Abstract
Background and Purpose
Vestibular rehabilitation strategies including gaze stabilization exercises have been shown to increase gain of the angular vestibulo-ocular reflex (aVOR) using a retinal slip error signal (ES). The identification of additional ESs capable of promoting substitution strategies or aVOR adaptation is an important goal in the management of vestibular hypofunction. Position ESs have been shown to increase both aVOR gain and recruitment of compensatory saccades (CSs) during passive whole body rotation. This may be a useful compensatory strategy for gaze instability during active head rotation as well. In vestibular rehabilitation, the imaginary target exercise is often prescribed to improve gaze stability. This exercise uses a position ES; however, the mechanism for its effect has not been investigated. We compared aVOR gain adaptation using 2 types of small position ES: constant versus incremental.
Methods
Ten subjects with normal vestibular function were assessed with unpredictable and active head rotations before and after a 20-minute training session. Subjects performed 9 epochs of 40 active, high-velocity head impulses using a position ES stimulus to increase aVOR gain.
Results
Five subjects demonstrated significant aVOR gain increases with the constant-position ES (mean, 2%; range, −18% to 12%) compared with another 5 subjects showing significant aVOR gain increases to the incremental-position ES (mean, 3.7%; range, −2% to 22.6%). There was no difference in aVOR gain adaptation or CS recruitment between the 2 paradigms.
Discussion and Conclusion
These findings suggest that some subjects can increase their aVOR gain in response to high-velocity active head movement training using a position ES. The primary mechanism for this seems to be aVOR gain adaptation because CS use was not modified. The overall low change in aVOR gain adaptation with position ES suggests that retinal slip is a more powerful aVOR gain modifier.
Keywords: vestibulo-ocular reflex gain, rehabilitation, adaptation, position error signal
Introduction
Gaze stability refers to the brain's ability to stabilize the eyes in space to ensure clear vision during head movement. When this function is compromised, as is common in individuals with vestibular hypofunction, one may experience “oscillopsia” or visual blurring during head movement. This blurring occurs as a result of excessive retinal slip, the movement of images off the fovea of the retina. Retinal slip can however, create a powerful error signal that the brain can use to modify neural processing within the angular vestibulo-ocular reflex (aVOR), necessary in the presence of disease and aging.
In addition to velocity ESs, patients with vestibular hypofunction are often asked to perform an exercise involving a position ES.5 This exercise is typically referred to as the “imaginary targets” paradigm. To perform this exercise, the individual is instructed to foveate a target, close the eyes, and turn the head while “imagining” that the eyes are still looking at the still target (the patient thus attempts to keep gaze fixed on the target without visual cues). When the individual opens the eyes, a well-compensated aVOR response will yield little to no error between the target position and the position of gaze. In contrast, a reduced aVOR will result in an error between the actual eye position and “imagined” target position, on completion of the head rotation. Patients are instructed to repeat this activity in multiple directions and at variable speeds to improve generalizability—but always with the intent to maintain stable gaze. This exercise has traditionally been considered to be a substitution type of exercise, recruiting an alternate gaze stability strategy, rather than an adaptation exercise designed to improve the gain of the aVOR. Identifying additional ESs capable of promoting substitution strategies or aVOR adaptation is an important goal for individuals with vestibular hypofunction.
Preliminary evidence suggests that using a position ES and passive, low-velocity head rotation (ie, 43 degrees/sec) results in partial adaptation of the aVOR.6 In this paradigm, the target was extinguished during head movement, then reappeared at the end of each ipsilateral half-cycle rotation, thereby exposing subjects to a position ES. The study did not evaluate responses to active head rotations or head velocities >43 degrees/sec. Interestingly, a greater amount of the stratúegy to improve gaze stability occurred from compensatory saccades (CSs), not from increased slow component eye velocity typically seen with aVOR gain adaptation. CSs are saccades that occur in the direction of the deficient aVOR during a head rotation. CSs have been shown to be inversely related to aVOR gain.4,7 The use of position ESs to drive aVOR adaptation during active, high-velocity head rotations has not been systematically investigated.
The purpose of this study was to characterize aVOR gain adaptation in response to 2 distinct position ES paradigms at head velocities characteristic of activities of daily living.8 We hypothesized that a position ES could be used to drive aVOR change with active, high-velocity head rotations. We further hypothesized that a gradually applied (incremental) position ES would lead to greater aVOR gain adaptation compared with a constant-position ES.
Methods
Ten subjects (mean age, 32.7 ± 9.4 years; range, 23–49 years) with no known history of vestibular pathology were studied. Participants with vestibular pathology were not recruited for the preliminary phase of this research so that experimenters could focus on the characterization of physiological responses to high-velocity, position ES in individuals without vestibular pathology. Participation in this study was voluntary. Informed consent was obtained as approved by The Johns Hopkins School of Medicine Institutional Review Board.
Head (custom-fit bite-block) and eye position data were collected using a monocular 2-dimensional scleral search coil during active and passive head impulse testing and adaptation trials. The procedures to collect scleral coil data were reported previously.2–4 Briefly, subjects were seated upright in a uniform magnetic field and instructed to fix their gaze on a rear-projected laser target 138 cm away. During testing, the laser target flashed when the head was immobile and centered, but extinguished during rotation. Forty passive (unpredictable timing and direction; 20 left, 20 right; mean amplitude: 25 degrees, mean velocity: 167 ± 27 degrees/sec, mean acceleration: 2700 degrees/sec2) and 40 active (20 left, 20 right; 25 degrees, 220 ± 34 degrees/sec, 3500 degrees/sec2) head impulses were collected before and after the training sessions in complete darkness. Only horizontal head impulses were studied. Passive head impulses were collected to assess the extent of vestibular function. Subjects were instructed to briefly pause in the center after the active head rotations. Active head impulses were obtained as the primary measure of adaptation to the active training paradigms. Active postadaptation testing was performed within 1 minute of the conclusion of the final training trial. Passive testing was performed after the active assessment (within 3 minutes).
Position Error Signal Training Paradigms
Each subject participated in 2 randomly ordered position ES paradigms. Training trials consisted entirely of active (subject-generated) head impulses as described previously. All training was performed in complete darkness. Each trial lasted approximately 90 seconds with roughly 30 seconds between trials, during which time the subject was permitted to rest his or her eyes, remove his or her custom bite block, and communicate with the tester. For both paradigms, the laser target flashed when the head was still but extinguished during head rotation to eliminate retinal slip. Once the head stopped (after the rotation), the target reappeared in a new position in the opposite direction of head rotation in an attempt to increase aVOR gain. During the training trials, subjects were allowed brief (1 to 2 second) pauses between head rotations. Sessions were separated by 4 to 16 days (mean 9 ± 4 days) to control for carryover effects between paradigms. Each testing session lasted approximately 45 minutes with 30–35 minutes of coil wear time. A complete session consisted of, active and passive head impulse testing performed both before and after the randomly selected training paradigm.
Constant-Position Error Signal
The constant ES paradigm consisted of 9 trials of adaptation using an ES that was always 5% greater than the head amplitude (in the opposite direction). This paradigm maintained a constant demand on the oculomotor response over the 9 trials.
Incremental-Position Error Signal
The incremental ES paradigm consisted of 3 sets of 3 trials of adaptation progressing from 5% to 10% and then to 15% greater than head amplitude. This paradigm demanded a gradual increase in the oculomotor response.
Data Analysis
Data analysis was performed using custom software written in Labview.1 Data were only calculated for those trials in which the subject's head velocity exceeded 120 degrees/sec.
aVOR Gain
aVOR gain is defined as a ratio of slow-component eye velocity to head velocity during the first 20 to 40 ms after the onset of head rotation. CSs were defined as quick eye rotations with accelerations exceeding 3000 degrees/sec2, absence of a biphasic waveform (consistent with blinks), and no evidence of vertical excursion.9 CSs have been characterized as occurring during head rotation, in a direction opposite the head rotation.4 CSs were not included in the calculation of aVOR gain.
Research Design and Statistical Analysis
These experiments incorporated a prospective, randomized, repeated-measures design. Individual differences between aVOR gain were assessed using 2-tailed t tests assuming equal variance. The level of significance was set at α = 0.05 for all tests. Based on pilot data, 10 subjects were needed to establish 80% statistical power.
Results
Does Position Error Lead to aVOR Gain Change?
Constant-Position Error Signal
Five of 10 subjects demonstrated significantly greater (P < .05) aVOR gains during active head impulses (Fig. 1). One subject demonstrated a 10.8% increase in his active aVOR gain in response to the constant-position ES paradigm (Fig. 2). One subject demonstrated a significant (P = .0003) decrease in aVOR gain to active head impulses. Across all subjects, mean aVOR gain adaptation to active impulses increased by 2 ± 5% (range, −18% to 12%). Across all subjects, there was a mean decreased in aVOR gain adaptation to passive impulses after the constant-position ES paradigm (mean, 5 ± 7.8%; range, −20% to +7%). This decrement was statistically (P < .05) significant in 6 of 10 subjects.
FIGURE 1.
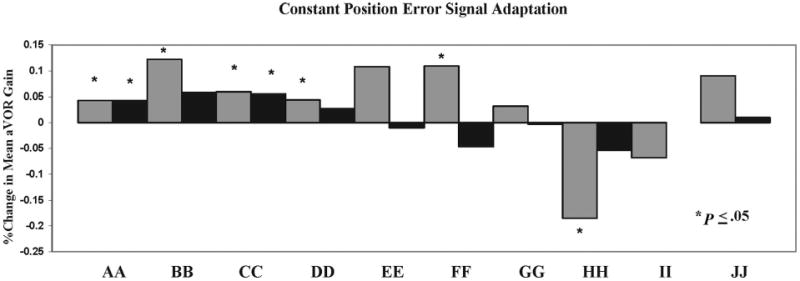
Percentage of aVOR gain adaptation in normal subjects after 9 active training trials with a constant-position error signal (5%). Gray boxes denote active impulses to the right and black boxes signify active impulses to the left. Capital letters (AA-JJ) represent individual subjects. Subject BB demonstrated no adaptation to left head impulses after the constant-position error signal paradigm. Asterisks denote significant (P < .05) adaptation (positive or negative) between pre- and postadaptation active impulse testing.
FIGURE 2.
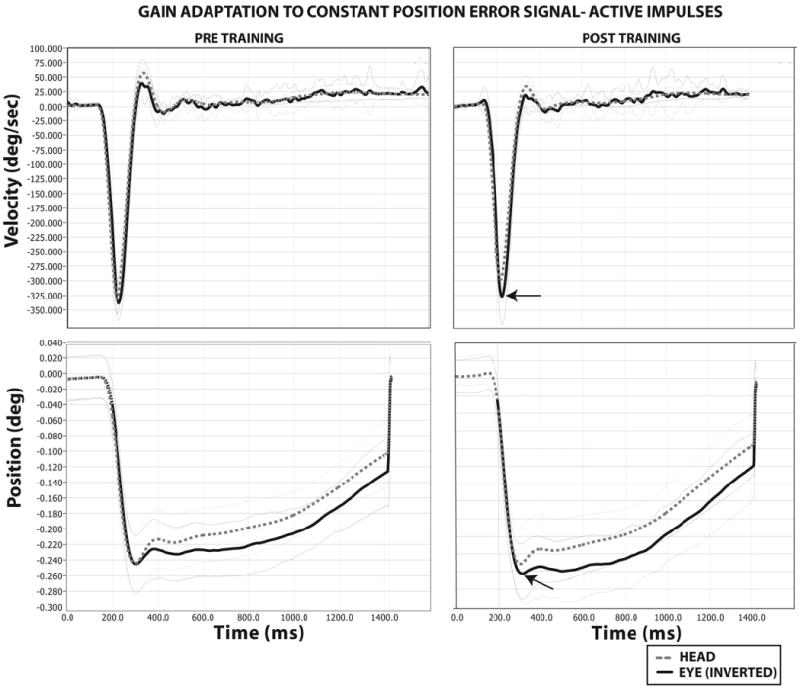
Gain adaptation to a constant-position error signal in a single subject. Pre- and posttraining head impulse testing presented for the velocity and position domains. Bold black trace denotes the compensatory eye movement (velocity in degrees per second, or position in degrees). The bolded-stippled gray trace denotes head movement (velocity in degrees per second, or position in degrees). One standard deviation for both eye and head values are presented as single black- and gray-stippled traces, respectively. The eye traces have been inverted for ease of comparison. Arrows denote a 10% increase in the amplitude of compensatory eye position and velocity responses to active head movements relative to the pretraining condition.
Incremental-Position Error Signal
Five subjects demonstrated significant (P < .05) increases in active aVOR gains in response to the incremental-position ES. Across all subjects, mean aVOR gain to active head impulses increased by 3.7 ± 4% (range, −2% to 22.6%). No subjects demonstrated significant decreases in aVOR gains (Fig. 3). Across all subjects, mean aVOR gain adaptation to passive impulses were unchanged (mean, 0 ± 6%; range, −7% to +7%). Two subjects demonstrated significant (P < .05) improvement and 2 subjects demonstrated significant decrement in passive aVOR gain responses with this paradigm.
FIGURE 3.
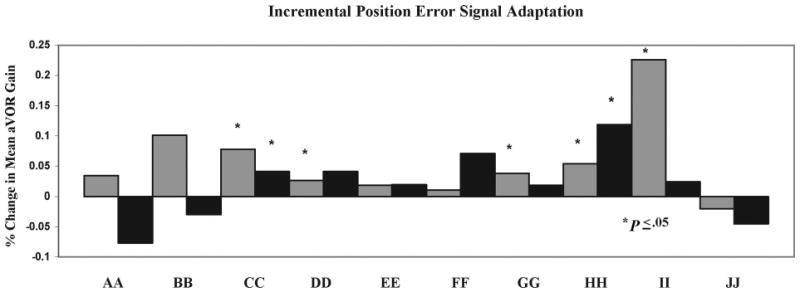
Percentage of aVOR gain adaptation in normal subjects after 9 active training trials with an incremental-position error signal (5% × 3 trials, 10% × 3 trials, and 15% × 3 trials). Gray boxes represent active impulses to the right and black boxes signify active impulses to the left. Capital letters (AA-JJ) identify individual study participants. Asterisks denote significant (P < .05) adaptation (positive or negative) between pre- and postadaptation active impulse testing.
Is There any Difference Between Position-Error Paradigms?
There was no difference in aVOR gain between the constant or incremental-position ES paradigms (2-tailed t test, P = .52). Similarly, there was no difference in the frequency of CS recruitment during postadaptation active head impulse testing (2-tailed t test, P = .98).
Discussion
Our data support the hypothesis that in some subjects, position ESs cause aVOR adaptation to high-velocity head rotation. However, we found no significant differences between the incremental and constant-position ES adaptation paradigms. In this study, both head and target movement contributed to the position ES. In contrast, for the imaginary target exercise prescribed by clinicians only the head moves. Although not completely similar, our findings suggest that the imaginary target exercise may in fact help increase aVOR gain. In particular, we observed that individuals without vestibular pathology seem to have a preference for how the aVOR gain is changed (eg, some subjects' responded more robustly to constant versus incremental ESs). This has also been established in individuals with vestibular hypofunction.1
Previous work suggests that some individuals with vestibular pathology may preferentially recruit unique gaze stability strategies after vestibular rehabilitation. Individuals with chronic vestibular hypofunction increased both slow-component eye velocity (increased aVOR gain) and the frequency of CSs.4,7 In contrast, those individuals with partial, natural recovery of the aVOR used fewer CSs. These findings suggest that individuals may have a unique “preferred” gaze stability strategy. Rehabilitation, therefore, may best be applied through initial identification of the individual's preferred compensatory strategy.
Although the use of retinal slip-based rehabilitation protocols is well established, there is emerging evidence for using position ESs to adapt the aVOR Position vestibular pause neurons are a class of central vestibular neurons that respond to ipsirotational head velocity and contralateral eye position signals to drive the aVOR.10 Position ESs may be another stimulus to drive gaze stabilization in rehabilitation.
We reported that ∼50% of subjects demonstrated significant adaptation to each condition. It is conceivable that the frequency and magnitude of aVOR adaptation may have been partially affected by subject tolerance for coil wear and/or attentiveness in the final conditions of the experiment. Diminished aVOR gain responses as a result of wandering or lessened attention is a critical problem when conducting vestibular function testing11 and may have been a factor in the decreased aVOR responses in some subjects.
We recognize the mean magnitudes of aVOR adaptation using a position ES (2%–3.7%) are less robust than similar protocols using a retinal slip signal.1 However, some subjects did have adaptation approaching 15%, suggesting that gaze stability exercises should incorporate exercises that use position ESs. Given these limited data, we recommend that clinicians treating individuals with aVOR deficits start with gaze stabilization exercises using velocity ESs. In addition, our data suggest that patients should also be instructed to perform gaze stability exercises using position ESs.
As the individual performs position ES gaze stabilization exercises, the clinician should observe the individual's eyes to ascertain the degree of error between gaze position and target location. Large errors should be minimized by reducing the amount of head rotation and progressed when the patient can perform the exercise without a noticeable corrective saccade. Treatment progression may include increasing head velocity, head amplitude, and plane of head rotation. Head velocity should be increased from moderate (80–100 degrees/sec) to fast (120–180 degrees/sec) when the patient is able to consistently acquire the target at the slower rate. Clinicians may also impart variable surface conditions to advance postural stability treatment goals.
In a previous adaptation experiment involving the use of position ESs, Eggers et al6 reported an increase in the recruitment of CSs. These subjects without vestibular pathology were exposed to passive, low-velocity (43 degrees/sec) head impulses. We did not find a similar result for either paradigm used in this study. One explanation for this difference in results is the higher head velocities that we used, which may lead to an ES that is preferentially generated from the aVOR system. In contrast, for lower velocity head rotations, both aVOR and smooth pursuit ESs may have combined to present a greater demand on the oculomotor system to recruit a CS.
Conclusions
These findings suggest that the aVOR can be increased during high-velocity active head rotations using a position ES. These data may have implications for gaze stability exercises in individuals with vestibular hypofunction. Although some individuals demonstrated large aVOR gain change, position ESs do not seem to be as robust as retinal slip ESs for aVOR gain adaptation. Position ES–driven interventions (ie, imaginary targets paradigm) may have an important role in gaze stability exercise programs; further research is necessary to explore this potential in subjects with vestibular deficits.
Acknowledgments
Supported by National Institutes of Health, National Institute on Deafness and Other Communication Disorders (NIDCD) award K23DC007926.
References
- 1.Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191:435–446. doi: 10.1007/s00221-008-1537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herdman S, Hall C, Schubert M, Das V, Tusa R. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007;133:383–389. doi: 10.1001/archotol.133.4.383. [DOI] [PubMed] [Google Scholar]
- 3.Herdman S, Hall C, Schubert M, Das V, Tusa R. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129:819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 4.Schubert MC, Migliaccio AA, Clendaniel RC, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89:500–507. doi: 10.1016/j.apmr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert MC, Das V, Tusa RJ, Herdman SJ. Cervico-ocular reflex in normal subjects and patients with unilateral vestibular hypofunction. Otol Neurotol. 2004;25:65–71. doi: 10.1097/00129492-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Eggers SD, De Pennington N, Walker MF, Shelhamer M, Zee DS. Short term adaptation of the VOR: non-retinal slip error signals and saccade substitution. Ann N Y Acad Sci. 2003;1004:94–110. [PubMed] [Google Scholar]
- 7.Schubert M, Migliaccio A, Della Santina C. Modification of compensatory saccades after a VOR gain recovery. J Vestib Res. 2006;16:1–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- 9.Scherer MR, Migliaccio A, Schubert MC. Effect of vestibular rehabilitation on passive dynamic visual acuity. J Vestib Res. 2008;18:147–157. [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–1933. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- 11.Baloh RB, Honrubia V. Clinical Neurophysiology of the Vestibular System. 3rd. New York, NY: Oxford University Press; 2003. [Google Scholar]


