This article identifies membrane skeleton proteins, adducins, as important regulators of epithelial cell–cell adhesions that promote assembly and antagonize stimulus-induced disassembly of adherens and tight junctions.
Abstract
Epithelial adherens junctions (AJs) and tight junctions (TJs) are dynamic structures that readily undergo disintegration and reassembly. Remodeling of the AJs and TJs depends on the orchestrated dynamics of the plasma membrane with its underlying F-actin cytoskeleton, and the membrane–cytoskeleton interface may play a key role in junctional regulation. Spectrin–adducin–ankyrin complexes link membranes to the actin cytoskeleton where adducins mediate specrtrin–actin interactions. This study elucidates roles of adducins in the remodeling of epithelial junctions in human SK-CO15 colonic and HPAF-II pancreatic epithelial cell monolayers. These cells expressed the α and γ isoforms of adducin that positively regulated each others protein level and colocalized with E-cadherin and β-catenin at mature, internalized and newly assembled AJs. Small interfering RNA-mediated down-regulation of α- or γ-adducin expression significantly attenuated calcium-dependent AJ and TJ assembly and accelerated junctional disassembly triggered by activation of protein kinase C. Two mechanisms were found to mediate the impaired AJ and TJ assembly in adducin-depleted cells. One mechanism involved diminished expression and junctional recruitment of βII-spectrin, and the other mechanism involved the decrease in the amount of cellular F-actin and impaired assembly of perijunctional actin bundles. These findings suggest novel roles for adducins in stabilization of epithelial junctions and regulation of junctional remodeling.
INTRODUCTION
The integrity and functions of simple epithelia that line the majority of luminal organs of the body depend on formation of adhesive contacts between neighboring epithelial cells. These adhesive contacts are composed of multiprotein complexes known as intercellular junctions. The junctional complexes span the lateral plasma membrane to interact with opposing complementary junctions in the intercellular space and associate with various cytoskeletal, signaling, and trafficking components at the cytosolic face of the membrane (Gonzalez-Mariscal et al., 2008; Hartsock and Nelson, 2008; Giepmans and van Ijzendoorn, 2009). The most apically located tight junctions (TJs) and subjacent adherens junctions (AJs) are considered as critical regulators of the integrity and barrier properties of differentiated epithelial layers (Tsukita et al., 2001; Pokutta and Weis, 2007; Hartsock and Nelson, 2008; Anderson and Van Itallie, 2009).
TJs and AJs are composed of several types of adhesive and scaffolding proteins. Major transmembrane components of TJ that mediate intercellular adhesion represent different claudin isoforms, occludin, and junctional adhesion molecule-A (Tsukita et al., 2001; Hartsock and Nelson, 2008; Paris et al., 2008; Anderson and Van Itallie, 2009). Members of the zonula occludens (ZO) protein family are prototypical components of the cytosolic plaque of TJs (Tsukita et al., 2001; Hartsock and Nelson, 2008; Paris et al., 2008; Anderson and Van Itallie, 2009). Adhesive properties of epithelial AJs are determined by E-cadherin and nectin proteins, whereas the AJ cytosolic scaffolds α, β, and p120 catenins link this junctional complex to the cytoskeleton and intracellular trafficking machinery (Pokutta and Weis, 2007; Hartsock and Nelson, 2008; Niessen and Gottardi, 2008). Once considered as static glue-like structures, TJs and AJs are now known to be very dynamic. Such dynamics include the steady-state remodeling of junctional complexes that is driven by a continuous renewal of the adhesive cadherin–cadherin interactions (Le et al., 1999; Troyanovsky et al., 2006; Hong et al., 2010). Furthermore, large-scale junctional rearrangements play crucial roles in normal epithelial morphogenesis and epithelial barrier breakdown in many pathological conditions (Perez-Moreno et al., 2003; Bryant and Stow, 2004; Ivanov et al., 2005b; Cavey and Lecuit, 2009; Turner, 2009). Understanding the mechanisms that underlie the dynamics of TJs and AJs under normal and diseased conditions is an important challenge in epithelial cell biology.
Epithelial cell–cell adhesions are known to be calcium dependent. Indeed, removal of extracellular calcium results in rapid TJ and AJ disassembly, whereas readdition of this cation leads to complete recovery of junctional structure and functions (Cereijido et al., 1978; Pitelka et al., 1983; Gonzalez-Mariscal et al., 1990; Ivanov et al., 2004b, 2005a). Such alterations in extracellular calcium level (known as the “calcium switch”) represent a powerful model to study mechanisms of TJ and AJ remodeling in vitro, which helped to reveal major mechanisms that drive reorganizations of epithelial junctions. One such mechanism involves reorganization of perijunctional actin filaments (Volberg et al., 1986; Ma et al., 2000; Ivanov et al., 2004a, 2005a; Smutny et al., 2010), and another mechanism involves remodeling of the plasma membrane (Pitelka et al., 1983; Le et al., 1999; Ivanov et al., 2004b; Troyanovsky et al., 2006; Nejsum and Nelson, 2007). Interestingly, these processes seem to be interconnected. For example, increase or decrease of the membrane protrusiveness is well coordinated with reorganization of F-actin bundles during disassembly and reformation of AJs and TJs (Pitelka et al., 1983; Volberg et al., 1986; Bershadsky, 2004; Ivanov et al., 2004a, 2005a; Nejsum and Nelson, 2007; Cavey and Lecuit, 2009). Furthermore, inhibition of F-actin reorganization was shown to block membrane dynamics during junctional remodeling and vice versa (Ma et al., 2000; Ivanov et al., 2004a,b, 2005a; Nejsum and Nelson, 2007; Perez et al., 2008). These findings suggest that physical interactions between plasma membrane and the underlying F-actin cytoskeleton are critical for transduction of signals, and forces that drive reorganization of epithelial junctions.
The most abundant interactions of actin filaments with the cytosolic face of the plasma membrane are mediated by the spectrin-based membrane skeletal network (Bennett and Baines, 2001; Thomas, 2001; Bennett and Healy, 2008; Baines, 2009). The major component of this network is spectrin tetramer that is formed via head-to-head interactions of two α- and β-spectrin heterodimers (Bennett and Baines, 2001; Thomas, 2001; Baines, 2009). The tetramers have actin-binding sites, and they are linked to the plasma membrane via specialized scaffolding proteins, such as ankyrin and protein 4.1 (Bennett and Baines, 2001; Thomas, 2001; Baines, 2009). The spectrin-based membrane skeleton has been extensively studied in erythrocytes, where it stabilizes the cell membrane and is involved in regulation of cell shape and volume (Mohandas and Gallagher, 2008).
Beside erythrocytes, the membrane-associated spectrin network is present in virtually all animal cells, including mammalian epithelia. Spectrin/ankyrin polymers are known to be enriched at the lateral plasma membrane of differentiated epithelial cells, where they colocalize with E-cadherin and α-catenin (Nelson and Veshnock, 1986; Drenckhahn and Bennett, 1987; Marrs et al., 1993; Pradhan et al., 2001). Furthermore, recent RNA interference (RNAi) studies demonstrated that βII-spectrin and ankyrin G are essential for recruitment of E-cadherin to intercellular junctions in cultured bronchial epithelial cell monolayers and early mice embryo (Kizhatil and Bennett, 2004; Kizhatil et al., 2007a,b). Given these results, one can suggest that spectrin-based membrane skeleton cooperates with perijunctional F-actin to regulate AJ and TJ functions and remodeling.
Spectrin rods only weakly associate with actin filaments, but this association is significantly increased by several accessory proteins, most notably, by adducins (Matsuoka et al., 2000; Bennett and Baines, 2001). The adducin protein family consists of three homologous proteins termed α-, β-, and γ-adducins (Matsuoka et al., 2000; Bennett and Baines, 2001). The α and γ isoforms are expressed in various tissues, whereas expression of the β-isoform is more limited, being abundant in erythrocytes and the brain (Joshi et al., 1991; Dong et al., 1995). Adducins readily oligomerize to form heterodimers and heterotetramers of either α/β or α/γ subunits (Dong et al., 1995; Hughes and Bennett, 1995). They recruit spectrin to actin filaments and promote assembly of spectrin lattice at the plasma membrane (Hughes and Bennett, 1995; Li et al., 1998; Abdi and Bennett, 2008). Besides mediating spectrin–F-actin linkage, adducins also are involved in actin filament bundling and capping (Mische et al., 1987; Taylor and Taylor, 1994; Kuhlman et al., 1996). Overall, these data highlight adducins as important regulators of both the spectrin-based membrane skeleton and the actin cytoskeleton.
It has been long recognized that α- and γ-adducins are enriched at intercellular junctions in cultured epithelial cells and simple mucosal epithelia in vivo (Kaiser et al., 1989; Marrs et al., 1993; Dong et al., 1995). Despite this junctional affiliation, the role of adducins in regulation of epithelial AJs and TJs remains poorly understood. The only recent study addressing this subject has shown that RNAi-mediated down-regulation of α-adducin expression in confluent human bronchial epithelial cell monolayers increased long-range intramembrane mobility of E-cadherin, without altering AJ and TJ structure (Abdi and Bennett, 2008). However, these results probably reflect high-redundancy mechanisms that control the integrity of AJs and TJs and therefore should not be interpreted as a lack of role for adducins in the regulation of epithelial junctions. Indeed, even depletion of the most abundant junctional components, such as E-cadherin and ZO-1, or a key cytoskeletal regulator, myosin IIA, did not prevent formation of morphologically normal epithelial junctions but instead interrupted junctional remodeling (assembly and disassembly) (McNeil et al., 2006; Capaldo and Macara, 2007; Ivanov et al., 2007, 2010). These data suggest that studying AJ/TJ dynamics would be a more relevant approach to discover new regulators of epithelial junctions.
In the present study, we examined the role of α- and γ-adducins in remodeling of AJs and TJs in cultured human intestinal and pancreatic epithelial cells. We report that adducins positively regulate intercellular junctions by accelerating AJ and TJ assembly and antagonizing stimuli-induced junctional disassembly. These effects of adducins on epithelial junctions are probably mediated by recruitment, stabilization, or both of βII-spectrin at the areas of cell–cell contact, as well as by controlling proper assembly of perijunctional F-actin bundles.
MATERIALS AND METHODS
Antibodies and Other Reagents
The following primary polyclonal antibodies (pAbs) and monoclonal antibodies (mAbs) were used to detect junctional and membrane skeleton proteins: anti-occludin, ZO-1, E-cadherin, and β-catenin mAbs and pAbs (Invitrogen, Carlsbad, CA); anti-α-adducin, phospho-adducin (Ser726), ankyrin-G pAbs, and γ-adducin mAb (Santa Cruz Biotechnology, Santa Cruz, CA); anti-αII-spectrin and βII-spectrin mAbs (BD Biosciences, San Jose, CA); and anti-tubulin and anti-actin mAbs (Sigma-Aldrich, St. Louis, MO). Alexa-488 or Alexa-568 dye-conjugated donkey anti-rabbit and goat anti-mouse secondary antibodies, as well as Alexa dye-conjugated phalloidin were obtained from Invitrogen. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Recombinant human epidermal growth factor (EGF) was obtained from R&D Systems (Minneapolis, MN). All other reagents were obtained from Sigma-Aldrich.
Cell Culture and Induction of Junctional Remodeling
SK-CO15 human colonic epithelial cells (a gift from Dr. E. Rodriguez-Boulan, Weill Medical College of Cornell University, New York City, NY) were cultured as described previously (Le Bivic et al., 1989; Ivanov et al., 2007). HPAF-II human pancreatic epithelial cells and A549 human lung epithelial cells were obtained from American Type Culture Collection, (Manassas, VA). HPAF-II cells were cultured in RPMI 1640 medium supplemented with 10 mM HEPES, 10% fetal bovine serum (FBS), and sodium pyruvate (Rajasekaran et al., 2004; Ivanov et al., 2009). A549 cell were cultured in DMEM/F-12 supplemented with 10% FBS. For immunolabeling experiments, epithelial cells were grown on either collagen-coated, permeable polycarbonate filters (0.4-μm pore size; Costar, Cambridge, MA) or on collagen-coated coverslips. For biochemical experiments, the cells were cultured on six-well plastic plates.
To study formation of epithelial TJs and AJs, confluent SK-CO15 monolayers were first depolarized by overnight incubation in low-calcium medium (calcium-free Eagle's minimal essential medium for suspension culture [Sigma-Aldrich] supplemented with 10 mM HEPES, 14 mM NaHCO3, 5 μM CaCl2, and 10% dialyzed fetal bovine serum, pH 7.4). To induce junctional reassembly, the cells were returned to normal cell culture media with high (∼1.8 mM) calcium concentration for the indicated times (referred to hereafter as “calcium repletion”). To induce protein kinase C (PKC)-dependent junctional disassembly, polarized HPAF-II cell monolayers were incubated for 1–3 h with 12-O-tetradecanoylphorbol-13-acetate (TPA; 1 μM). To analyze growth factor-induced junctional remodeling, A549 cells were serum starved overnight followed by a 24-h treatment with EGF (100 ng/ml) in serum-free medium supplemented with 0.1% bovine serum albumin. Afterward, EGF was washed out, and the cells were incubated for an additional 24 h in complete medium.
Immunofluorescence Labeling and Image Analysis
Cell monolayers were fixed and permeabilized in 100% ethanol for 20 min at −20°C. Fixed cells were blocked in HEPES-buffered HBSS (HBSS+) containing 1% bovine serum albumin (blocking buffer) for 60 min at room temperature and incubated for another 60 min with primary antibodies diluted in the blocking buffer. Cells were then washed, incubated for 60 min with Alexa dye-conjugated secondary antibodies, rinsed with blocking buffer, and mounted on slides with ProLong Antifade medium (Invitrogen). F-Actin was labeled by Alexa-488–conjugated phalloidin. Fluorescently labeled cell monolayers were examined using an LSM510 laser scanning confocal microscope (Carl Zeiss Microimaging, Thornwood, NY) or a FluoView 1000 confocal microscope (Olympus America, Center Valley, PA). The Alexa Fluor-488 and -568 signals were imaged sequentially in frame-interlaced mode to eliminate cross talk between channels. Images were processed using an LSM5 image browser (Carl Zeiss Microimaging) or FV1000 software (Olympus America) and Photoshop (Adobe Systems, Mountain View, CA). Images shown are representative of at least three experiments, with multiple images taken per slide. AJ and TJ reassembly was quantified by measuring the length of E-cadherin, β-catenin, and occludin-based intercellular contacts by using ImageJ software (National Institutes of Health, Bethesda, MD). Cell height was quantified by using FV1000 software (Olympus America).
Immunoblotting
Cells were homogenized in a radioimmunoprecipitation assay lysis buffer (20 mM Tris, 50 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% sodium deoxycholate, 1% Triton X-100 [TX-100], and 0.1% SDS, pH 7.4), containing a protease inhibitor cocktail (1:100; Sigma-Aldrich) and phosphatase inhibitor cocktails 1 and 2 (both at 1:200; Sigma-Aldrich). Lysates were cleared by centrifugation (20 min at 14,000 × g), diluted with 2× SDS sample buffer, and boiled. SDS-polyacrylamide gel electrophoresis and immunoblotting were conducted by standard protocols with an equal amount of total protein (10 or 20 μg) per lane. Protein expression was quantified by densitometric analysis of at least three immunoblot images, each representing an independent experiment, by using Scion Image (Scion, Frederick, MD) and UN-SCAN-IT digitizing software (Silk Scientific, Orem, UT).
G/F-Actin Fractionation
Quantification of G-actin and F-actin was performed by TX-100 fractionation of intracellular actin as described previously (Ivanov et al., 2005a). In brief, SK-CO15 cell monolayers were washed with Hanks' balanced salt solution (HBSS), and G-actin was extracted by gentle shaking for 5 min at room temperature with the cytoskeleton stabilization buffer [10 mM 2-(N-morpholino)ethanesulfonic acid, 140 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 280 mM sucrose, pH 6.1] supplemented with 0.5% TX-100, proteinase inhibitor cocktail, and 1 μg/ml phalloidin to prevent filament disassembly. The TX-100–soluble G-actin fraction was mixed with an equal volume of SDS sample buffer and boiled. Cells were then briefly washed with HBSS, and the TX-100–insoluble F-actin fraction collected by scraping cells in two volumes of SDS sample buffer, and boiled. The amount of actin in each fraction was determined by gel electrophoresis, immunoblotting, and densitometry as described above.
RNA interference
Small interfering RNA (siRNA)-mediated knockdown of α-adducin, γ-adducin, and βII-spectrin was carried out as described previously (Ivanov et al., 2007, 2009, 2010) using isoform-specific siRNA pools (Santa Cruz Biotechnology) or SmartPools (Dharmacon RNA Technologies, Lafayette, CO). Cyclophilin B siRNA SmartPool was used as a control. SK-CO15, HPAF-II, and A549 cells were transfected using the DharmaFect 1 transfection reagent (Dharmacon RNA Technologies) in Opti-MEM I medium (Invitrogen) according to the manufacturer's protocol, with a final siRNA concentration of 100 nM. Cells were used in experiments 3–4 d after transfection.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time PCR Analysis of Gene Expression
Total RNA was isolated using the RNeasy mini kit (QIAGEN, Valencia CA) followed by DNAse treatment to remove traces of genomic DNA. Total RNA (1 μg) was reverse transcribed into cDNA using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Conventional RT-PCR was performed using HotStart TAQ plus kit (QIAGEN) in MJ-mini thermal cycler (Bio-Rad Laboratories) and the amplified fragments were visualized in ethidium bromide-stained gels. Quantitative real-time PCR was performed with 2 μg of cDNA per reaction by using IQ SYBR Green Supermix (Bio-Rad Laboratories) and Opticon DNA Engine Opticon thermocycler (MJ Research, Watertown, MA). The following primers were used: human β-actin (NM_001101.3), forward-CGAGGCCCAGAGCAAGAGAG, reverse-CGGTTGGCCTTAGGGTTCAG; α-adducin (NM_176801.1), forward-TCTGGGCTACAGAACTGGCT, reverse-TCTTCGACTTGGGACTGCTT; β-adducin (NM_017484.2), forward-GAAAATGAGCGAAGAGACGG, reverse-TTCAGGGAGTCAGCTGTGTG; γ-adducin (NM_016824.3), forward-CACCTCCTCTCAGTCTTGGC, reverse-GCTGTTGCAAGGGTATGGAT; and βII-spectrin (nonerythroid; NM_006946.2), forward-CTTCAAGCTGGGCTTACAGG, reverse-TCTCGTTCTCTGCCGTCTTT. In all cases, primers were designed to amplify all known transcript variants of the selected genes. Threshold cycle number for the gene of interest was calculated based on the amplification curve representing a plot of the fluorescent signal intensity versus the cycle number. Delta threshold cycle number was calculated as a difference between threshold cycle numbers of adducin-siRNA– and control siRNA-transfected cells, and each value was normalized by the difference in the threshold cycle number for the housekeeping gene amplification in the same samples.
Epithelial Barrier Permeability Measurements
Transepithelial electrical resistance (TEER) was measured using an EVOMX voltohmmeter (World Precision Instruments, Sarasota, FL). The resistance of cell-free collagen-coated filters was subtracted from each experimental point.
Statistics
Numerical values from individual experiments were pooled and expressed as mean ± SEM throughout. Obtained numbers were compared by either two-tailed Student's t test or one-way analysis of variance with Bonferroni's multiple comparison test, with statistical significance assumed at p < 0.05.
RESULTS
Coexpression and Junctional Localization of α- and γ-Adducins in Human Intestinal Epithelial Cells
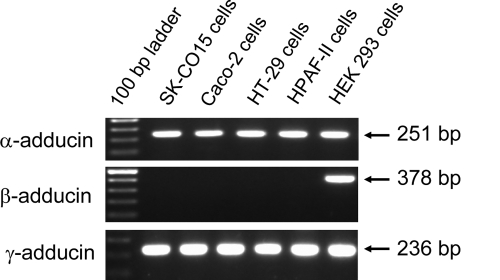
Because adducins function as oligomers composed of different subunits, we first investigated which of their subunits are expressed in human epithelial cells. RT-PCR analysis with isoform-specific primers was performed on RNA samples isolated from human intestinal (SK-CO15, Caco-2, and HT-29), pancreatic (HPAF-II), and renal (human embryonic kidney [HEK] 293) epithelial cells. Figure 1 shows abundant expression of α- and γ-adducin mRNA in all epithelial cells tested. By contrast, expression of β-adducin message was observed in HEK293 cells but was undetectable in intestinal and pancreatic epithelial cell lines. These results are consistent with previously published tissue expression data (Joshi et al., 1991; Dong et al., 1995; Ferrandi et al., 2010) and indicate that α- and γ-adducins are the predominant isoforms of this protein in human epithelia.
Figure 1.
mRNA expression of different adducin isoforms in cultured human epithelial cells. Expression of different adducin isoforms was analyzed by RT-PCR using isoform-specific primers. Note abundant expression of α- and γ-adducin mRNA in cultured human intestinal, pancreatic, and renal epithelial cells and β-adducin expression restricted to HEK293 renal epithelial cells.
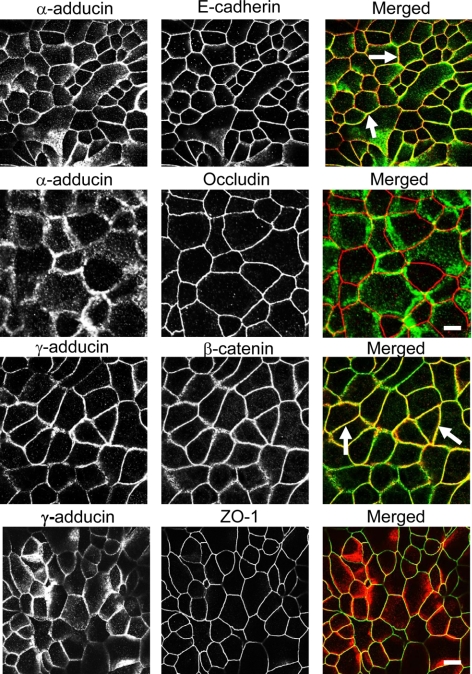
To examine the role of adducins in the regulation of apical junctions we used the human colonic epithelial cell line SK-CO15. These cells are known to rapidly form high-resistance monolayers with well-defined AJs and TJs (Le Bivic et al., 1989; Ivanov et al., 2007, 2010). Furthermore, SK-CO15 cells are amenable for siRNA-mediated gene knockdown (Ivanov et al., 2007, 2009, 2010). These features make SK-CO15 cells a valuable model to study the structure and regulation of epithelial junctions. By using dual immunolabeling and confocal microscopy, we found that α- and γ-adducin labeling was restricted to the areas of cell–cell contact in high-resistance SK-CO15 cell monolayers where these proteins significantly colocalized with the AJ components E-cadherin and β-catenin (Figure 2, arrows). By contrast, no significant colocalization of adducins with the TJ proteins occludin and ZO-1 was observed (Figure 2), which suggests a selective association of adducins with AJ.
Figure 2.
Adducins colocalize with AJs in polarized intestinal epithelial cell monolayers. Confluent, high-resistance SK-CO15 cell monolayers were dual immunolabeled for adducin isoforms and either AJ or TJ proteins. Note significant colocalization of α-adducin (green) with E-cadherin (red) and γ-adducin (red) with β-catenin (green) at mature AJs (arrows) and lack of significant colocalization adducins with TJ proteins. Bar, 10 μm.
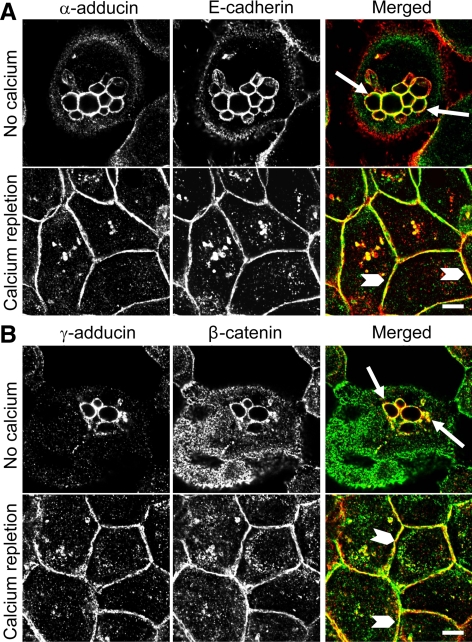
We next used the calcium switch model to investigate whether adducins are recruited to AJs at early stages of junctional assembly. Figure 3 shows that in contact-naïve, calcium-depleted SK-CO15 cells, E-cadherin and β-catenin localized predominantly in the cytoplasm where they frequently appeared within large vacuoles (arrows). These vacuoles were devoid of TJ proteins (data not shown) and may represent a storage compartment for the lateral plasma membrane components that has been identified previously in nonpolarized epithelial cells (Low et al., 2000; Ivanov et al., 2004b). Interestingly, α- and γ-adducins accumulated in the intracellular vacuoles together with AJ proteins (Figure 3, arrows). Readdition of extracellular calcium (calcium repletion) triggered rapid (within 1 h) translocation of cytosolic E-cadherin and β-catenin to the areas of cell–cell contact (Figure 3, arrowheads). Similarly, α- and γ-adducins demonstrated a dramatic relocalization to the newly forming AJ-like junctions (Figure 3). These data suggest a close association of adducins with epithelial AJ at different stages of junctional biogenesis.
Figure 3.
Adducins colocalize with AJ proteins in contact-naive epithelial cells and are recruited to newly forming AJ-like junctions. SK-CO15 cell monolayers were subjected to overnight extracellular calcium depletion to disassemble all intercellular contacts followed by 1 h of calcium repletion to induce junctional reassembly. Immunolabeling and confocal microscopy show colocalization of α-adducin (green) and E-cadherin (red) or γ-adducin (red) and β-catenin (green) in contact-naive epithelial cells (arrows) and in newly assembled AJ-like junctions (arrowheads). Bar, 5 μm.
Depletion of α- and γ-Adducins Mutually Decreased Each Others Protein Level without Affecting the Development of the Epithelial Barrier and Establishment of Apical Junctions
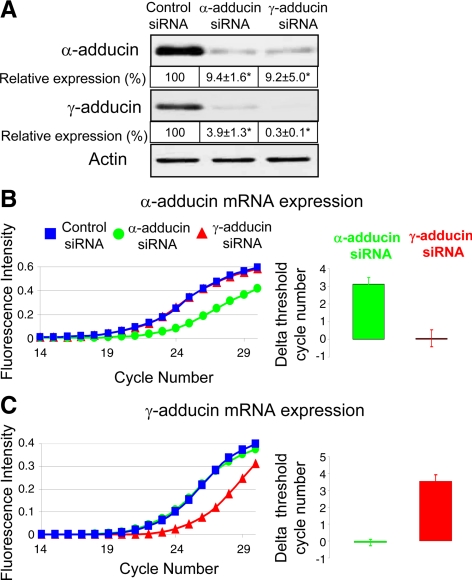
To identify the roles adducins play in regulation of apical junctions we down-regulated expression of these proteins in SK-CO15 cells by using the RNAi approach. Different siRNA pools specific for α- and γ-adducins and composed of either three (Santa Cruz Biotechnology) or four (Dharmacon RNA Technologies) distinct duplexes, were used to minimize possible off-target effects of RNAi (Jackson and Linsley, 2010). All data presented were obtained using siRNA pools from Santa Cruz Biotechnology. However, similar results were obtained with siRNA SmartPools (data not shown). Figure 4A shows that α- and γ-adducin–specific siRNA decreased expression of targeted proteins by ∼90 and 99%, respectively, on day 4 after transfection. Interestingly, α-adducin siRNA dramatically decreased protein levels of the γ isoform and vice versa (Figure 4A). Such mutual down-regulation of adducin subunits was evident only at their protein levels, but did not affect mRNA expression (Figure 4, B and C). This indicates that the observed phenomenon was not due to poor selectivity of adducin isoform-specific siRNAs but rather reflects the fact that expression of one adducin subunit stabilizes the other subunit in the cell. Our results are consistent with recent data obtained with adducin isoform knockout mice. Indeed, lack of β and γ subunits have been observed in erythrocytes and platelets of homozygous α-adducin null mice (Robledo et al., 2008), whereas the α subunit level has been found to be decreased in platelets and kidneys of γ-adducin null animals (Sahr et al., 2009). The observed mutual stabilization of adducin isoforms together with the aforementioned localization data strongly suggests that α and γ heterooligomers represent the major functional adducin module in human intestinal epithelial cells.
Figure 4.
siRNA-mediated depletion of α- and γ-adducins decreases each others protein level but does not affect mRNA expression. (A) Immunoblotting analysis shows that α-adducin–specific siRNAs decrease protein expression of both α and γ isoforms of adducin in SK-CO15 cells. Similar effects can be observed after siRNA-mediated knockdown of γ-adducin; *p < 0.05 compared with the control siRNA-treated group (n = 3). (B and C) Real-time quantitative RT-PCR data presented as individual amplification graphs and the calculated normalized delta threshold cycle number demonstrates specific decrease in mRNA levels of α-adducin (B) and γ-adducin (C) by corresponding siRNAs without altering mRNA expression of the other isoform.
To study the effects of adducin knockdown on the development of epithelial barrier, SK-CO15 cells plated on membrane filters were transfected with either control or adducin isoform-specific siRNA, and barrier development was monitored by measuring TEER on days 2–4 after transfection. No significant differences in TEER values between control and α- or γ-adducin–deficient cell monolayers was observed (Supplemental Figure 1). Furthermore, immunofluorescence labeling demonstrated that depletion of α- (Supplemental Figure 2) or γ-adducins (Supplemental Figure 3) did not result in major defects to AJ or TJ structure on day 4 after transfection. In addition, confocal xz sections showed either no effect of α-adducin depletion on the cell height or just a small (<10%) decrease of the cell height in confluent γ-adducin deficient SK-CO15 cell monolayers (Supplemental Figure 4). Our data contrast with previous observations that α-adducin knockdown caused a collapse of the lateral plasma membrane in human bronchial epithelial cells (Abdi and Bennett, 2008), which can be attributed to the cell specificity of such a response. Together, these results indicate that adducins are dispensable for development of the paracellular barrier and formation of normal apical junctions in SK-CO15 cell monolayers.
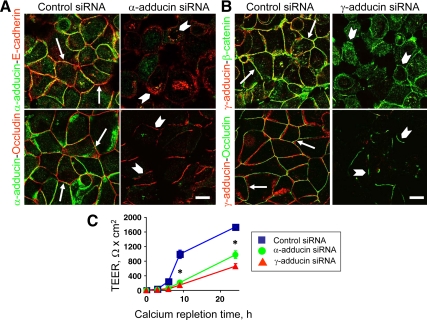
Depletion of α- and γ-Adducins Attenuated Reassembly of AJs and TJs in SK-CO15 Cell Monolayers Subjected to the Calcium Switch
A prolonged junctional assembly that occurs while epithelial cells reach confluence and acquire apicobasal polarity probably involves several redundant mechanisms, which can compensate for/mask the loss of important A and /TJ components and regulators (McNeil et al., 2006; Capaldo and Macara, 2007; Ivanov et al., 2007, 2010). However, functional roles of junction-affiliated proteins can be unmasked during a rapid and orchestrated calcium-dependent remodeling (disassembly and reassembly) of cell–cell contacts (McNeil et al., 2006; Capaldo and Macara, 2007; Ivanov et al., 2007, 2010). Therefore, we next analyzed whether adducins are essential for junctional reassembly by using the calcium switch model. SK-CO15 cells were transfected with control, α– or γ-adducin–specific siRNAs and on day 3 after transfection were subjected to overnight calcium depletion followed by calcium repletion for 1 or 3 h to allow reassembly of AJs or TJs, respectively. Figure 5, A and B shows that control cells rapidly reestablished the majority of their AJs, which was manifested by accumulation of E-cadherin and β-catenin in the areas of cell–cell contact (Figure 5, A and B arrows). By contrast, significant fractions of AJ proteins remained intracellularly and did not translocate to intercellular junctions in adducin-depleted cells (Figure 5, A and B arrowhead). Morphometric analysis of confocal images presented in Table 1 shows a significantly smaller length of reassembled AJs in α- or γ-adducin–depleted SK-CO15 cells compared with control siRNA-transfected cells.
Figure 5.
Down-regulation of adducin expression attenuates reassembly of AJs and TJs and reestablishment of the paracellular barrier. Reassembly of AJs and TJs was examined in control, α-adducin– (A) or γ-adducin (B)–depleted SK-CO15 cells during 1 and 3 h of calcium repletion, whereas recovery of the paracellular barrier was monitored during 24 h of calcium repletion (C). Immunofluorescence labeling and confocal microscopy demonstrates accumulation of the majority of AJ and TJ proteins at the intercellular contacts in control cell monolayers (arrows), whereas in α- and γ-adducin–deficient cells substantial amounts of E-cadherin and β-catenin remain in the cytoplasm and only short defective occludin-based TJs are formed (arrowheads). Bar, 10 μm. Permeability measurements show significant attenuation of the TEER increase in adducin-depleted cell monolayers. *p < 0.05 compared with the control siRNA-treated group (n = 3).
Table 1.
Effect of adducin knockdown on the length of adherens junctions and tight junctions assembled after 1 and 3 h of calcium repletion, respectively
| Avg. junctional length (μm) | Control siRNA | α-Adducin siRNA | γ-Adducin siRNA |
|---|---|---|---|
| Adherens junctions | 603 ± 73 | 96 ± 70* | 57 ± 25* |
| Tight junctions | 755 ± 114 | 159 ± 37* | 88 ± 39* |
Data are presented as mean ± SE (n = 9).
* p < 0.0001.
After 3 h of calcium repletion, control SK-CO15 cells demonstrated a characteristic “chicken wire” labeling pattern for occludin (Figure 5, A and B arrows) that is indicative of TJ reassembly. By contrast, α- or γ-adducin–deficient cell monolayers revealed only short, disconnected occludin-based TJ strands (Figure 5, A and B arrowheads). A quantitative image analysis shows that depletion of adducins significantly decreased the length of TJ that were reestablished during 3 h of calcium repletion (Table 1). These morphological data were reinforced by permeability measurements that demonstrated a significant delay in TEER recovery in α- or γ-adducin–depleted SK-CO15 cell monolayers subjected to the calcium switch (Figure 5C).
To ensure physiological relevance of our results obtained using the calcium switch model, we analyzed effects of α-adducin knockdown on AJ recovery after exposure of epithelial cells to a junction-disrupting EGF. Because EGF failed to induce AJ and TJ disassembly in SK-CO15 cells even after 48-h treatment (data not shown), these experiments were performed using EGF-sensitive A549 human lung epithelial cells. Exposure of serum-starved A549 cells for 24 h to EGF caused disruption of cell–cell contacts as was indicated by the diffuse β-catenin labeling (Supplemental Figure 5). Removal of EGF resulted in restoration of the continuous AJ belt in control A549 cells 24 h after the growth factor wash out (arrows). siRNA-mediated knockdown of α-adducin in A549 cells decreased by ∼98% its protein level (data not shown). By contrast, post-EGF reassembly of AJs was attenuated in α-adducin–depleted cells that were able to restore only discontinuous spot-like junctions (Supplemental Figure 5, arrowheads). Together, these results strongly suggest that α and γ-adducins regulate the velocity of AJ and TJ assembly and formation of the paracellular barrier.
Knockdown of Adducins Altered Expression and Junctional Localization of βII-Spectrin and Down-Regulation of βII-Spectrin Mimicked Effects of Adducin Depletion on Junctional Assembly
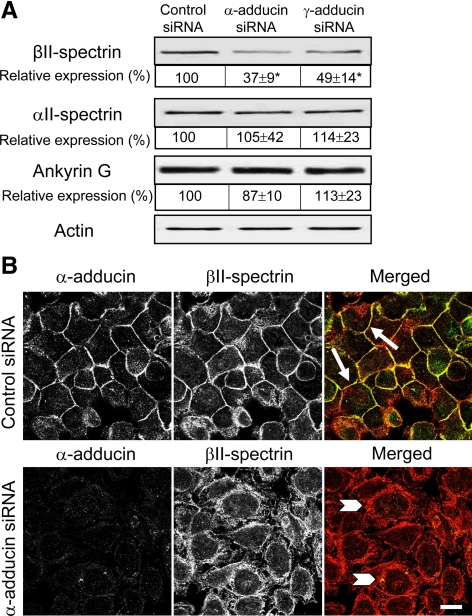
We next sought to dissect mechanisms that are responsible for the observed delay of AJ and TJ reassembly in adducin-depleted SK-CO15 cells. Given the known role of adducins in regulating the spectrin-based plasma membrane skeleton (Matsuoka et al., 2000; Bennett and Baines, 2001), it is reasonable to suggest that decreased expression of adducins may affect organization and stability of the membrane-associated spectrin lattice. To test this hypothesis, we first analyzed whether down-regulation of adducins alters expression of spectrin subunits and ankyrin G, which is a key linker of the spectrin skeleton to the plasma membrane in epithelial cells (Bennett and Baines, 2001; Bennett and Healy, 2008). Figure 6A shows that expression of βII-spectrin was significantly decreased by ∼63 and 51% in α- and γ-adducin–depleted SK-CO15 cells, respectively. By contrast, the protein levels of αII-spectrin and ankyrin G were unaffected by adducin knockdown. Such down-regulation of βII-spectrin was evident only at the protein level and did not involve changes in mRNA expression (Supplemental Figure 6).
Figure 6.
siRNA-mediated depletion of adducins attenuates expression and junctional recruitment of βII-spectrin. (A) Immunoblots of cell lysates collected from control and α- and γ-adducin–depleted SK-CO15 cell monolayers demonstrates selective decrease of βII-spectrin protein level in adducins knockdowns. *p < 0.05 compared with the control siRNA-treated group (n = 3). (B) Immunofluorescence labeling and confocal microscopy show βII-spectrin recruitment to intercellular junctions during 1 h of calcium repletion in control cells (arrows). By contrast, βII-spectrin remains intracellularly in α-adducin–depleted cells (arrowheads). Bar, 10 μm.
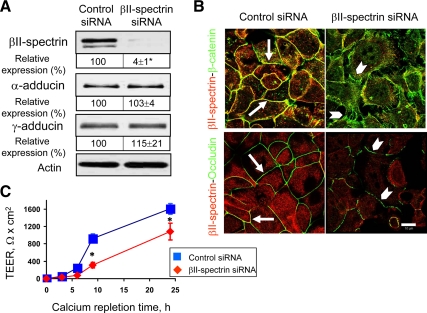
Importantly, adducin knockdown not only decreased the total amount of βII-spectrin protein but also impaired its junctional recruitment. Figure 6B shows that in control SK-CO15 cell monolayers, βII-spectrin rapidly accumulated at newly-assembled AJs during 1 h of calcium repletion (arrows). By contrast, βII-spectrin was retained in a perinuclear compartment and did not translocate to intercellular junctions in α-adducin–deficient cells (Figure 6B, arrowheads). If the decreased expression and impaired junctional recruitment of βII-spectrin contribute to attenuated AJ and TJ assembly caused by down-regulation of adducins, one can predict that down-regulation of βII-spectrin should also inhibit junctional reassembly. To test this hypothesis, βII-spectrin expression was down-regulated in SK-CO15 cells by using specific siRNAs. Figure 7A shows that such RNAi resulted in ∼96% decrease in βII-spectrin protein level without significant effects on α- and γ-adducin expression. As expected, depletion of βII-spectrin attenuated calcium-dependent recruitment of β-catenin and occludin to the areas of cell–cell contact (Figure 7B, arrowheads) compared with AJ and TJ reassembly in control cells (arrows). Furthermore, βII-spectrin knockdown significantly inhibited recovery of TEER in calcium-repleted SK-CO15 cell monolayers (Figure 7C). Together, these data suggest that down-regulation of βII-spectrin is likely to mediate delayed reassembly of apical junctions and reformation of epithelial barrier in adducin-depleted SK-CO15 cells.
Figure 7.
Down-regulation of βII-spectrin expression attenuates junctional reassembly and reestablishment of the paracellular barrier. (A) Immunoblots show a dramatic decrease in βII-spectrin protein expression on day 4 of siRNA-mediated depletion of βII-spectrin in SK-CO15 cells, whereas protein levels of α- and γ-adducin remain unaffected; *p < 0.05 compared with the control siRNA-treated group (n = 3). (B) Immunofluorescence labeling and confocal microscopy show rapid reassembly of β-catenin-based AJs and occludin-based TJs (green) during 1- and 3-h calcium-repletion of control cells (arrows) and attenuation of AJ and TJ reassembly (arrowheads) in βII-spectrin–depleted cell monolayers. Bar, 10 μm. (C) Permeability measurements show significant attenuation in the increase of TEER in βII-spectrin–deficient cell monolayers. *p < 0.05 compared with the control siRNA-treated group (n = 3).
Depletion of Adducins Impaired Formation of Perijunctional F-Actin Bundles
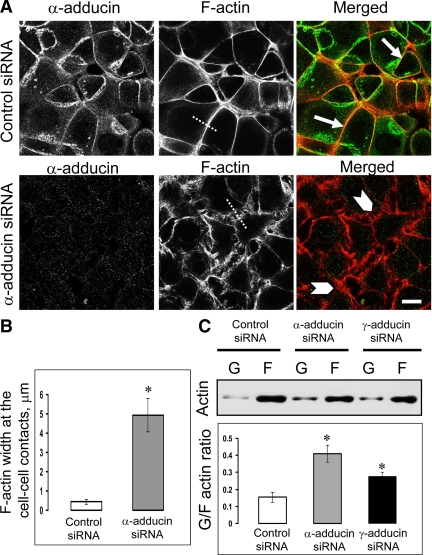
Given extensive biochemical studies that have implicated adducins in mediating associations between the spectrin lattice and actin filaments, one can expect that depletion of adducins affects not only assembly of spectrin polymers but also F-actin organization at intercellular junctions. To test this hypothesis, we examined the architecture of the actin cytoskeleton associated with newly formed AJs after 1 h of calcium repletion. Figure 8A shows that control SK-CO15 cells readily assembled prominent F-actin bundles that run in parallel to the cell–cell contact area (arrows). By contrast, a more diffuse network of actin filaments running either in parallel or perpendicular to intercellular contacts was observed in α-adducin–depleted cells (Figure 8A, arrowheads). Measurements of the lateral thickness of actin filaments populating the cell–cell contact zone demonstrated a much narrower distribution of perijunctional F-actin in the control as compared with α-adducin–depleted cells (Figure 8B); this indicates disorganization of the perijunctional F-actin bundles caused by α-adducin knockdown. siRNA-mediated knockdown of γ-adducin similarly impaired the assembly of actin filaments associated with newly forming junctions (data not shown). Furthermore, biochemical analysis of the amount of monomeric (G-) and polymeric (F)-actin in SK-CO15 cells demonstrated that down-regulation of adducins significantly increased the G/F actin ratio (Figure 8C), thereby indicating defects in actin filament assembly. Interestingly, stationary adducin-depleted SK-CO15 cells exhibited a well-formed perijunctional F-actin belt (Supplemental Figure 7, arrows), which indicates that these proteins are essential for the remodeling but can be dispensable for the steady-state integrity of junction-associated F-actin bundles.
Figure 8.
Down-regulation of adducins affects organization of the actin cytoskeleton. (A) Fluorescence labeling shows rapid formation of the circumferential F-actin belt at the intercellular junctions of control SK-CO15 cells during 1 h of calcium repletion (arrows). By contrast, α-adducin–depleted cells demonstrate diffuse and disorganized perijunctional actin filaments (arrowheads). Bar, 10 μm. (B) Measurements of the lateral width of perijunctional F-actin on cross-sections through cell-cell contacts (indicated by dotted lines on Figure 8A) show significantly less compact F-actin bundles in α-adducin–deficient SK-CO15 cells compared with control cell monolayers. *p < 0.0001 compared with control siRNA-transfected cells (n = 50). (C) Immunoblotting analysis of monomeric (G-) and filamentous (F)-actin fractions demonstrates significant increase in the G/F actin ratio in α- and γ-adducin–depleted cells. *p < 0.05 (n = 3).
Depletion of Adducins Accelerated PKC-induced Junctional Disassembly in Pancreatic Epithelial Cells
Given our findings that adducins can promote the establishment of epithelial AJs and TJs, it is reasonable to suggest that these proteins also should antagonize junctional disassembly. We tested this by examining the effects of adducin knockdown on disruption of AJs and TJs induced by PKC-activating phorbol ester in HPAF-II human pancreatic epithelial cells. This model of junctional disassembly was chosen for two major reasons. First, PKC is known to phosphorylate adducins at several serine residues (Ser726, Ser712, and Ser660) in their C-terminal MARCKS domain (Fowler et al., 1998; Matsuoka et al., 1998). Such phosphorylation has been shown to inhibit the functions of adducins by decreasing their associations with actin filaments and spectrin (Fowler et al., 1998; Matsuoka et al., 1998; Barkalow et al., 2003). Second, PKC activation has been shown to induce AJ and TJ disruption in several types of epithelia in a cytoskeleton-dependent manner (Kamei et al., 1999; Krendel et al., 1999; Ivanov et al., 2009). HPAF-II cells were chosen for this experiment because they readily respond to TPA treatment with AJ and TJ disassembly (Ivanov et al., 2009), whereas SK-CO15 cell junctions appear to be less sensitive to such PKC activation (data not shown).
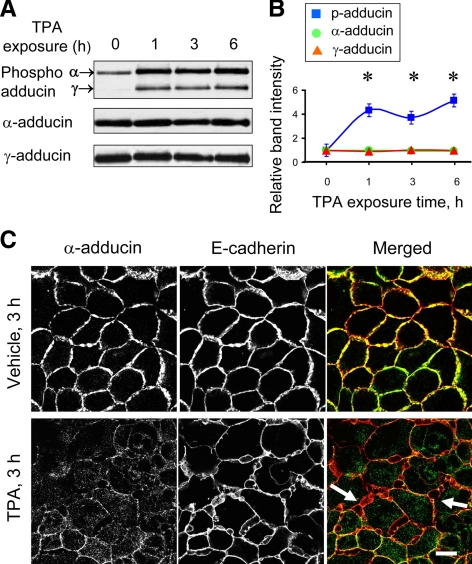
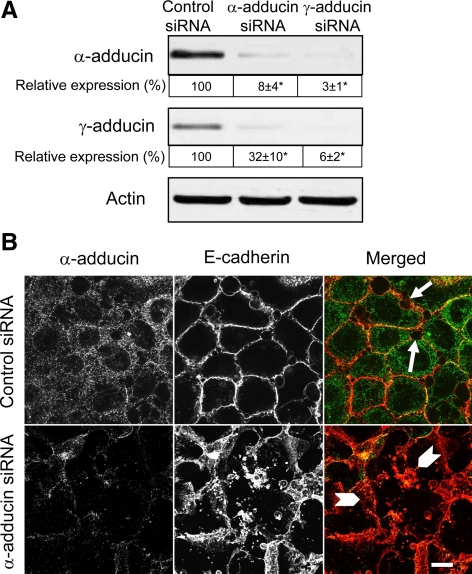
Exposure of HPAF-II cells to TPA-induced rapid (within 1 h) and substantial (up to fivefold) increase in the level of phosphorylated adducin without significantly changing the total protein level of the α and γ subunits (Figure 9, A and B). The phospho-antibody, which is specific for the Ser726 PKC-phosphorylation site of α-adducin, recognized two bands at ∼120 and 90 kDa, the phosphorylated α and γ subunits, respectively (Figure 9A). Furthermore, dual immunofluorescence labeling and confocal microscopy demonstrated that PKC activation altered both the structure of epithelial junctions and adducin localization in confluent HPAF-II cell monolayers. Indeed, Figure 9C shows that TPA exposure caused formation of extensive invaginations of E-cadherin–based intercellular contacts (arrows) that were eventually transformed into intracellular vacuoles. This process was accompanied by fragmentation and cytosolic translocation of TJ (data not shown). Interestingly, both α-adducin (Figure 9C) and γ-adducin (data not shown) disappeared from disassembling junctions in TPA-exposed epithelial cells. Such relocalization of adducins paralleled the increase in their phosphorylation and was evident already after 1 h of TPA treatment before signs of significant junctional disassembly (data not shown). The early disappearance of junctional adducins after PKC activation suggests that this event may contribute to subsequent AJ and TJ disassembly. We tested this by examining the effects of adducin depletion on TPA-dependent junctional breakdown. Similar to SK-CO15 cells, α- and γ-adducins were dramatically and interdependently down-regulated by isoform-specific siRNAs in HPAF-II cells (Figure 10A). Furthermore, depletion of α-adducin substantially exaggerated disruption of E-cadherin–based AJs after 3 h of TPA exposure (Figure 10B). Similar results were obtained after depletion of the γ isoform (data not shown). Overall, these data suggest that phosphorylation-dependent removal of adducins from intercellular junctions is a prerequisite for the disruption of AJs and TJs caused by PKC activation.
Figure 9.
PKC activation induces adducin phosphorylation and triggers disappearance of α-adducin from intercellular junctions and AJ disassembly. (A) Representative immunolots. (B) Densitometric quantification shows an increase in the amount of phosphorylated (p) but not total α- and γ-adducins in HPAF-II cells treated with TPA (1 μM). *p < 0.05 compared with the vehicle-treated control (n = 3). (C) Double-fluorescence labeling of E-cadherin (red) and α-adducin (green) shows that TPA exposure triggers α-adducin disappearance from intercellular junctions and disassembly/internalization of AJs (arrows) in HPAF-II cell monolayers. Bar, 10 μm.
Figure 10.
siRNA-mediated depletion of α-adducin accelerates TPA-induced AJ disassembly. (A) Immunoblots of cell lysates collected from control and α- or γ-adducin siRNA-transfected HPAF-II pancreatic cell monolayers demonstrate a dramatic decrease in adducins expression on day 4 after transfection. *p < 0.05 compared with control siRNA-transfected cells (n = 3). (B) Control and α- adducin–depleted HPAF-II cells were exposed to TPA for 3 h. Immunofluorescence labeling and confocal microscopy show phorbol ester-induced AJ fragmentation and internalization of E-cadherin-containing vesicles (arrows) in control cell monolayer. Such AJ disintegration is significantly exaggerated in α-adducin–depleted HPAF-II cells (arrowheads). Bar, 10 μm.
DISCUSSION
Adducins Regulate Remodeling of AJs and TJs in Human Epithelial Monolayers
Although several previous studies have demonstrated the association of adducins with intercellular contacts in different mammalian epithelia (Kaiser et al., 1989; Dong et al., 1995; Abdi and Bennett, 2008), the functional roles of this association remain elusive. Our study provides the first evidence that α- and γ-adducins positively regulate cell–cell adhesions in model human epithelia by promoting assembly and impeding disassembly of AJs and TJs. This conclusion is supported by the findings that RNAi-mediated depletion of adducins attenuated calcium-dependent assembly of apical junctions in SK-CO15 intestinal epithelial cells (Figure 5) as well as post-EGF reassembly of AJs in A549 cells (Supplemental Figure 5). Interestingly, adducin depletion impaired formation of both AJs and TJs, although this effect on TJ assembly is probably indirect. Indeed, adducins consistently colocalized with AJ proteins in cytoplasmic vacuoles of contact-naïve cells (Figure 3), nascent AJ-like junctions (Figure 3), and mature intercellular contacts in polarized cell monolayers (Figure 2). In contrast, no significant colocalization of adducins with TJ proteins has been observed (Figure 2; data not shown). Furthermore, AJ assembly is known to be an early step of epithelial morphogenesis, which regulates subsequent formation of TJs (Takai and Nakanishi, 2003; Capaldo and Macara, 2007). Based on these data, we believe that depletion of adducins directly impairs the establishment of epithelial AJs which in turn attenuates TJ assembly.
Consistent with their positive role in formation of AJs and TJs, adducins can antagonize junctional disassembly. This protective effect of adducins was obvious during disruption of the epithelial barrier by PKC-activating phorbol ester, which readily phosphorylates adducins (Figure 9, A and B), thereby inhibiting their associations with spectrin and actin filaments (Fowler et al., 1998; Matsuoka et al., 1998; Barkalow et al., 2003). Indeed, we found that TPA-induced phosphorylation of adducins resulted in their rapid disappearance from the intercellular junctions (Figure 9C) of pancreatic epithelial cells, and that this loss of adducins preceded junctional disassembly (data not shown). Furthermore, adducin depletion significantly accelerated disruption of AJs and TJs induced by PKC activation (Figure 10B). It is likely therefore that PKC-dependent phosphorylation of adducins triggers their early release from complexes with perijunctional spectrin and actin filaments, thereby destabilizing AJ and TJ structure and accelerating junctional disassembly. Destabilization of epithelial junctions after adducin depletion is consistent with the results of a previous study in bronchial epithelial cells where α-adducin knockdown increased long-range intramembrane diffusion of E-cadherin (Abdi and Bennett, 2008). It should be noted that other kinases, such as protein kinase A and Rho-dependent kinase, phosphorylate α-adducin and alter its cellular distribution and activity (Kimura et al., 1998; Matsuoka et al., 1998). It is tempting to speculate that adducins can be important downstream effectors of different protein kinases that regulate integrity and remodeling of epithelial junctions.
Adducins Regulate Epithelial AJs and TJs by Altering Organization of Perijunctional Spectrin and F-Actin
The present study provides an important insight into the mechanisms that mediate the effects of adducins on epithelial junctions. Given known associations of adducins with different cytoskeletal structures, it is reasonable to hypothesize that they can regulate epithelial junctions by controlling either organization of the spectrin lattice or assembly of actin filaments in the areas of cell–cell contact. Our data support the interplay between these two mechanisms.
Indeed, we found that depletion of adducins decreased expression of βII-spectrin in intestinal epithelial cells (Figure 6A) and attenuated recruitment of this protein to newly forming AJ (Figure 6B). This observation, together with the previously reported increase in detergent solubility of βII-spectrin in α-adducin–deficient bronchial epithelial cells (Abdi and Bennett, 2008), suggests that adducin depletion impairs formation of the highly ordered spectrin lattice at the plasma membrane of contacting epithelial cells. Such disorganization of the spectrin lattice is probably responsible for the delayed junctional assembly, because depletion of βII-spectrin consistently attenuated reestablishment of AJs and TJs and reformation of the paracellular barrier in human intestinal epithelial cells (Figure 7) as well as inhibited formation of AJs in human bronchial epithelium (Kizhatil et al., 2007b), and early mouse embryo (Kizhatil et al., 2007a). In epithelial cells, spectrin is known to form complexes with E-cadherin (Nelson et al., 1990; Kizhatil et al., 2007a) and α-catenin (Pradhan et al., 2001). Such complexes that also involve a spectrin accessory protein ankyrin G are thought to be important for E-cadherin trafficking from the Golgi-to-intercellular junctions (Kizhatil and Bennett, 2004; Kizhatil et al., 2007a). Hence, decreased assembly of the spectrin lattice in adducin-depleted epithelial cells may result in attenuated delivery or diminished retention of AJ proteins at the areas of cell–cell contact.
Another important mechanism that can mediate destabilization of apical junctions in adducin-depleted epithelial cells involves impaired formation of the perijunctional F-actin belt. A key role of F-actin in junctional regulation is supported by an extensive literature that describes impaired formation of AJs and TJs after pharmacological depolymerization of actin filaments or genetic depletion of a number of actin-binding proteins (Ivanov et al., 2005a, 2007; Shen and Turner, 2005; Shewan et al., 2005; Scott et al., 2006; Smutny et al., 2010). Previous biochemical studies described the ability of α-adducin to cap (Kuhlman et al., 1996; Li et al., 1998) and bundle (Mische et al., 1987; Taylor and Taylor, 1994) actin filaments in cell-free systems. The present study demonstrates for the first time that adducins regulate organization of the F-actin cytoskeleton in epithelial cells. This conclusion is supported by findings that siRNA-mediated depletion of α- and γ-adducins increased the G/F actin ratio (Figure 8C), which indicates either impaired polymerization or enhanced depolymerization of actin filaments. Furthermore, down-regulation of adducins attenuated assembly of cell–cell contact-associated F-actin bundles during reestablishment of epithelial AJs (Figure 8, A and B) without preventing the eventual formation of the perijunctional F-actin belt (Supplemental Figure 7). Given the previous results from our group (Ivanov et al., 2005a, 2007) and others (Perez-Moreno et al., 2003; Yap et al., 2007; Cavey and Lecuit, 2009) showing that impaired formation of circumferential F-actin bundles prevent junctional assembly, it is likely that this mechanism contributes to the attenuated formation of AJs and TJs in adducin-depleted intestinal epithelial cells.
Despite the well-known actin binding ability of purified adducins, only a few studies have demonstrated a role for these proteins in the regulation of F-actin organization and remodeling in a cellular context. For example, mutations of a Drosophila homologue of adducin has been shown to prevent formation of characteristic F-actin rings associated with the egg chamber of flies (Yue and Spradling, 1992). Furthermore, γ-adducin has been recently implicated in F-actin rearrangements that lead to endothelial tube formation during angiogenesis (Matou-Nasri et al., 2009). What type of adducin-F-actin interactions (filament capping or cross-linking) are involved in such cytoskeletal remodeling and whether spectrin is essential for these events remain to be determined. It is also unclear whether the effects of adducin depletion on reorganization of actin filaments and assembly of spectrin lattice at intercellular junctions represent two distinct mechanisms or whether they are mutually dependent. However, based on a classical model of adducin action, it is likely that lack of these scaffolding proteins breaks a physical link between spectrin oligomers and actin filaments that is important for proper organization of both cytoskeletal structures at the plasma membrane.
In conclusion, our study reveals novel roles for adducins in regulation of apical junction in model human epithelia. We observed that adducins promote assembly of AJs and TJs and antagonize stimulus-induced junctional disassembly. This novel function of adducins could be important for remodeling of epithelial junctions during embryonic morphogenesis, a steady-state rejuvenation of epithelial layers, and restoration of epithelial barrier during wound healing. Furthermore, given the fact that adducins can be rapidly removed from intercellular junctions and degraded by cytokines (Pariser et al., 2005), invading bacteria (Chu et al., 2008), and proapoptotic agents (van de Water et al., 2000), adducins dysfunctions could mediate disruption of epithelial barriers during infection and inflammation. Further studies are required to test the roles of these membrane skeleton proteins in mucosal diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Linda Callahan for help with Olympus confocal microscope, Claudia Jasalavich for editing this manuscript, and Dr. Victor Morales for statistical data analysis. This work was supported by a National Institutes of Health grant DK-084953 and a Senior Research Award from the Crohn's and Colitis Foundation of America (to A.I.I.).
Abbreviations used:
- AJ
adherens junction
- PKC
protein kinase C
- RNAi
RNA interference
- TEER
transepithelial, electrical resistance
- TJ
tight junction
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TX-100
Triton X-100
- ZO
zonula occludens.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-03-0259) on August 25, 2010.
REFERENCES
- Abdi K. M., Bennett V. Adducin promotes micrometer-scale organization of beta2-spectrin in lateral membranes of bronchial epithelial cells. Mol. Biol. Cell. 2008;19:536–545. doi: 10.1091/mbc.E07-08-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M. Physiology and function of the tight junction. Cold Spring Harbor Perspect. Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines A. J. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem. Soc. Trans. 2009;37:796–803. doi: 10.1042/BST0370796. [DOI] [PubMed] [Google Scholar]
- Barkalow K. L., Italiano J. E., Jr, Chou D. E., Matsuoka Y., Bennett V., Hartwig J. H. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J. Cell Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Baines A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bennett V., Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol. Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Stow J. L. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Capaldo C. T., Macara I. G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M., Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harbor Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H. G., Weeks S. K., Gilligan D. M., Rockey D. D. Host alpha-adducin is redistributed and localized to the inclusion membrane in chlamydia- and chlamydophila-infected cells. Microbiology. 2008;154:3848–3855. doi: 10.1099/mic.0.2008/020941-0. [DOI] [PubMed] [Google Scholar]
- Dong L., Chapline C., Mousseau B., Fowler L., Ramsay K., Stevens J. L., Jaken S. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J. Biol. Chem. 1995;270:25534–25540. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Bennett V. Polarized distribution of Mr 210,000 and 190,000 analogs of erythrocyte ankyrin along the plasma membrane of transporting epithelia, neurons and photoreceptors. Eur. J. Cell Biol. 1987;43:479–486. [PubMed] [Google Scholar]
- Ferrandi M., et al. alpha- and beta-Adducin polymorphisms affect podocyte proteins and proteinuria in rodents and decline of renal function in human IgA nephropathy. J. Mol. Med. 2010;88:203–217. doi: 10.1007/s00109-009-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler L., Dong L., Bowes R. C., 3rd, van de Water B., Stevens J. L., Jaken S. Transformation-sensitive changes in expression, localization, and phosphorylation of adducins in renal proximal tubule epithelial cells. Cell Growth Differ. 1998;9:177–184. [PubMed] [Google Scholar]
- Giepmans B. N., van Ijzendoorn S. C. Epithelial cell-cell junctions and plasma membrane domains. Biochim. Biophys. Acta. 2009;1788:820–831. doi: 10.1016/j.bbamem.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Contreras R. G., Bolivar J. J., Ponce A., Chavez De Ramirez B., Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am. J. Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Tapia R., Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Troyanovsky R. B., Troyanovsky S. M. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc. Natl. Acad. Sci. USA. 2010;107:3528–3533. doi: 10.1073/pnas.0911027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J. Biol. Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., Bachar M., Babbin B. A., Adelstein R. S., Nusrat A., Parkos C. A. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Hunt D., Utech M., Nusrat A., Parkos C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2005a;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., McCall I. C., Parkos C. A., Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2004a;15:2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Nusrat A., Parkos C. A. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell. 2004b;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Nusrat A., Parkos C. A. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005b;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., Samarin S. N., Bachar M., Parkos C. A., Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Young C., Beste K. D., Capaldo C. T., Humbert P. O., Brennwald P., Parkos C. A., Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am. J. Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. L., Linsley P. S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- Joshi R., Gilligan D. M., Otto E., McLaughlin T., Bennett V. Primary structure and domain organization of human alpha and beta adducin. J. Cell Biol. 1991;115:665–675. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H. W., O'Keefe E., Bennett V. Adducin: Ca++-dependent association with sites of cell-cell contact. J. Cell Biol. 1989;109:557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei T., Matozaki T., Sakisaka T., Kodama A., Yokoyama S., Peng Y. F., Nakano K., Takaishi K., Takai Y. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells–regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- Kimura K., Fukata Y., Matsuoka Y., Bennett V., Matsuura Y., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J. Biol. Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. J. Biol. Chem. 2004;279:16706–16714. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Davis J. Q., Davis L., Hoffman J., Hogan B. L., Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J. Biol. Chem. 2007a;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Yoon W., Mohler P. J., Davis L. H., Hoffman J. A., Bennett V. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J. Biol. Chem. 2007b;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- Krendel M., Gloushankova N. A., Bonder E. M., Feder H. H., Vasiliev J. M., Gelfand I. M. Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell-cell contacts in cultured epitheliocytes. Proc. Natl. Acad. Sci. USA. 1999;96:9666–9670. doi: 10.1073/pnas.96.17.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman P. A., Hughes C. A., Bennett V., Fowler V. M. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J. Biol. Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc. Natl. Acad. Sci. USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. L., Yap A. S., Stow J. L. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Li X., Matsuoka Y., Bennett V. Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS-related domain and a newly defined oligomerization domain. J. Biol. Chem. 1998;273:19329–19338. doi: 10.1074/jbc.273.30.19329. [DOI] [PubMed] [Google Scholar]
- Low S. H., Miura M., Roche P. A., Valdez A. C., Mostov K. E., Weimbs T. Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol. Biol. Cell. 2000;11:3045–3060. doi: 10.1091/mbc.11.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T. Y., Tran D., Hoa N., Nguyen D., Merryfield M., Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc. Res. Tech. 2000;51:156–168. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Marrs J. A., Napolitano E. W., Murphy-Erdosh C., Mays R. W., Reichardt L. F., Nelson W. J. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K(+)-ATPase distributions in polarized epithelia. J. Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matou-Nasri S., Gaffney J., Kumar S., Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int. J. Oncol. 2009;35:761–773. doi: 10.3892/ijo_00000389. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Li X., Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J. Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y., Li X., Bennett V. Adducin: structure, function and regulation. Cell Mol. Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil E., Capaldo C. T., Macara I. G. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2006;17:1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mische S. M., Mooseker M. S., Morrow J. S. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J. Cell Biol. 1987;105:2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Gallagher P. G. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum L. N., Nelson W. J. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J. Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1986;103:1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen C. M., Gottardi C. J. Molecular components of the adherens junction. Biochim. Biophys. Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris L., Tonutti L., Vannini C., Bazzoni G. Structural organization of the tight junctions. Biochim. Biophys. Acta. 2008;1778:646–659. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Pariser H., Herradon G., Ezquerra L., Perez-Pinera P., Deuel T. F. Pleiotrophin regulates serine phosphorylation and the cellular distribution of beta-adducin through activation of protein kinase C. Proc. Natl. Acad. Sci. USA. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora C., Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Perez T. D., Tamada M., Sheetz M. P., Nelson W. J. Immediate-early signaling induced by E-cadherin engagement and adhesion. J. Biol. Chem. 2008;283:5014–5022. doi: 10.1074/jbc.M705209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitelka D. R., Taggart B. N., Hamamoto S. T. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J. Cell Biol. 1983;96:613–624. doi: 10.1083/jcb.96.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Weis W. I. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Pradhan D., Lombardo C. R., Roe S., Rimm D. L., Morrow J. S. alpha-Catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J. Biol. Chem. 2001;276:4175–4181. doi: 10.1074/jbc.M009259200. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S. A., Gopal J., Espineda C., Ryazantsev S., Schneeberger E. E., Rajasekaran A. K. HPAF-II, a cell culture model to study pancreatic epithelial cell structure and function. Pancreas. 2004;29:e77–e83. doi: 10.1097/00006676-200410000-00016. [DOI] [PubMed] [Google Scholar]
- Robledo R. F., Ciciotte S. L., Gwynn B., Sahr K. E., Gilligan D. M., Mohandas N., Peters L. L. Targeted deletion of alpha-adducin results in absent beta- and gamma-adducin, compensated hemolytic anemia, and lethal hydrocephalus in mice. Blood. 2008;112:4298–4307. doi: 10.1182/blood-2008-05-156000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahr K. E., Lambert A. J., Ciciotte S. L., Mohandas N., Peters L. L. Targeted deletion of the gamma-adducin gene (Add3) in mice reveals differences in alpha-adducin interactions in erythroid and nonerythroid cells. Am. J. Hematol. 2009;84:354–361. doi: 10.1002/ajh.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. A., Shewan A. M., den Elzen N. R., Loureiro J. J., Gertler F. B., Yap A. S. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell. 2006;17:1085–1095. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Turner J. R. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan A. M., Maddugoda M., Kraemer A., Stehbens S. J., Verma S., Kovacs E. M., Yap A. S. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M., Cox H. L., Leerberg J. M., Kovacs E. M., Conti M. A., Ferguson C., Hamilton N. A., Parton R. G., Adelstein R. S., Yap A. S. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Taylor D. W. Formation of two-dimensional complexes of F-actin and crosslinking proteins on lipid monolayers: demonstration of unipolar alpha-actinin-F-actin crosslinking. Biophys. J. 1994;67:1976–1983. doi: 10.1016/S0006-3495(94)80680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H. Spectrin: the ghost in the machine. Bioessays. 2001;23:152–160. doi: 10.1002/1521-1878(200102)23:2<152::AID-BIES1022>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Troyanovsky R. B., Sokolov E. P., Troyanovsky S. M. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol. Biol. Cell. 2006;17:3484–3493. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Turner J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- van de Water B., Tijdens I. B., Verbrugge A., Huigsloot M., Dihal A. A., Stevens J. L., Jaken S., Mulder G. J. Cleavage of the actin-capping protein alpha-adducin at Asp-Asp-Ser-Asp633-Ala by caspase-3 is preceded by its phosphorylation on serine 726 in cisplatin-induced apoptosis of renal epithelial cells. J. Biol. Chem. 2000;275:25805–25813. doi: 10.1074/jbc.M001680200. [DOI] [PubMed] [Google Scholar]
- Volberg T., Geiger B., Kartenbeck J., Franke W. W. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J. Cell Biol. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A. S., Crampton M. S., Hardin J. Making and breaking contacts: the cellular biology of cadherin regulation. Curr. Opin. Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Spradling A. C. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992;6:2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.