Abstract
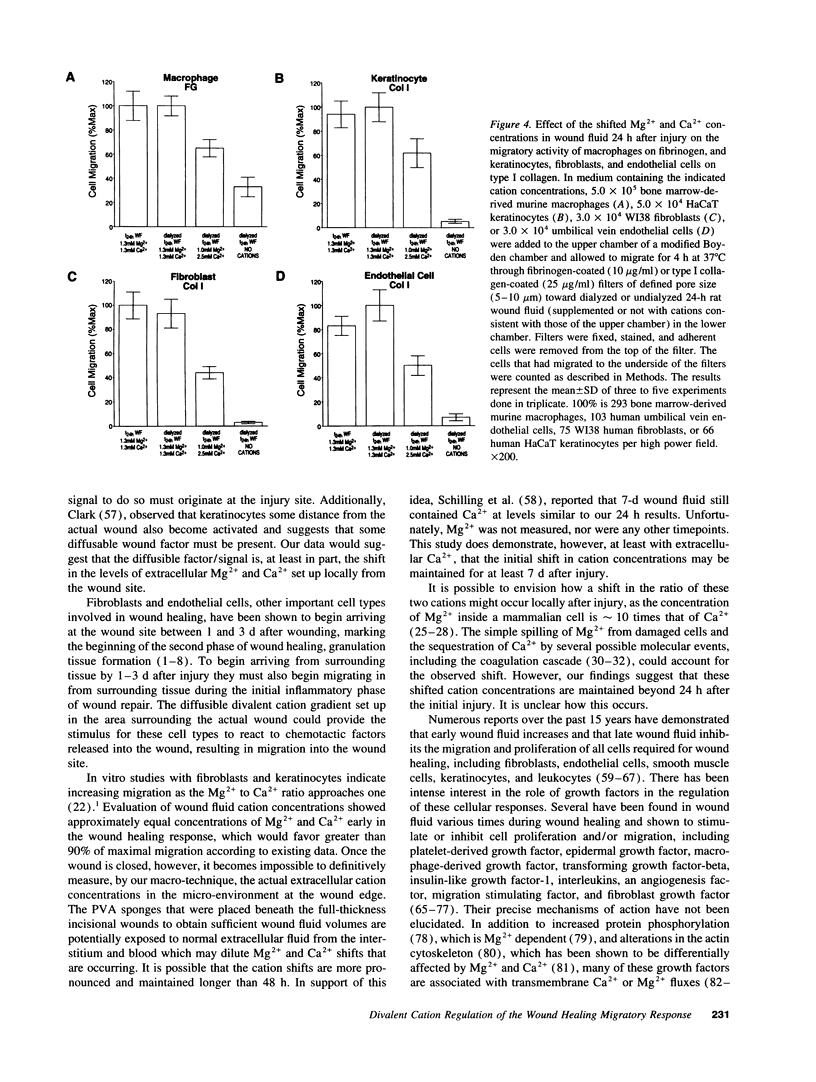
Accruing evidence indicates that the levels of extracellular Mg2+ and Ca2+ can have a distinct impact on the adhesive and migratory activities of many cell types. The physiological relevance of these observations, however, has remained largely unexplored. In the present study, wound fluids collected throughout the early stages of cutaneous wound repair were examined for possible Mg2+ and Ca2+ fluctuations. Early in the process, when cell migration into the wound site is initiated, Mg2+ is elevated and Ca2+ is reduced (Mg2+:Ca2+ = 1). As wound healing progresses, wound fluid concentrations of Mg2+ and Ca2+ begin to return to normal plasma levels (Mg2+:Ca2+ = 0.4). When macrophages, keratinocytes, fibroblasts, and endothelial cells were exposed to dialyzed wound fluid, the migration stimulated by undialyzed wound fluid was lost. Addition back to dialyzed wound fluid of 24 h, postinjury concentrations of Mg2+ and Ca2+ restored all migratory stimulus. This observed migration is approximately twofold greater than when normal plasma Mg2+ and Ca2+ concentrations are present. Changes in the levels of Mg2+ and Ca2+ in wound fluid occur during the same period that inflammatory cells, keratinocytes, fibroblasts, and neovasculature have been shown to migrate during wound healing in vivo. Together, these data suggest that the impact of these changes on integrins and E-cadherin may play a direct role in the activation and maintenance of the migratory phenotypes of the cells involved in the wound healing process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper J. C., Tibbetts L. L., Sarazen A. A., Jr The in vitro response of fibroblasts to the fluid that accumulates under a vapor-permeable membrane. J Invest Dermatol. 1985 Jun;84(6):513–515. doi: 10.1111/1523-1747.ep12273497. [DOI] [PubMed] [Google Scholar]
- Altieri D. C. Occupancy of CD11b/CD18 (Mac-1) divalent ion binding site(s) induces leukocyte adhesion. J Immunol. 1991 Sep 15;147(6):1891–1898. [PubMed] [Google Scholar]
- Altieri D. C., Plescia J., Plow E. F. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (alpha M beta 2, Mac-1) and promotes leukocyte adhesion. J Biol Chem. 1993 Jan 25;268(3):1847–1853. [PubMed] [Google Scholar]
- Baird A., Walicke P. A. Fibroblast growth factors. Br Med Bull. 1989 Apr;45(2):438–452. doi: 10.1093/oxfordjournals.bmb.a072333. [DOI] [PubMed] [Google Scholar]
- Banai S., Haggroth L., Epstein S. E., Casscells W. Influence of extracellular magnesium on capillary endothelial cell proliferation and migration. Circ Res. 1990 Sep;67(3):645–650. doi: 10.1161/01.res.67.3.645. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Knighton D. R., Hunt T. K., Werb Z. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7773–7777. doi: 10.1073/pnas.79.24.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Caddell J. L., Reed G. F. Validity of the parenteral magnesium load test for mature mammals. Magnesium. 1989;8(2):65–70. [PubMed] [Google Scholar]
- Celada A., Maki R. A. Transforming growth factor-beta enhances the M-CSF and GM-CSF-stimulated proliferation of macrophages. J Immunol. 1992 Feb 15;148(4):1102–1105. [PubMed] [Google Scholar]
- Chen W. Y., Rogers A. A., Lydon M. J. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol. 1992 Nov;99(5):559–564. doi: 10.1111/1523-1747.ep12667378. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Cutaneous tissue repair: basic biologic considerations. I. J Am Acad Dermatol. 1985 Nov;13(5 Pt 1):701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990 Jun;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Wound repair. Curr Opin Cell Biol. 1989 Oct;1(5):1000–1008. doi: 10.1016/0955-0674(89)90072-0. [DOI] [PubMed] [Google Scholar]
- Cromack D. T., Sporn M. B., Roberts A. B., Merino M. J., Dart L. L., Norton J. A. Transforming growth factor beta levels in rat wound chambers. J Surg Res. 1987 Jun;42(6):622–628. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Davis G. E., Camarillo C. W. Regulation of integrin-mediated myeloid cell adhesion to fibronectin: influence of disulfide reducing agents, divalent cations and phorbol ester. J Immunol. 1993 Dec 15;151(12):7138–7150. [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992 Jan;116(1):219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989 Dec 1;8(12):3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvonch V. M., Murphey R. J., Matsuoka J., Grotendorst G. R. Changes in growth factor levels in human wound fluid. Surgery. 1992 Jul;112(1):18–23. [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Use of an antiserum against phosphotyrosine for the identification of phosphorylated components in human fibroblasts stimulated by platelet-derived growth factor. J Biol Chem. 1984 Sep 10;259(17):11145–11152. [PubMed] [Google Scholar]
- Elemer G. S., Edgington T. S. Microfilament reorganization is associated with functional activation of alpha M beta 2 on monocytic cells. J Biol Chem. 1994 Feb 4;269(5):3159–3166. [PubMed] [Google Scholar]
- Fitzgerald L. A., Phillips D. R. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 15;260(20):11366–11374. [PubMed] [Google Scholar]
- Ford H. R., Hoffman R. A., Wing E. J., Magee D. M., McIntyre L., Simmons R. L. Characterization of wound cytokines in the sponge matrix model. Arch Surg. 1989 Dec;124(12):1422–1428. doi: 10.1001/archsurg.1989.01410120068014. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Fujimura K., Kuramoto A. Electrophysiological evidence that glycoprotein IIb-IIIa complex is involved in calcium channel activation on human platelet plasma membrane. J Biol Chem. 1991 Sep 5;266(25):16370–16375. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- Gow C. B., Wilkinson M., Silvapulle M. J., Moore G. P. Fluid balance, electrolyte profiles and plasma parathyroid hormone concentrations in ewes treated with epidermal growth factor. J Endocrinol. 1992 Oct;135(1):91–101. doi: 10.1677/joe.0.1350091. [DOI] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Ho C. H., Wysocki A. Degradation of fibronectin and vitronectin in chronic wound fluid: analysis by cell blotting, immunoblotting, and cell adhesion assays. J Invest Dermatol. 1992 Apr;98(4):410–416. doi: 10.1111/1523-1747.ep12499839. [DOI] [PubMed] [Google Scholar]
- Grinnell F. The activated keratinocyte: up regulation of cell adhesion and migration during wound healing. J Trauma. 1990 Dec;30(12 Suppl):S144–S149. [PubMed] [Google Scholar]
- Grinnell F., Toda K., Takashima A. Activation of keratinocyte fibronectin receptor function during cutaneous wound healing. J Cell Sci Suppl. 1987;8:199–209. doi: 10.1242/jcs.1987.supplement_8.11. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992 Jan;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Soma Y., Takehara K., Charette M. EGF and TGF-alpha are potent chemoattractants for endothelial cells and EGF-like peptides are present at sites of tissue regeneration. J Cell Physiol. 1989 Jun;139(3):617–623. doi: 10.1002/jcp.1041390323. [DOI] [PubMed] [Google Scholar]
- Grzesiak J. J., Davis G. E., Kirchhofer D., Pierschbacher M. D. Regulation of alpha 2 beta 1-mediated fibroblast migration on type I collagen by shifts in the concentrations of extracellular Mg2+ and Ca2+. J Cell Biol. 1992 Jun;117(5):1109–1117. doi: 10.1083/jcb.117.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Toda K., Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990 Jun;96(Pt 2):197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Henrotte J. G. Genetic regulation of blood and tissue magnesium content in mammals. Magnesium. 1988;7(5-6):306–314. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Daniel T. O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Haapanen T., Lyytikäinen A. M., Larjava H. Wound fluids mediate granulation tissue growth phases. Cell Biol Int Rep. 1983 Sep;7(9):745–753. doi: 10.1016/0309-1651(83)90202-3. [DOI] [PubMed] [Google Scholar]
- Juhasz I., Murphy G. F., Yan H. C., Herlyn M., Albelda S. M. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993 Nov;143(5):1458–1469. [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Koyasu S., Nishida E., Sakai H., Takaku F., Yahara I., Kasuga M. Insulin-like growth factors, insulin, and epidermal growth factor cause rapid cytoskeletal reorganization in KB cells. Clarification of the roles of type I insulin-like growth factor receptors and insulin receptors. J Biol Chem. 1986 Dec 5;261(34):16141–16147. [PubMed] [Google Scholar]
- Katz M. H., Alvarez A. F., Kirsner R. S., Eaglstein W. H., Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol. 1991 Dec;25(6 Pt 1):1054–1058. doi: 10.1016/0190-9622(91)70306-m. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Tseng E., Springer T. A. Cloning of the murine lymphocyte function-associated molecule-1 alpha-subunit and its expression in COS cells. J Immunol. 1991 Jul 1;147(1):369–374. [PubMed] [Google Scholar]
- Kirchhofer D., Grzesiak J., Pierschbacher M. D. Calcium as a potential physiological regulator of integrin-mediated cell adhesion. J Biol Chem. 1991 Mar 5;266(7):4471–4477. [PubMed] [Google Scholar]
- Lages B., Scrutton M. C., Holmsen H. Studies on gel-filtered human platelets: isolation and characterization in a medium containing no added Ca2+, Mg2+, or K+. J Lab Clin Med. 1975 May;85(5):811–825. [PubMed] [Google Scholar]
- Larjava H., Salo T., Haapasalmi K., Kramer R. H., Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993 Sep;92(3):1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Silverstein R., Wright S. D., Weitz J. I., Huang A. J., Silverstein S. C. The role of protected extracellular compartments in interactions between leukocytes, and platelets, and fibrin/fibrinogen matrices. Ann N Y Acad Sci. 1992 Dec 4;667:163–172. doi: 10.1111/j.1749-6632.1992.tb51608.x. [DOI] [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Matsuoka J., Grotendorst G. R. Two peptides related to platelet-derived growth factor are present in human wound fluid. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4416–4420. doi: 10.1073/pnas.86.12.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- Olinger M. L. Disorders of calcium and magnesium metabolism. Emerg Med Clin North Am. 1989 Nov;7(4):795–822. [PubMed] [Google Scholar]
- Orlova A., Egelman E. H. A conformational change in the actin subunit can change the flexibility of the actin filament. J Mol Biol. 1993 Jul 20;232(2):334–341. doi: 10.1006/jmbi.1993.1393. [DOI] [PubMed] [Google Scholar]
- Orredson S. U., Knighton D. R., Scheuenstuhl H., Hunt T. K. A quantitative in vitro study of fibroblast and endothelial cell migration in response to serum and wound fluid. J Surg Res. 1983 Sep;35(3):249–258. doi: 10.1016/s0022-4804(83)80011-0. [DOI] [PubMed] [Google Scholar]
- Picardo M., Grey A. M., McGurk M., Ellis I., Schor S. L. Detection of migration stimulating activity in wound fluid. Exp Mol Pathol. 1992 Aug;57(1):8–21. doi: 10.1016/0014-4800(92)90044-c. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Page E. Magnesium in heart muscle. Circ Res. 1973 Oct 5;33(4):367–374. doi: 10.1161/01.res.33.4.367. [DOI] [PubMed] [Google Scholar]
- Pricolo V. E., Caldwell M. D., Mastrofrancesco B., Mills C. D. Modulatory activities of wound fluid on fibroblast proliferation and collagen synthesis. J Surg Res. 1990 Jun;48(6):534–538. doi: 10.1016/0022-4804(90)90226-r. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILLING J. A., FAVATA B. V., RADAKOVICH M. Studies of fibroplasia in wound healing. Surg Gynecol Obstet. 1953 Feb;96(2):143–149. [PubMed] [Google Scholar]
- SCHILLING J. A., MILCH L. E. Fractional analysis of experimental wound fluid. Proc Soc Exp Biol Med. 1955 Jun;89(2):189–192. doi: 10.3181/00379727-89-21753. [DOI] [PubMed] [Google Scholar]
- Sank A., Chi M., Shima T., Reich R., Martin G. R. Increased calcium levels alter cellular and molecular events in wound healing. Surgery. 1989 Dec;106(6):1141–1148. [PubMed] [Google Scholar]
- Scharffetter-Kochanek K., Klein C. E., Heinen G., Mauch C., Schaefer T., Adelmann-Grill B. C., Goerz G., Fusenig N. E., Krieg T. M., Plewig G. Migration of a human keratinocyte cell line (HACAT) to interstitial collagen type I is mediated by the alpha 2 beta 1-integrin receptor. J Invest Dermatol. 1992 Jan;98(1):3–11. doi: 10.1111/1523-1747.ep12493266. [DOI] [PubMed] [Google Scholar]
- Simon D. I., Ezratty A. M., Francis S. A., Rennke H., Loscalzo J. Fibrin(ogen) is internalized and degraded by activated human monocytoid cells via Mac-1 (CD11b/CD18): a nonplasmin fibrinolytic pathway. Blood. 1993 Oct 15;82(8):2414–2422. [PubMed] [Google Scholar]
- Soma Y., Dvonch V., Grotendorst G. R. Platelet-derived growth factor AA homodimer is the predominant isoform in human platelets and acute human wound fluid. FASEB J. 1992 Aug;6(11):2996–3001. doi: 10.1096/fasebj.6.11.1644262. [DOI] [PubMed] [Google Scholar]
- Spencer E. M., Skover G., Hunt T. K. Somatomedins: do they play a pivotal role in wound healing? Prog Clin Biol Res. 1988;266:103–116. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lloyd W., Derynck R., Winkler M. E., Levine L. Actions of growth factors on plasma calcium. Epidermal growth factor and human transforming growth factor-alpha cause elevation of plasma calcium in mice. J Clin Invest. 1986 Nov;78(5):1405–1409. doi: 10.1172/JCI112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezzini C., Schüepp B., Maly F. E., Jungi T. W. Evidence that exposure to fibrinogen or to antibodies directed against Mac-1 (CD11b/CD18; CR3) modulates human monocyte effector functions. Br J Haematol. 1991 Jan;77(1):16–24. doi: 10.1111/j.1365-2141.1991.tb07942.x. [DOI] [PubMed] [Google Scholar]
- Van Strijp J. A., Russell D. G., Tuomanen E., Brown E. J., Wright S. D. Ligand specificity of purified complement receptor type three (CD11b/CD18, alpha m beta 2, Mac-1). Indirect effects of an Arg-Gly-Asp (RGD) sequence. J Immunol. 1993 Sep 15;151(6):3324–3336. [PubMed] [Google Scholar]
- Viljanto J. Cellstic: A device for wound healing studies in man. Description of the method. J Surg Res. 1976 Feb;20(2):115–119. doi: 10.1016/0022-4804(76)90107-4. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Woodley D. T., Bachmann P. M., O'Keefe E. J. Laminin inhibits human keratinocyte migration. J Cell Physiol. 1988 Jul;136(1):140–146. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., O'Keefe E. J., Prunieras M. Cutaneous wound healing: a model for cell-matrix interactions. J Am Acad Dermatol. 1985 Feb;12(2 Pt 2):420–433. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]
- Wysocki A. B., Grinnell F. Fibronectin profiles in normal and chronic wound fluid. Lab Invest. 1990 Dec;63(6):825–831. [PubMed] [Google Scholar]


