane is coupled to both Na and Cl. J Biol Chem. 1986 Dec 5;261(34):16060–16066. [PubMed] [Google Scholar]
- Uchida S., Kwon H. M., Preston A. S., Handler J. S. Expression of Madin-Darby canine kidney cell Na(+)-and Cl(-)-dependent taurine transporter in Xenopus laevis oocytes. J Biol Chem. 1991 May 25;266(15):9605–9609. [PubMed] [Google Scholar]
- Wade J. V., Olson J. P., Samson F. E., Nelson S. R., Pazdernik T. L. A possible role for taurine in osmoregulation within the brain. J Neurochem. 1988 Sep;51(3):740–745. doi: 10.1111/j.1471-4159.1988.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Wolff N. A., Kinne R. Taurine transport by rabbit kidney brush-border membranes: coupling to sodium, chloride, and the membrane potential. J Membr Biol. 1988 May;102(2):131–139. doi: 10.1007/BF01870451. [DOI] [PubMed] [Google Scholar]
- Wolff N. A., Perlman D. F., Goldstein L. Ionic requirements of peritubular taurine transport in Fundulus kidney. Am J Physiol. 1986 Jun;250(6 Pt 2):R984–R990. doi: 10.1152/ajpregu.1986.250.6.R984. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Burg M. B., Bagnasco S. M. Effects of NaCl, glucose, and aldose reductase inhibitors on cloning efficiency of renal medullary cells. Am J Physiol. 1990 Jan;258(1 Pt 1):C156–C163. doi: 10.1152/ajpcell.1990.258.1.C156. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Burg M. B. Counteracting effects of urea and betaine in mammalian cells in culture. Am J Physiol. 1990 Jan;258(1 Pt 2):R198–R204. doi: 10.1152/ajpregu.1990.258.1.R198. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zelikovic I., Stejskal-Lorenz E., Lohstroh P., Budreau A., Chesney R. W. Anion dependence of taurine transport by rat renal brush-border membrane vesicles. Am J Physiol. 1989 Apr;256(4 Pt 2):F646–F655. doi: 10.1152/ajprenal.1989.256.4.F646. [DOI] [PubMed] [Google Scholar]