Cdk5 plays a role in nervous system development; its role in the initial stages of neural differentiation is poorly understood. We isolated neural stem cells from E13 Cdk5 WT and KO mouse and observed them as they switched from proliferating stage to neural differentiation. We show that Cdk5 phosphorylation of p27kip1 at Thr187 is crucial to neural differentiation.
Abstract
Cyclin-dependent kinase 5 (Cdk5) plays a key role in the development of the mammalian nervous system; it phosphorylates a number of targeted proteins involved in neuronal migration during development to synaptic activity in the mature nervous system. Its role in the initial stages of neuronal commitment and differentiation of neural stem cells (NSCs), however, is poorly understood. In this study, we show that Cdk5 phosphorylation of p27Kip1 at Thr187 is crucial to neural differentiation because 1) neurogenesis is specifically suppressed by transfection of p27Kip1 siRNA into Cdk5+/+ NSCs; 2) reduced neuronal differentiation in Cdk5−/− compared with Cdk5+/+ NSCs; 3) Cdk5+/+ NSCs, whose differentiation is inhibited by a nonphosphorylatable mutant, p27/Thr187A, are rescued by cotransfection of a phosphorylation-mimicking mutant, p27/Thr187D; and 4) transfection of mutant p27Kip1 (p27/187A) into Cdk5+/+ NSCs inhibits differentiation. These data suggest that Cdk5 regulates the neural differentiation of NSCs by phosphorylation of p27Kip1 at theThr187 site. Additional experiments exploring the role of Ser10 phosphorylation by Cdk5 suggest that together with Thr187 phosphorylation, Ser10 phosphorylation by Cdk5 promotes neurite outgrowth as neurons differentiate. Cdk5 phosphorylation of p27Kip1, a modular molecule, may regulate the progress of neuronal differentiation from cell cycle arrest through differentiation, neurite outgrowth, and migration.
INTRODUCTION
Cyclin-dependent kinase 5 (Cdk5), a unique member of the cyclin-dependent kinase family is a multifunctional kinase whose principal activities are restricted to the nervous and muscular systems where its activators, p35 and p39, are specifically expressed (Tsai et al., 1994; Tang et al., 1995; Ohshima et al., 1996; Philpott et al., 1999). Cdk5 is essential for neuronal survival and migration and regulates synaptic transmission (Dhavan and Tsai, 2001; Grant et al., 2001; Cheng and Ip, 2003; Kesavapany et al., 2004).
Studies relating Cdk5 activity to embryonic corticogenesis have yielded mixed results, however. Though neurons do differentiate in the Cdk5−/− mouse and form an initial cortical preplate, all subsequent postmitotic neurons fail to migrate normally into their respective cortical layers (Ohshima et al., 1996). This suggests neuronal migration as the target of Cdk5 activity (Ayala et al., 2007). In Xenopus neurogenesis, Cdk5/p35 activity is induced after expression of neural differentiation transcription factors, neurogenin and neuroD, consistent with its role in migration rather than early neural commitment (Philpott et al., 1999). A more active role for Cdk5 has been proposed in preventing cell cycle reentry in postmitotic neurons to protect neurons from cell death (Cicero and Herrup, 2005). A comparison of the E16.5 cortical neurons in Cdk5+/+ and Cdk5−/− mice showed that a greater number of Cdk5−/− neurons persisted in the cell cycle compared with Cdk5+/+ neurons. Here, it is suggested that control of cell cycle exit is a site of Cdk5 regulation independent of its kinase activity; rather its action is correlated with its subcellular localization (Zhang and Herrup, 2008). According to this model, neurons are protected from cell cycle reentry when Cdk5/p35 is localized in nuclei bound to p27Kip1 in the absence of kinase activity. Under stress, Cdk5 transfers into the cytoplasm, neurons reenter the cell cycle to undergo a delayed apoptosis (Zhang et al., 2010).
Adult neurogenesis is also dependent on Cdk5 activity. A conditional knockout (KO) of Cdk5 in the dentate gyrus (DG) of adult hippocampus inhibits granule cell neurogenesis, results in fewer immature DG neurons without affecting the level of cell proliferation (Lagace et al., 2008). Survival of newly generated granule cells seems to be a major function of Cdk5 activity in the subgranular zone (SGZ) of the adult hippocampus. In a related study, stem cell/progenitors from adult rat and mouse brains, a mixed population of proliferating NSCs and neuroglial progenitors, presumed to be derived from hippocampus, displayed no difference in proliferation or differentiation whether Cdk5 was overexpressed or down-regulated (Jessberger et al., 2008). Heterogeneity of this population, however, tends to confound this conclusion because the identity of cells transfected was unknown. On the other hand transfection of proliferating SGZ cells with dominant negative Cdk5 in situ indicated that Cdk5 was essential for growth and maturation of dendritic processes and spines that correlated with abnormal migration patterns. In the adult, as in the embryo, it seems that neuronal migration, maturation, and survival are targets of Cdk5 activity.
The question still arises as to whether Cdk5 is a positive regulator of neural differentiation by modulating cell cycle exit or by regulation of proneural gene expression. Most studies of embryonic and adult neurogenesis deal with heterogeneous populations of ventricular zone (VZ or SVZ) cells, a mixture of noncommitted NSC and proliferating neuroglial progenitors (some multipotential, others lineage restricted), a situation difficult to interpret (Lillien, 1998a,b). Ideally, experiments carried out with pure populations of noncommitted NSCs with and without Cdk5 should provide a more unambiguous test of early events in neuronal differentiation. Hence, our approach to studying Cdk5 in neural fate determination from NSCs at the ventricular zone is to isolate a homogeneous population of uncommitted NSCs from the early E13 telencephalon of Cdk5+/+ and Cdk5−/− mice and compare their progression from proliferation to differentiation in vitro. Using this strategy, it should be possible to identify some of the key molecular players as they are expressed during the transition from cell cycle exit to generation of postmitotic neurons. To test this hypothesis, it was first necessary to isolate a homogeneous population of uncommitted cells from the early telencephalon that meets the rigorous criteria defining true NSCs (Reynolds and Rietze, 2005). This was successfully accomplished by a fluorescence-activated cell sorting (FACS) protocol using a negative selection procedure with a set of antibodies specific for neuronal and glial cell surface epitopes (Maric et al., 2000, 2003; Maric and Barker, 2004).
Mammals express three members of the Cip/Kip family that regulate cell cycle exit: p21Cip1, p27Kip1, and p57Kip1 (Sherr and Roberts, 1999). A series of recent studies have implicated p27Kip1 in regulation of neurogenesis in several ways, from cell cycle arrest, to cell fate determination and neuronal migration (Ohnuma et al., 1999; Nguyen et al., 2006b,c, 2007 Kawauchi et al., 2006). It has been suggested that the modular nature of p27Kip1 in which N- and C-terminal domains exercise different but overlapping roles may account for coupling these events of neurogenesis (Nguyen et al., 2006b). The coordination of these multiple pathways is poorly understood, although evidence is accumulating that Cdk5 phosphorylates p27Kip1 at Ser10 and Thr187, with the former site shown to play a role in neuronal migration (Kawauchi et al., 2006). Because the role of phosphorylation at the Thr187 site was not further studied, we used the FACS procedure (Maric et al., 2003, 2007) to isolate homogeneous populations of uncommitted NSCs from Cdk5+/+ and Cdk5−/− mouse telencephalons at E13 and explored whether Cdk5-mediated phosphorylation of p27Kip1 at Thr 187 does, in fact, play a role in the initial stages of neuronal differentiation. The present study shows that Cdk5-mediated phosphorylation of the cell cycle inhibitor, p27Kip1, at Thr187 in the C-terminal domain, together with phosphorylation of p27Kip1at Ser10, is correlated with cell cycle exit and with the progression to neuronal differentiation, neurite outgrowth, and migration.
MATERIALS AND METHODS
Antibodies
Anti-Cdk5 (J-3, C-8), anti-p35 (C-19), anti-His, and anti-Myc were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho (P)-p27Kip1 (T187) and total (T)-p27Kip1 antibodies were also obtained from Santa Cruz Biotechnology. Phospho-p27(Ser10) antibody was purchased from Zymed-Invitrogen (Carlsbad, CA). Anti-TuJ1 was obtained from Promega (Madison, WI); anti-BrdU was obtained from Novus Biologicals (Littleton, CO); anti-GFAP and anti-nestin were purchased from Millipore (Billerica, MA); β-actin was purchased from Invitrogen. Anti-NF-M antibody was obtained from Biocompare (San Francisco, CA). Secondary horseradish peroxidase–conjugated antibodies were obtained from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Secondary fluorescence-conjugated Oregon Green and Texas Red antibodies and Lipofectamine (2000) were purchased from Invitrogen.
Cell Preparation, Flow Cytometric Analysis, and Cell Sorting
Experiments were performed on embryos recovered from timed pregnant Cdk5+/+ and Cdk5−/− mice. Dissociated cell suspensions from Cdk5+/+ and Cdk5−/− embryonic day 13 (E13) telencephalic tissues were used as primary sources of uncommitted NSCs. Cells were surface-labeled using a cocktail of lineage-specific surface markers for identifying early and late neuroglial and glial progenitors (CD15/LeX/SSEA-1, CDw60/9-O-acetylated GD3/Jones, A2B5, and O4) and for differentiating postmitotic neurons (tetanus toxin fragment C/TnTx, cholera toxin B subunit/CnTx). Lineage negative NSCs were then isolated by preparative FACS protocol using a negative selection program, as previously described (Maric et al., 2003, 2007; Maric and Barker, 2004, 2005).
Cell Culture
Sort-purified Cdk5+/+ and Cdk5−/− NSCs were plated at clonal density (1 × 103 cells/cm2) in a Neurobasal/B27 medium, which was supplemented with 10 ng/ml human recombinant basic fibroblast growth factor (bFGF ; Intergen, Purchase, NY). In this medium cells were proliferated and expanded. Withdrawal of bFGF after 24 h and 5 d in culture tested the potential of these cells to differentiate into neuronal, astroglial progenitors, and postmitotic neurons. They were identified using classical markers β-III tubulin (Tuj1), glial fibrillary acidic protein (GFAP), and neurofilament (NF-M), respectively (Maric et al., 2003).
Bromodeoxyuridine Labeling of E13 Brains In Situ
Acute pulse labeling of bromodeoxyuridine (BrdU) was carried out in time pregnant dams (50 mg/kg body weight of the animal). After 24 h the embryos were genotyped and fixed with 4% PFA overnight and cryopreserved for another 4 h in 30% sucrose. Eight-micrometer-thick sections were taken from E13 Cdk5+/+ and Cdk5−/− embryos and immunostained for BrdU incorporation as previously described (Shukla et al., 2005).
Preparation of Primary and Secondary Neurospheres
Both primary and secondary neurosphere preparation and immunostaining was carried out as described by Mishra et al. (2006) with modification. Tissue from telencephalon of E13 embryos recovered from timed pregnant Cdk5+/+ and Cdk5−/− mice were homogenized to prepare neurospheres. Mouse mAb was applied to immunodetect dividing cells using intermediate filament Nestin (1:50, clone 401; Chemicon, Temecula, CA).
Immunocytochemistry
Immunocytochemistry was performed largely as previously described (Zheng et al., 2005). All fluorescent images were observed using 63× oil immersion objective on a Zeiss LSM-510 laser-scanning confocal microscope (Thornwood, NY). Images were combined using Zeiss LSM 510 image software and managed in Adobe Photoshop.
Immunoprecipitation
Whole-cell lysates were incubated with antibodies for 1–2 h at 4°C on a rotating wheel, followed by 1-h incubation with protein A/G agarose beads. Beads were subsequently centrifuged and washed, and the protein was eluted and subjected to immunoblotting.
Immunoblotting
Western blot analysis was performed as described previously (Zheng et al., 2002).
Transfections and Kinase Assays
Transfections were performed with Lipofectamine 2000 (Invitrogen) reagents according to a previously published report (Li et al., 2002), and kinase assays were performed according to previously published methods (Li et al., 2002).
Small Interfering RNA Preparation and Transfections
The small interfering RNA (siRNA) sequences used for targeted silencing of p27Kip1 (5′-aagtacgagtggcaagaggtg-3′) were recommended by the siRNA supplier (Xeragon, Germantown, MD). Transfections of p27Kip1 siRNA for endogenous gene targeting were carried out as previously described (Zheng et al., 2007).
Cdk5 and Cdk2 Activity Assay
Two hundred micrograms of protein was immunoprecipitated for each sample by anti-Cdk2 and anti-Cdk5 antibody. The immunoprecipitated protein was resuspended in 50 μl of kinase buffer (20 mM HEPES, pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM DTT, 7.5 mM MgCl2, and 50 μM ATP) containing 10 μCi of (γ-32P)ATP (3000 Ci/mmol; Amersham, Piscataway, NJ) and incubated for 1 h at 30°C. Reaction was stopped by adding 15 μl of 4× NuPAGE SDS sample buffer. Samples were boiled for 5 min, and incorporation of radioactive phosphate was determined by 10% NuPAGE. Analysis of the dried gel was performed using a PhosphoImager (Molecular Dynamics, Piscataway, NJ).
RESULTS
Cdk5+/+ and Cdk5−/− NSCs Exhibit Similar Proliferation Patterns
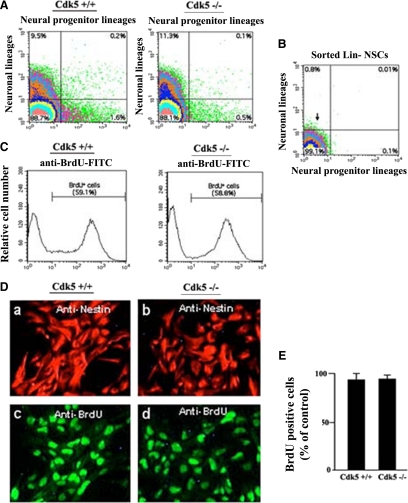
To investigate the role of Cdk5 in neural differentiation during early corticogenesis, we isolated NSCs from Cdk5+/+ and Cdk5−/− mouse embryos at E13 using a negative selection sorting strategy against a multitude of surface markers (CD15/LeX/SSEA-1, CDw60/9-O-acetylated GD3/Jones, A2B5, O4, TnTX, CnTx). We have also used this strategy previously, to identify the lineage-restricted and differentiating progeny of NSCs (Maric et al., 2003, 2007; Maric and Barker, 2004, 2005). Before sorting, these lineage-negative NSCs comprised >88% of E13 telencephalic dissociates, and there was no significant difference in the enumeration of these cells between Cdk5+/+ and Cdk5−/− mice (Figure 1A). After sorting, NSCs from Cdk5+/+ mice were more than 99% pure, as revealed by their lack of surface markers used for sorting (Figure 1B). Pulse-labeling the telencephalic dissociates with BrdU for 2 h, revealed that both Cdk5+/+ and Cdk5−/− NSCs were equally proliferative (Figure 1C). This proliferative potential was retained even after culturing the NSCs for 3 d with bFGF as revealed by cumulative BrdU labeling for 24 h (Figure 1, D and E). Under these permissive growth conditions, most of the NSCs were maintained in an undifferentiated state, as revealed by nestin expression (Figure 1D). These results suggest that Cdk5 is not essential for the proliferation and self-renewal of NSCs.
Figure 1.
Sorting and characterization of Cdk5+/+ and Cdk5−/− NSCs. Telencephalic dissociates from E13 Cdk5+/+ and Cdk5−/− mice were surface-labeled using a cocktail of surface markers identifying the neuronal or neuroglial progenitor lineages (see Materials and Methods). (A) FACS analysis of unsorted cells reveals that uncommitted lineage-negative NSCs (Lin− NSCs) comprise the majority (>88%) of total cells (see bottom left quadrant in each bivariate plot) in E13 telencephalic dissociates of both Cdk5+/+ (left) and Cdk5−/− mice (right). (B) FACS analysis of sorted neural stem cells from Cdk5+/+ and Cdk5−/− revealed 99% pure population (see bottom left quadrant in bivariate plot). (C) Acute pulse-labeling of NSCs with BrdU revealed that the majority (>60%) of these cells are actively proliferating in both Cdk5+/+ and Cdk5−/− mice. (D) An aliquot of the sorted Cdk5+/+ and Cdk5−/− NSCs was expanded with bFGF (10 ng/ml) for 3 d in culture, and the cells were cumulatively labeled with BrdU for 24 h and then immunostained for nestin expression and BrdU incorporation. The great majority of cells are nestin+ (a and b) and BrdU+ (c and d), demonstrating that both Cdk5+/+ and Cdk5−/− NSCs efficiently self-renew in the presence of bFGF. (E) The bar graph quantifies the % of cells expressing BrdU staining after 3 d of expansion in bFGF. WT and Cdk5−/− NSCs are nearly 100% BrdU+ after 24 h of cumulative labeling.
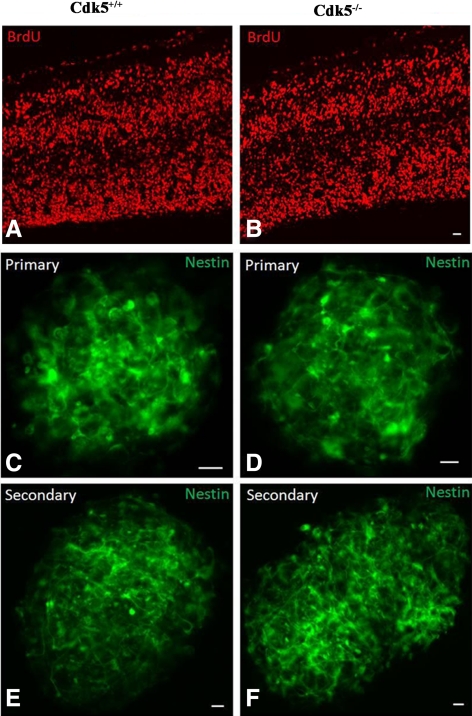
Consistent with these results on NSCs, in situ proliferation of cortical neurons was examined in E13 embryos. Pregnant dams were injected with BrdU, and Cdk5+/+ and Cdk5−/− embryos were fixed, sectioned, and examined with BrdU antibody (Figure 2, A and B). The extent of BrdU cortical labeling was similar in Cdk5+/+ and Cdk5−/− brains. A further test of the equivalent self renewal properties of wild-type (WT) and KO cells was examined in a neurosphere assay using cells isolated from E13 WT and KO telencephalons. Neurosphere assay was based on a published protocol (Reynolds and Weiss, 1992). In both WT and KO, primary and secondary neurosphere expression was similar (Figure 2, C–F), further supporting the equivalent self-renewal potential of KO and WT neuronal progenitors.
Figure 2.
Self renewal of E13 cortex is equivalent in Cdk5+/+ and Cdk5−/− brains. (A and B) In situ incorporation of BrdU into E13 telencephalons of Cdk5+/+ and Cdk5−/− mouse brains is equivalent. After intraperitoneal (i.p.) injection of BrdU into pregnant dams, embryos were fixed and sectioned. Immunodetection of BrdU incorporation into cortices revealed no difference between Cdk5+/+ and Cdk5−/− brains. Scale bar, 20 μm. (C–F) Neurospheres from Cdk5+/+ and Cdk5−/− E13 telencephalons were generated according to published procedures (Reynolds and Weiss, 1992). (C and D) In the presence of growth factors, dissected and dissociated telencephalons gave rise to primary neurospheres after 5 d in culture and were stained with nestin. (E and F) Primary neurospheres were dissociated and subpassaged to generate secondary neurospheres. After 7 d in culture secondary neurospheres from Cdk5+/+ and Cdk5−/− were immunopositive for nestin. All scale bars, 10 μm.
Cdk5 Is Required for Differentiation of NSCs
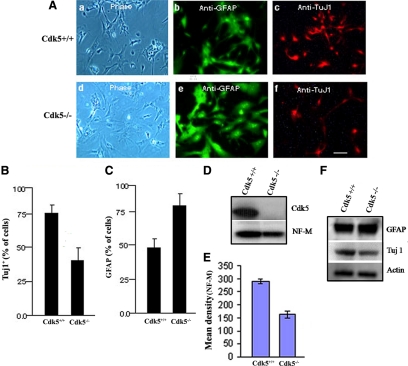
We next determined whether Cdk5 might modulate neurogenesis. Both Cdk5+/+ and Cdk5−/− NSCs were cultured in the absence of bFGF for 4–6 d and prepared for immunocytochemical (ICC) analysis using antibodies to βIII tubulin (TuJ1) and GFAP, a marker for astroglial progenitors (Figure 3A). The results show that the Cdk5+/+ NSCs produced 75% TuJ1-positive neurons and 50% GFAP-positive glia, compared with 35% TuJ1-positive neurons and 80% GFAP-positive glia in the Cdk5−/− NSCs (Figure 3, B and C). These data suggest that the preferred default differentiation of Cdk5−/− NSCs is primarily in the direction of astroglia, although a smaller percentage of cells do show the neuronal phenotype. Expression of neurofilament NF-M (Figure 3, D and E), a marker of more differentiated neurons, confirmed that neuronal differentiation of Cdk5−/− NSCs is significantly reduced. To further confirm the difference in phenotypic expression in cortices of WT and KO brains, the expression of additional neuronal and glial phenotypic markers were examined in brain lysates (telencephalon) from Cdk5+/+ and Cdk5−/− E13 embryos. Western blots of neuronal and astroglial antigenic markers were obtained showing greater expression of neuronal markers (Tuj1) in WT brains compared with the robust expression of GFAP in Cdk5−/− brain lysates (Figure 3F), a result consistent with the ICC phenotypic expression of WT and KO NSCs illustrated in Figure 3, A–C. The results suggest that in the absence of Cdk5, the default expression of differentiation is strongly in favor of a glial phenotype.
Figure 3.
Comparison of the differentiation potential of Cdk5+/+ and Cdk5−/− NSCs. Aliquots of the sorted WT and Cdk5−/− NSCs were cultured with bFGF (10 ng/ml) for 3 d, and then bFGF was removed from the culture medium followed by an additional 5 d of culture. The cells were subjected to immunocytochemistry with anti-GFAP and TuJ1 antibodies. (A) ICC expression patterns of Cdk5+/+ (a–c) and Cdk5−/− (d–f), respectively. (B) Bar graph shows the percent of TuJ1 staining cells in each population. (C) Percent of GFAP staining cells in each population obtained from A. (D) Western blots of Cdk5+/+ and Cdk5−/− NSC lysates showing expression of Cdk5 and the neurofilament NF-M. (E) Densitometry analysis of NF-M/actin obtained from Figure 2D. (F) Western blots of lysates derived from Cdk5+/+ and Cdk5−/− forebrains (cerebella and brain stem removed) to show additional neuronal and glial phenotypic markers.
Apoptosis of Cdk5+/+ and Cdk5−/− NSC Is Similar
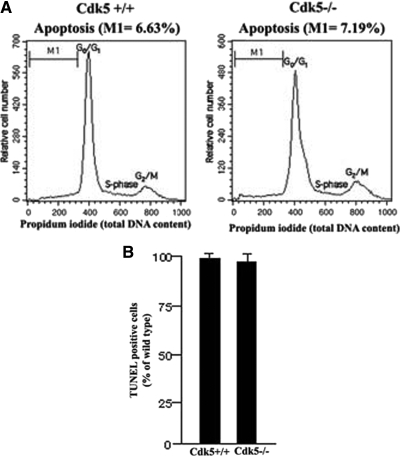
It is possible that the decreased production of the neuronal phenotype in Cdk5−/− NSCs reflects an increased level of apoptosis in that population. Initially, we compared the percentage of apoptotic cells with hypodiploid DNA content and the cell cycle kinetics in Cdk5+/+ and Cdk5−/− NSCs using propidium iodide staining and flow cytometry, which showed that both the cell cycle and apoptotic patterns of the two populations were virtually identical (Figure 4A). This suggests that the decreased neurogenic potential as revealed by lesser TuJ1-positive cells in Cdk5−/− cultures was not due to a selective increase in numbers of apoptotic cells after 4–6 d of proliferation in culture. The more relevant question is whether the induction of differentiation by withdrawal of bFGF induces more cell death among the differentiating Cdk5−/− NSCs, particularly those committed to a neuronal phenotype. To test this possibility, Cdk5+/+ and Cdk5−/− NSCs were cultured in the absence of bFGF for 4–6 d and then analyzed for apoptosis using the TdT-mediated dUTP nick end labeling (TUNEL) assay. In the absence of bFGF, there was no increased cell death in Cdk5−/− NSCs, compared with Cdk5+/+ NSCs; the percentage of cell death in the two populations was virtually identical (Figure 4B). This suggests that Cdk5−/− NSCs show no preferred cell death as compared with Cdk5+/+ and that the reduced number of neural progenitors is due to a failure in neural differentiation in the absence of Cdk5 (Figure 4, A and B).
Figure 4.
The extent of apoptosis is similar in cultured Cdk5+/+ and Cdk5−/− NSCs. After sorting, aliquots of NSCs were cultured for 3 d in bFGF (or without bFGF) and prepared for a TUNEL assay to determine the extent of apoptosis. (A) Using propidium iodide to measure total DNA content, a flow cytometric histogram analysis shows that the levels of apoptotic (hypodiploid) cells is similar in both NSC populations (compare M1 values). Peak of diploid G0/G1 cells, S-phase cells, and small peak of tetraploid G0/M cells are also similar, indicating no appreciable difference in cell cycle kinetics between Cdk5+/+ and Cdk5−/− NSCs. (B) Bar graph shows the percent of TUNEL-positive cells of Cdk5+/+ and Cdk5−/− cultures after assay normalized to the Cdk5+/+ control.
P27Kip1 Is a Target of Cdk5 Phosphorylation during Neural Differentiation
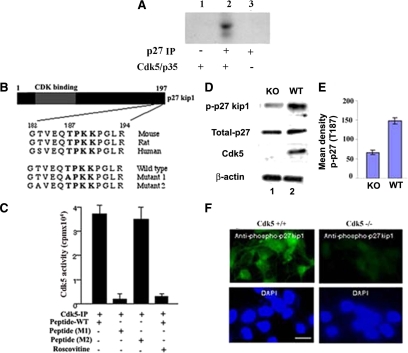
Because it has already been demonstrated that p27Kip1 is a target of Cdk5 phosphorylation at sites Ser10 and Thr187 (Kawauchi et al., 2006), with phosphorylation at the former site involved in modulating neuronal cell migration, we first determined whether p27Kip1 is also involved in neural differentiation of NSCs in our system. In a microarray analysis of proliferating Cdk5+/+ NSC cultured with or without bFGF for 4–6 d after neural differentiation was initiated, we noted that all cell cycle genes were down-regulated except for p27Kip1 (Supplemental Table 1). This correlated with a significant up-regulation of neuronal cytoskeletal and synaptic protein genes, suggesting that these events may be causally related. We first wanted to address, does Cdk5 directly phosphorylate p27Kip1? To do this we have performed an in vitro kinase assay using immunoprecipitated p27Kip1 from E16 brain lysate. We confirm that Cdk5 phosphorylates p27Kip1 in vitro using a standard kinase assay with active Cdk5/p35 and radioactive ATP. In the absence of any recombinant p27Kip1, we used instead a p27Kip1 IP of an E16 WT brain lysate as a substrate. The radioautograph shows that p27Kip1 is phosphorylated in the presence of Cdk5/p35 (Figure 5A).
Figure 5.
Phosphorylation of p27Kip1 and analysis of peptides derived from p27Kip1 to identify the potential site phosphorylated by Cdk5. (A) Phosphorylation of p27Kip1 by Cdk5. In the absence of recombinant p27Kip1, we used a p27Kip1 immunoprecipitation (IP) of E16 brain lysate as substrate for a Cdk5 kinase assay. The radioautograph shows that p27Kip1 is phosphorylated by the active Cdk5/p35 complex. (B) A diagram of the p27Kip1 protein sequence is shown with its Cdk5-binding domain at the N-terminus. Below, a Cdk5 consensus sequence is shown within conserved residues 182-194 of p27Kip1 from mouse, rat, and human. Thr187 in p27Kip1 occupies a Cdk5 consensus motif, TPKK. Two mutants were constructed as shown: mutant 1 with alanine replacing threonine 187 and mutant 2 with an alanine substitution at threonine 183 as a control. (C) The Cdk5+/+ and mutant peptides were used as substrates in vitro kinase assays using Cdk5 immunoprecipitated from brain lysates. The bar graph, based on three experiments, shows the control mutant 2 (183A) exhibited a level of phosphorylation comparable to a wild-type (WT) peptide, whereas mutant 1 (187A) showed activity equivalent to the roscovitine-inhibited activity of the WT peptide. (D) Western blots of lysates from E16 Cdk5+/+ and Cdk5−/− cultured cortical neurons were probed with antibodies to p27Kip1 and a specific phospho-p27Kip1, phosphorylated at Thr187. Compared with the WT, a reduced expression of phospho-p27Kip1 (approximately one-third, as indicated in the bar graph, n = 3) could be detected in the Cdk5−/− lysates, although the expression of total p27Kip1 was equivalent in both. (E) Densitometry analysis of p-p27(T187) from Cdk5+/+ and Cdk5−/− cultured cortical neurons (F) Immunocytochemical assays of cortical neurons using the phospho-Thr187-p27Kip1 antibody to confirm the absence of expression in the Cdk5−/− neurons compared with Cdk5+/+ neurons. Bar, 10 μm.
Because phosphorylation of Ser10 in p27Kip1 has already been implicated in neuronal migration, we decided to use a site directed mutagenesis approach to analyze the role of Thr187 phosphorylation. It is noteworthy that the site is conservatively maintained in a comparison of three vertebrate species, rat, mouse, and human (Figure 5B) and has a perfect Cdk5 consensus sequence. To investigate whether Thr187 is the target site for Cdk5 phosphorylation, we compared phosphorylation of the WT peptide GTVEQTPKKPGLR with two mutant peptides, GTVEQAPKKPGLR where Thr187 was mutated to Ala (T187A, M1) and a related mutant peptide GAVEQTPKKPGLR, where Thr183 was mutated to Ala (T183A, M2). We performed an in vitro kinase assay with Cdk5 immunoprecipitates to check phosphorylation (Figure 5C). Cdk5 robustly phosphorylated WT p27Kip1 peptide and the M2(183A) mutant peptide, but not the mutant M1(Thr187). We confirmed this reduced phosphorylation using roscovitine, a Cdk5 specific inhibitor, and the results suggested that Thr187 is one of the target sites phosphorylated by Cdk5. To examine further how Cdk5 activity is necessary for phosphorylation of p27Kip1 at Thr187 during neural differentiation, we compared the expression and phosphorylation state of p27Kip1 of E16 cortical neurons from Cdk5+/+ and Cdk5−/− grown 5 d in culture using an antibody specific for p27Kip1 phosphorylated at Thr187 (Figure 5, D–F). Cdk5+/+ and Cdk5−/− cells exhibited similar levels of total p27Kip1 protein expression (Figure 5D), but Cdk5−/− cells showed a decrease in the expression levels of p27Kip1 phosphorylated at Thr187 (Figure 5, D–F). These findings suggest that Thr187 of p27Kip1 is a Cdk5 specific site for phosphorylation during neural differentiation.
Colocalization of Cdk5/p35 with p27Kip1
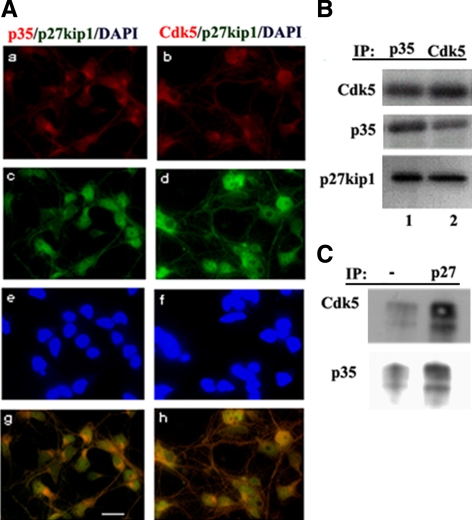
To investigate whether p27 is associated with Cdk5 and p35 in postmitotic neurons, E18 mouse cortical neurons from a Cdk5+/+ mouse brain were grown in culture for 7 d, and then fixed and prepared for ICC analysis (Figure 6A). The expression of p35, Cdk5, and p27Kip1 was determined using specific antibodies. P35 and Cdk5 were visualized by a rhodamine-labeled secondary antibody (Figure 6A, a and b), whereas p27Kip1 was labeled with an FITC antibody (Figure 6A, c and d). In Figure 6A, g and h, the overlap indicates that p27Kip1 does in fact colocalize with Cdk5/p35 in mature neurons. To confirm this, lysates of Cdk5+/+ NSCs after 5-d bFGF withdrawal were immunoprecipitated with Cdk5 or with p35 and control antibodies and were detected by Western blot with p27Kip1 antibody (Figure 6B). We found that p27Kip1 was coimmunoprecipitated with both Cdk5 and p35 but not with the control antibody. The reciprocal immunoprecipitation of Cdk5+/+ NSC lysate with antibody to p27Kip1 pulls down Cdk5 and p35, although the negative IgG control showed some expression (Figure 6C). This is consistent with the ICC data and confirms in vivo colocalization of the active kinase with its specific substrate, p27Kip1.
Figure 6.
P27Kip1 colocalized and associated with Cdk5 and p35. (A) Cortical neurons were prepared for immunocytochemistry and labeled with antibodies to p35 or Cdk5 (rhodamine) together with an antibody to p27Kip1 (FITC). Colocalization is seen for p27Kip1 and the Cdk5/p35 complex. Compare a, c, e, and g with b, d, f, and h. Bar, 10 μm. (B) Cdk5+/+ NSC were deprived of bFGF for 5 d and fixed. NSCs were lysed and used for a p35 immunoprecipitation (IP; antibody C19) and a Cdk5 IP (C8) and probed with an antibody to p27Kip1. P27Kip1 coimmunoprecipitates in each case. (C) A reciprocal IP was carried out on lysates of Cdk5+/+ NSCs deprived of bFGF for 3 d using antibody to p27Kip1. The blots were probed with antibodies to Cdk5 and p35.
Cdk5 Activity Correlates with Increased p27Kip1 Expression during Neural Differentiation
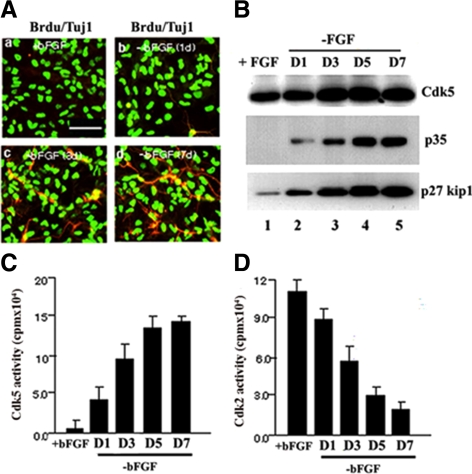
To determine whether regulation of Cdk5 and its activator, p35, were related to neural stem cell differentiation, bFGF was removed from Cdk5+/+ NSC cultures for 7 d to initiate differentiation. Using this strategy, we were able to follow the timing of the expression of Cdk5, p35, and p27Kip1 during the course of differentiation. Figure 7A shows the double-labeled ICC assays used to determine the expression of BrdU (FITC) and Tuj1 (rhodamine) on day 0 (a), day 1 (b), day 3 (c), and day 7 (d). We observed the appearance of TuJ1-labeled neurites at day 3 and day 7. Lysates of similar cultures were used for Western blot analysis, which showed that endogenous Cdk5 was expressed in bFGF-treated NSC cultures, that p35 was absent, and that p27Kip1 was weakly expressed (Figure 7B, lane 1). After removal of bFGF, p35 was expressed weakly only at day 1, and Cdk5 expression was modestly increased between day 3 and 7. p35, however, robustly increased from day 1 to day 7, correlating with the appearance of neurites. p27Kip1 expression also increased significantly from day 1 to day 7 (Figure 7B). Cdk5 kinase activity also increased after removal of bFGF, matching the up-regulation of p35 (Figure 7C). In contrast to Cdk5, Cdk2 kinase activity, a key cell cycle kinase, decreased dramatically after removal of bFGF, presumably reflecting the kinetics of p27Kip1 expression (Figure 7D). These data are consistent with the view that the process of exiting the cell cycle is tightly coupled to the early stages of neural stem cell differentiation and the robust expression of p27Kip1.
Figure 7.
P27Kip1 expression is up-regulated during NSC differentiation. (A) Cdk5+/+ NSC were depleted of bFGF and cultured for 7 d to promote neural differentiation. At different time points cells were assayed immunocytochemically with antibodies to BrdU and TuJ1 so as to monitor proliferating and differentiating cells. Panels a–d display a progressive increase in TuJ1 expression in neurites as cells differentiate. Bar, 50 μm. (B) During the same time course, cells were lysed and prepared for Western blots using an antibody to p27Kip1. p27Kip1 expression increased dramatically during the same time course together with p35 and Cdk5. (C) Cdk5 IPs were prepared from lysates at different times after bFGF depletion and assayed for kinase activity. The bar graph indicates that Cdk5 activity increases rapidly over the same time course of neural differentiation. (D) Cdk2 immunoprecipitations were prepared from cells over the same time course and assayed for kinase activity. Decline in activity correlates with cell cycle exit and neural differentiation. Data in C and D represent mean ± SEM of three experiments.
Cdk5 Phosphorylation of p27Kip1 at Thr187 Correlates with the Onset of NSC Differentiation
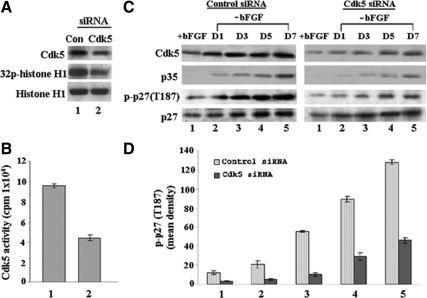
Because Cdk5−/− embryos were limited, sizeable KO NSCs were not always readily available, making it difficult to compare the time course of differentiation with the WT cultures studied above. Instead, we could easily prepare Cdk5+/+ NSCs according to our negative selection procedure and grow these into relatively large proliferating populations in the presence of bFGF. Our intent was to transfect these Cdk5+/+ NSC cultures with Cdk5 siRNA as a model of Cdk5−/− NSCs, recognizing the unlikelihood of completely down-regulating Cdk5 activity to a level equivalent to that of a Cdk5−/−. We prepared control nonsilencing and Cdk5 siRNAs (silencing) sense and antisense sequences, with Cdk5 siRNA designed against the mouse Cdk5 cDNA nucleotide sequence spanning base pairs 732–757. The control nonsilencing sequences were, sense and antisense 5′r(UUCUCCGAACGUGUCACGU)d(TT)3′ and 5′r(ACGUGACACGUUCGGAGAA)d(TT)-3′, respectively, whereas Cdk5 siRNA sense and antisense sequences were 5′r(CAUGACCAAGCUGCCAGACUAUAAG)d(TT)3′ and 5′r(CUUAUAGUCUGGCAGCUUGGUCAUG)d(TT)-3′, respectively. The sense and antisense strands were annealed to create the double-stranded siRNA at a 20 μM concentration. Final concentrations (20 nM) of siRNAs were transfected into WT E13 mouse NSCs (after sorting) using the Lipofectamine 2000 reagent according to the manufacturer's (Invitrogen) instructions. After 48-h transfection, bFGF was removed, and cells were harvested and lysed at 1, 3, 5, and 7 d for Western blot analyses. Initially we compared the expression levels and activities of Cdk5 in cultures transfected with control and silencing Cdk5 siRNA (Figure 8, A and B). The results show that Cdk5 activity was down-regulated ∼50% in Cdk5 siRNA-transfected cultures, a level that is significantly greater than that seen in the Cdk5−/− cells. Nevertheless, when induced to differentiate in the absence of bFGF for 7 d, the expression of Cdk5 was significantly reduced in the Cdk5 siRNA-treated cells compared with control (Figure 8C), accompanied by a reduction in neurite outgrowth and Tuj1 expression. This correlated with the reduced expression of phosphorylated p27Kip1 (p-p27Kip1; at Thr187) and even the total p27Kip1 (Figure 8C). The histogram in Figure 8D quantifies the change in density of p-p27Kip1 expression in control and siRNA-treated NSC during the 7-d period of induced differentiation. The dramatic reduction of p-p27Kip1 expression, as a consequence of the reduced Cdk5 activity, is consistent with the hypothesis that in addition to the up-regulation of p27Kip1, its phosphorylation by Cdk5 at Thr187 is necessary for the induction of neural differentiation in NSCs.
Figure 8.
p27Kip1 is decreased during neural differentiation in Cdk5 siRNA knockdown of NSCs. E13 telencephalic NSCs were isolated, induced to proliferate for 4 d with bFGF, and sorted by negative selection (see Materials and Methods). After sorting, cells were replated with bFGF and transfected with control and Cdk5 siRNA. After 48-h transfection, bFGF was removed and cells were harvested at days 0 (+bFGF), 1, 3, 5, and 7. Cell lysates were subjected to Western blotting and kinase assays. (A) Expression of Cdk5 and histone H1 phosphorylation in control and Cdk5 siRNA-transfected cells. (B) Autoradiograph and bar graph show that Cdk5 activity in Cdk5 siRNA-transfected cells is reduced by more than 50%. (C) A time course of Cdk5 phosphorylation at Thr187 after removal of bFGF in both control siRNA and Cdk5 siRNA cells. The first two panels show the expression of Cdk5 and p35 respectively; the third panel is the expression of phosph-p27Kip1 (Thr187), and the bottom panel represents total p27Kip1. (D) A bar graph shows a quantitative comparison of p27Kip1 phosphorylation between control and Cdk5 knockdown cells as a ratio of p-p27Kip1 to total p27Kip1. Data represent mean ± SEM of three different experiments.
p27Kip1 Is Involved in NSC Differentiation into Neurons
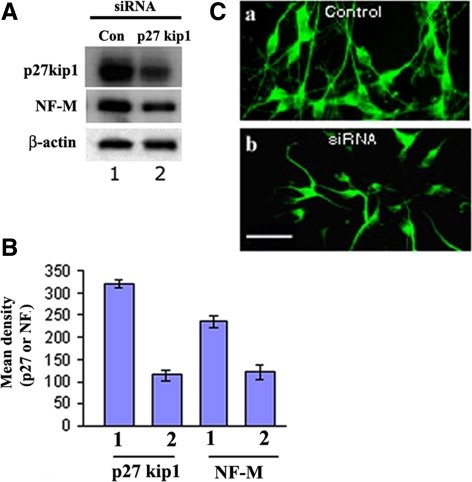
Previous reports have demonstrated the key role of p27Kip1 in neural differentiation (Nguyen et al., 2006 a.b.c.; Kawauchi et al., 2006). In our study, we set out to determine whether p27Kip1 was indeed involved in differentiation of NSCs. Our approach was based on the use of a siRNA to knock down p27Kip1 expression in Cdk5+/+ neural stem cells. We directly transfected the p27Kip1 siRNAs (Sense: 5′-AAGTACGAGTGGCAAGAGGTG-3′ and antisense: 5′-CACCTCTTGCCACTCGTACTT-3′) into expanded Cdk5+/+ NSCs with Trans Messenger transfection reagent (Qiagen, MD). To monitor p27Kip1 expression and NSC differentiation, bFGF was withdrawn for 5 d 72 h after transfection, in order to promote neural differentiation. The expression of p27Kip1 and NF-M was detected by Western blot analysis of lysates (Figure 9, A and B). Both p27Kip1 and NF-M expression were significantly inhibited, consistent with the ICC experiment in Figure 9C, which shows a reduced population of neurons expressing p27Kip1 in the presence of p27Kip1 siRNA. These results are consistent with the view that p27Kip1 is a key player in NSC neurogenesis (Kawauchi et al., 2006; Nguyen et al., 2006a) and that Thr187 is a key site of Cdk5 phosphorylation.
Figure 9.
Inhibition of neurogenesis by small interfering RNA (siRNA) of p27Kip1. (A) Expanded populations of Cdk5+/+ NSCs were transfected with the sense and antisense p27Kip1 siRNAs, respectively, and then deprived of bFGF for 5 d to induce neural differentiation. Western blots of lysates of each cell population were probed with antibodies to p27Kip1 and NF-M. Sample blots of three separate experiments are shown. (B) Bar graphs showing the data (mean density) from A, quantified from three experiments (means ± SEM). (C) Aliquots of cells treated as in A above were prepared for immunocytochemistry and stained with antibody to p27Kip1 to confirm reduced p27Kip1 expression in cells transfected with p27Kip1 siRNA. Scale bar, 20 μm.
p27kip1 Phosphorylated at Thr187 by Cdk5 Is Sufficient for NSC Differentiation
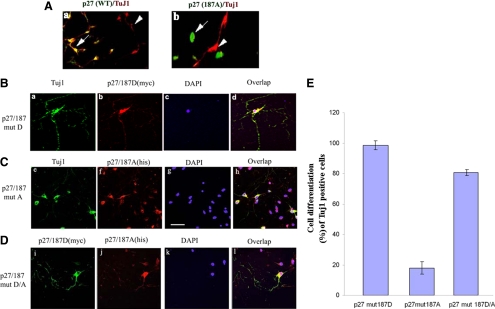
To directly investigate whether Cdk5 phosphorylation of p27 at Thr187 is involved in regulating neuronal differentiation of NSC, we transfected hemagglutinin (HA)-tagged p27 mutant Thr187A or the HA wild-type p27 expression plasmid into Cdk5+/+NSCs, which were expanded in presence of bFGF. After removal of bFGF for 3 d, the cells were double-labeled with anti-TuJ1 and anti-HA antibodies, and the respective number of double-labeled cells was compared (i.e., the numbers of HA-transfected cells expressing TuJ1; Figure 10A, a and b). To further confirm that Cdk5 phosphorylation of p27Kip1 at Thr187 plays a critical role in neuronal differentiation of NSCs, we conducted an experiment to determine whether neural stem cells prevented from differentiating, as above, by transfected mutant p27Kip1 (p27/187A) could be rescued by a phosphorylation-mimicking mutant p27/187D. We transfected expanded Cdk5+/+ neural stem cells with a nonphosphorylatable mutant, p27/187A (His-tag, mut A) or a phosphorylation-mimicking mutant, p27/187D (Myc-tag, mut D), respectively, or with both p27/187A and p27/187D together (mut D/A). Two days after transfection, bFGF was removed, and cells were expanded for 5 more days to differentiate. Afterward, cells were coimmunostained with anti-Myc, anti-His, and Tuj1 antibodies, respectively (Figure 10, B–E). Cells expressing mut D, were positive for both Myc and Tuj1 and showed many neurons with well-developed neurite processes (Figure 10B, a–d). On the other hand, His-positive cells expressing only the mutant p27/187A (Figure 10C, e–h) had blunted neuritic processes, indicating a stalled neuronal differentiation. Differentiation was reduced to ∼20% compared with the p27/187D control (Figure 10E). Double-labeled cells, expressing Myc and His, harboring both the mutants, p27/187D and p27/187A, revealed well-developed neurites (Figure 10D, i–l). When immunostained with the neuronal marker, Tuj1, almost all resembled the cells shown in (10Ba), indicating uncompromised neuronal differentiation; 80% of double-labeled cells were rescued (10E). These data suggest that the phosphorylation-mimicking form p27/187D can rescue the inhibitory effect on neurogenesis by the mutated p27/187A. Furthermore, these results implicate Cdk5-mediated phosphorylation of the p27Kip1 Thr 187 site as a key event in neural differentiation of NSC. Because Ser-10 is phosphorylated by Cdk5 under these conditions, it was necessary to determine whether Ser10 played any role in the differentiation process. Accordingly, a set of experiments were carried out using Ser10A (nonphosphorylatable mutant), and Ser10D (a phospho-mimicking mutant).
Figure 10.
Transfection of mutant p27Kip1 (p27/187A) into NSCs inhibits neurodifferentiation. (A) Expanded cultures of Cdk5+/+ NSCs were transfected with HA-tagged plasmids containing WT p27Kip1 (a) or mutant p27Kip1 (p27/187A) (b) then deprived of bFGF for 3 d to induce neuronal differentiation. In immunocytochemical assays, cells were double-labeled with HA antibody (FITC) to identify p27Kip1-transfected cells (arrows) and TuJ1 (rhodamine) to mark neurons or neural progenitors (arrowheads). A sample field of cells transfected with the WT p27Kip1 is compared with a field of cells with the mutant p27Kip1. (B) Cells expressing with the phosphorylation-mimicking form p27/187D (Myc-tagged) showed many neurons with well-developed neurite processes (a–d); (C) cells expressing the mutant p27/187A (His-tagged) had blunted neuritic processes (e–h); (D) cells with double expressing mutants p27/187D and A display well-developed TuJ1 stained neurites (i–l), which suggest that the phosphorylation-mimicking mutant 187D rescued the nonphosphorylation-mimetic form, 187A. (E) NSCs with well-developed neurites reflecting unperturbed neuronal differentiation were counted in order to determine the relative rate of differentiation in the total cell population. The histogram expresses the neurodifferentiation rate (% of cells with well-developed neurites as opposed to cells with blunted neurites or no neurites), as obtained from the experiment presented in A–D. Scale bar, 20 μm.
Effect of Cdk5 Phosphorylation of Ser10-p27Kip1 in NSCs Differentiation
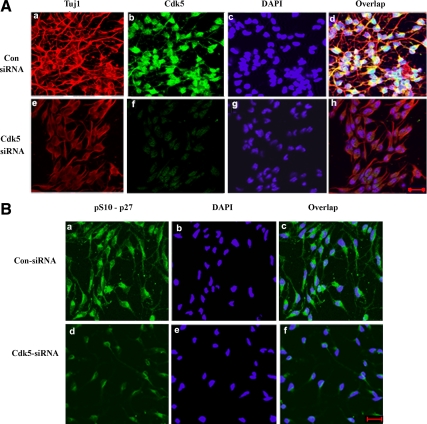
Because Cdk5 phosphorylates both Ser10 and Thr187, in the experiment above it is likely that Ser10 was phosphorylated in both expressed mutant proteins. If Ser10 phosphorylation is implicated in neuronal migration as reported (Kawauchi et al., 2006), it is possible that Ser10 phosphorylation also contributes to the process of neuronal differentiation. To explore the role of Ser10 phosphorylation in neural differentiation, we first examined the effect of Cdk5 siRNA on neurite outgrowth as seen in an ICC of Cdk5+/+ NSCs deprived of bFGF for 3 d (Figure 11A). In cells treated with Cdk5 siRNA, one can assume that both sites, Ser10 and Thr187, are poorly phosphorylated compared with controls. The elaborate TuJ1-stained neurite network seen in the control siRNA panel above (a and d) is absent in the lower panel of cells expressing Cdk5 siRNA. Though cell bodies express TuJ1 weakly (e and h), in the absence of Cdk5 activity, neurite outgrowth is inhibited and TuJ1 is not expressed in neurites. The phosphorylation of Ser10 p27Kip1 is seen in cell bodies and neurites of control cells (Figure 11B, a–c), whereas cells in which Cdk5 is down-regulated by the siRNA exhibit a weak expression of p-Ser10 p27Kip1 and no extensive neurite outgrowth (Figure 11B, d–f). These results suggest that neural differentiation as defined by TuJ1 expression and neurite outgrowth may depend on Cdk5 phosphorylation at both sites.
Figure 11.
Effect of Cdk5 phosphorylation of p27Kip1 Ser10 in neural stem cell differentiation. (A) E13 WT NSCs were expanded in bFGF, transfected with control siRNA and Cdk5 siRNA for 48 h and then deprived of bFGF for 3 d to differentiate as neurons. Cells were plated for ICC analysis using TuJ1 expression as a measure of differentiation. Control cells are in a–d (top) and Cdk5 siRNA-transfected cells in e–h (bottom). (B) A similar sample of transfected cells shows the expression pattern of phospho-Ser10-p27Kip1 antibody in the control cells (a–c, top) and in Cdk5 siRNA-transfected cells (d–f, bottom). Scale bar, 20 μm.
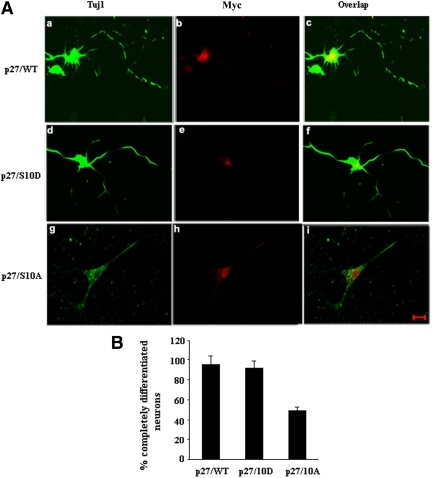
This conclusion was further tested by experiments with Ser10 mutants, a phosphomimetic Ser10D p27Kip1 and a nonphosphorylatable Ser10A p27 mutant, both Myc tagged. Mutants were individually transfected into expanded Cdk5+/+ NSCs followed by 3 d of bFGF withdrawal to promote neural differentiation (Figure 12). The WT p27Kip1 myc tag–transfected control cells display robust TuJ1 expression in perikarya and in long neurites (a–c). A similar pattern is observed in cells expressing the Ser10D phosphomimicking mutant (d–f). Cells transfected with the Ser10A nonphosphorylatable mutant, however, are not profoundly differentiated compared with S10D or WT. p27Kip1-transfected cells, as we can see form short neurites with a reduced TuJ1 expression (g–i). For each of these mutants, it is likely that the Thr187 site was phosphorylated, which suggests that although cells were induced to differentiate with activation of TuJ1 expression, in the absence of Ser10 phosphorylation in the Ser10A-p27Kip1 mutant, extensive neurite outgrowth with TuJ1 expression did not occur. The data suggest that phosphorylation of both sites by Cdk5 may be necessary and sufficient for the full expression of neuronal differentiation.
Figure 12.
Phosphorylation of Ser10 of p27Kip1 is involved in neurite outgrowth. (A) Cdk5+/+ NSCs were expanded in bFGF for 3 d and then transfected with Myc-tagged vectors bearing WT p27Kip1 (a–c), phospho-mimicking p27/10D (d–f) and nonphosphorylatable mutant the p27/10A (g–i). After removal of bFGF for 3 d to induce neuronal differentiation, cells were fixed and prepared for ICC, and immunoprobed with antibody to TuJ1 and Myc. Scale bar, 20 μm. (B) The histogram represents % of cells with well-developed neurites as opposed to cells with blunted neurites or no neurites, as obtained from four independent experiments.
DISCUSSION
To study the role of Cdk5 in early cortical neurogenesis, we isolated uncommitted NSCs from the telencephalon of E13 WT and Cdk5−/− mouse brains using a negative selection cell-sorting strategy previously developed for rat cortical neurons (Maric et al., 2003, 2007; Maric and Barker, 2004, 2005). Both cell populations proliferated actively for many generations in the presence of bFGF, were nestin positive, expressed no surface epitopes characteristic of neural or glial progenitors and were equally apoptotic. Self renewal of Cdk5+/+ and Cdk5−/− E13 cortex was further confirmed by BrdU expression and the equivalent formation of secondary neurospheres. When challenged with bFGF removal, these cells differentiated into lineage-restricted neuronal progenitors (TuJ1+, NF-M+) and astroglial (GFAP) phenotypes. These cells meet NSC criteria: they are self-renewing for many generations and differentiate into neuronal and glial phenotypes. Cdk5−/− cells, however, exhibited a reduction in the neurogenic output (TuJ1- and NF-M–positive cells) compared with Cdk5 WT. This suggested that Cdk5 is essential for neuronal differentiation.
Our data showing Cdk5 phosphorylation of p27Kip1 at Thr187 in NSC is consistent with previous studies on neural differentiation. p27Kip1 has been implicated in inducing cell cycle arrest in promoting neurogenesis in the retina and developing CNS (Ohnuma et al., 1999; Farah et al., 2000; Vernon et al., 2003; Vernon and Philpott, 2003). Though neurons do differentiate and migrate in the p27Kip1 null mouse (probably compensated by other cell cycle inhibitors such as p21Cip1), abnormal patterns of cell cycle exit affect the numbers of mature projection neurons that arise and populate specific cortical layers (Goto et al., 2004; Gui et al., 2007).
p27Kip1 plays a dual role in neurogenesis (Ohnuma et al., 1999; Vernon et al., 2003; Vernon and Philpott, 2003; Nguyen et al., 2006a,b). Overlapping domains in the N-terminal region of p27Kip1 independently terminate progenitor cell cycles and determine neuronal cell fate by stabilizing the proneural gene neurogenin2, an upstream bHLH transcription factor (Nguyen et al., 2006a). Recently, Cdk5 regulation of neuronal migration has been proposed by virtue of phosphorylating p27Kip1 at Ser10, thus stabilizing phospho-p27Kip1 and regulating actin dynamics (Kawauchi et al., 2006). The S10 phosphorylation of p27Kip1 by Cdk5 is shown to stabilize the p27Kip1 protein levels (Kawauchi et al., 2006).
We found that neuronal differentiation of Cdk5+/+ NSCs, in the absence of bFGF is dependent on the up-regulation of p27Kip1and its phosphorylation at Thr187. This correlates with cell cycle exit as seen in the progressive decline of Cdc2 activity, and the appearance of TuJ1-positive neurites. In the absence of Cdk5, NSCs exhibit a significant decrease in TuJ1 and NF-M–positive neurons and p27Kip1 phosphorylated at Thr187. More compelling is the demonstration of reduced TuJ1-positive neurons compared with the Cdk5+/+ control, after transfection of Cdk5+/+ NSCs with a mutant, nonphosphorylatable (Thr187A) p27Kip1. Moreover, such Cdk5+/+ NSC, blocked in neural differentiation by a transfected p27/187A, were rescued when cotransfected with the phosphomimetic p27/Thr187D mutant; cells exhibited a dramatic improvement in neuronal morphology compared with p27/Thr187A expressing cells, with extended neurites and intense TuJ1 expression. It would appear that it is sufficient to provide a phosphorylation-mimicking p27Kip1 Thr 187site in order for NSC to differentiate as neuronal progenitors. However, because both p27Kip1 mutants in these experiments possess a phosphorylatable N-terminal Ser10 site, it is likely that under the conditions of the experiment, this site was also phosphorylated by Cdk5 in all cells (Kawauchi et al., 2006). Because phosphorylation at the Ser10 site promotes neuronal migration, it was necessary to determine if this site plays any role in the differentiation process. Initially we showed that the Ser10 site is indeed phosphorylated by Cdk5 in NSC and promotes outgrowth of TuJ1-positive neurites. Cells expressing the Ser10A mutant, however, failed to form extended neurites. Because the Thr187 site on this mutant was phosphorylated, transfected cells exhibited a low level of TuJ1 expression as if differentiation had been initiated. Evidently for the formation of fully differentiated neurons, both the Ser10 and the Thr 187 site need to be phosphorylated.
In cycling cells, the levels of p27Kip1 regulate the division cycle; high levels promote cell cycle exit, whereas low levels of p27Kip1 characterize proliferating cells. p27Kip1 protein levels are regulated at translation and at protein turnover. p27Kip1 is phosphorylated by Cdk2-cyclin E at Thr 187, which prepares it for ubiquitination via a Skp-2 ligase complex during the S-G1 phase of the cell cycle in preparation for the next round of replication (Pagano et al., 1995; Sheaff et al., 1997; Vlach et al., 1997; Montagnoli et al., 1999; Tsvetkov et al., 1999). In the G0-G1 phase, however, p27Kip1 phosphorylation and degradation are low, which means that p27Kip1 levels are high presumably as cells leave the cell cycle, a condition one would expect to find in progenitor and postmitotic neurons. In fact in NSCs, phosphorylated p27/Thr187 accumulates as cells differentiate into neurons. In transgenic mice expressing the mutant p27/Thr187A gene, there are alternative Skp-2 independent pathways for p27Kip1 degradation that regulate proliferation during development (Malek et al., 2001).
In neurons Cdk5 phosphorylates p27Kip1 at both sites, Ser10 and Thr187 with the former phosphorylation site stabilizing and increasing p27Kip1 protein in the cytoplasm, as neurons migrate (Kawauchi et al., 2006). In the absence of Thr187 phosphorylation, as is the case in the p27/Thr187A mouse transgenic cited above, does Ser10 phosphorylation compensate by stabilizing p27Kip1 to promote neuronal differentiation as well as migration? Our data for NSC in vitro suggest that this does not occur; only a phosphomimetic Thr187 mutant (p27/Thr187D) together with Ser10 phosphorylated p27Kip1 is sufficient to rescue NSC blocked by the nonphosphorylatable mutant p27/Thr187A. It appears that regulation of p27Kip1 in neurons during differentiation differs fundamentally in vitro and in vivo. But how?
In neurons, in contrast to cell cycle Cdks, Cdk5/35 is protected from inhibition by p27Kip1 (Lee et al., 1996). This makes sense because Cdk5/p35 is essential for normal corticogenesis (Oshima et al., 1996). Because of its multiple domains, p27Kip1 is implicated in cell cycle exit, differentiation, and migration in neuronal development (Nguyen et al., 2006a,b). Each domain, N- as well as C-terminal, may require phosphorylation to launch neurogenesis. If phosphorylation at the Thr187 site is blocked as in the p27/Thr187A mutant, another site in the C-terminal region may be phosphorylated to compensate. In fact, a third proline-directed phosphorylation site at Ser178 does exist and is phosphorylatable (Rodier et al., 2001). Hence, in the absence of phosphorylation at Thr187, a compensatory phosphorylation at Ser178, together with the phosphorylation at Ser10, may be sufficient to initiate neurogenesis. Compensatory phosphorylation at Thr178 may come about only in situ where mitogens and other niche factors from radial glial, ependymal, and endothelial cells, including extracellular matrix, are known to play a key role in neural differentiation in the embryo and the adult (Goldberg and Hirschi, 2009; Illes et al., 2009; Miller and Gauthier-Fisher, 2009; Williams and Lavik, 2009). Accordingly, in the p27/Thr 187A transgenic in vivo, we suggest that a combination of niche factors and cell interactions signal a compensatory phosphorylation in the C-terminal domain or evoke an alternative pathway to neurogenesis. In the absence of such niche factors, NSCs in vitro, expressing the p27/Thr187A mutation, do not signal the compensatory phosphorylation and only respond positively to the introduction of the phospho-mimetic mutation p27/Thr187D. In cultured NSCs, phosphorylation of Thr 187 of p27Kip1 by Cdk5, seems to be necessary, and to promote neural differentiation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by intramural funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Abbreviations used:
- Cdk5
cyclin-dependent kinase 5
- FACS
fluorescence-activated cell sorting
- NSC
neural stem cell.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0054) on September 1, 2010.
REFERENCES
- Ayala R., Shu T., Tsai L. H. Trecking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Cheng K., Ip N. Y. Cdk5, a new player at synapses. Neurosignals. 2003;12:180–190. doi: 10.1159/000074619. [DOI] [PubMed] [Google Scholar]
- Cicero S., Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J. Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R., Tsai L. H. A decade of CDK5. Nat. Rev. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Farah M. H., Olson J. M., Sucic H. B., Hume R. I., Tapscott S. J., Turner D. L. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Goldberg J. S., Hirschi K. K. Diverse roles of the vasculature within the neural stem cell niche. Regen. Med. 2009;4:879–897. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Mitsuhashi T., Takahashi T. Altered patterns of neuron production in the p27 knockout mouse. Dev. Neurosci. 2004;26:208–217. doi: 10.1159/000082138. [DOI] [PubMed] [Google Scholar]
- Grant P., Sharma P., Pant H. C. Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur. J. Biochem. FEBS. 2001;268:1534–1546. [PubMed] [Google Scholar]
- Gui H., Li S., Matise M. P. A cell-autonomous requirement for Cip/Kip cyclin-kinase inhibitors in regulating neuronal cell cycle exit but not differentiation in the developing spinal cord. Dev. Biol. 2007;301:14–26. doi: 10.1016/j.ydbio.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes S., Theiss S., Hartung H. P., Siebler M., Dihne M. Niche-dependent development of functional neuronal networks from embryonic stem cell-derived neural populations. BMC Neurosci. 2009;10:93. doi: 10.1186/1471-2202-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Aigner S., Clemenson G. D., Jr, Toni N., Lie D. C., Karalay O., Overall R., Kempermann G., Gage F. H. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T., Chihama K., Nabeshima Y., Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Kesavapany S., Li B. S., Amin N., Zheng Y. L., Grant P., Pant H. C. Neuronal cyclin-dependent kinase 5, role in nervous system function and its specific inhibition by the Cdk5 inhibitory peptide. Biochim. Biophys. Acta. 2004;1697:143–153. doi: 10.1016/j.bbapap.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Lagace D. C., Benavides D. R., Kansy J. W., Mapelli M., Greengard P., Bibb J. A., Eisch A. J. Cdk5 is essential for adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Nikolic M., Baptista C.A., Lai E., Tsai L.H., Massague J. The brain-specific activator p35 allows Cdk5 to escape inhibition by p27Kip1 in neurons. Proc Natl Acad Sci USA. 1996;93:3259–3263. doi: 10.1073/pnas.93.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. S., Zhang L., Takahashi S., Ma W., Jaffe H., Kulkarni A. B., Pant H. C. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 2002;21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien L. Neural progenitors and stem cells: mechanisms of progenitor heterogeneity. Curr. Opin. Neurobiol. 1998a;8:37–44. doi: 10.1016/s0959-4388(98)80006-8. [DOI] [PubMed] [Google Scholar]
- Lillien L. Progenitor cells: what do they know and when do they know it? Curr. Biol. 1998b;8:R872–R874. doi: 10.1016/s0960-9822(07)00548-9. [DOI] [PubMed] [Google Scholar]
- Malek N.P., Sundberg H., McGrew S., Nakayama K., Kyriakides T.R., Roberts J.M. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature. 2001;413:323–327. doi: 10.1038/35095083. [DOI] [PubMed] [Google Scholar]
- Maric D., Barker J. L. Neural stem cells redefined: a FACS perspective. Mol. Neurobiol. 2004;30:49–76. doi: 10.1385/MN:30:1:049. [DOI] [PubMed] [Google Scholar]
- Maric D., Barker J. L. Fluorescence-based sorting of neural stem cells and progenitors. Curr. Protoc. Neurosci. 2005 doi: 10.1002/0471142301.ns0318s33. Chapter 3, Unit 3.18. [DOI] [PubMed] [Google Scholar]
- Maric D., Fiorio Pla A., Chang Y. H., Barker J. L. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J. Neurosci. 2007;27:1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D., Maric I., Chang Y. H., Barker J. L. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J. Neurosci. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D., Maric I., Chang Y.H, Barker J.L. Stereotypical physiological properties emerge during early neuronal and glial lineage development in the embryonic rat neocortex. Cereb. Cortex. 2000;10:729–747. doi: 10.1093/cercor/10.8.729. [DOI] [PubMed] [Google Scholar]
- Miller F. D., Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Braun N., Shukla V., Füllgrabe M., Schomerus C., Korf H.W., Gachet C., Ikehara Y., Sevigny J., Robson S.C., Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Montagnoli A., Fiore F., Eytan E., Carrano A.C., Draetta G.F., Hershko A., Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., Philpott A., Roberts J. M., Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006a;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., Philpott A., Roberts J. M., Guillemot F. [p27Kip1 independently promotes neuronal differentiation and migration in the cerebral cortex] Bulletin et memoires de l'Academie royale de medecine de Belgique. 2007;162:310–314. [PubMed] [Google Scholar]
- Nguyen L., Besson A., Roberts J. M., Guillemot F. Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle. 2006b;5:2314–2318. doi: 10.4161/cc.5.20.3381. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Borgs L., Vandenbosch R., Mangin J. M., Beukelaers P., Moonen G., Gallo V., Malgrange B., Belachew S. The yin and yang of cell cycle progression and differentiation in the oligodendroglial lineage. Mental Retardation Dev. Disabilities Res. Rev. 2006c;12:85–96. doi: 10.1002/mrdd.20103. [DOI] [PubMed] [Google Scholar]
- Ohnuma S., Philpott A., Wang K., Holt C. E., Harris W. A. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna, Pant H. C., Brady R. O., Martin L. J., Kulkarni A. B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Tam S.W., Theodoras A.M., Beer-Romero P., Del Sal G., Chau V., Yew P.R., Draetta G.F., Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Philpott A., Tsai L., Kirschner M. W. Neuronal differentiation and patterning in Xenopus: the role of cdk5 and a novel activator xp35.2. Dev. Biol. 1999;207:119–132. doi: 10.1006/dbio.1998.9146. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Rietze R. L. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rodier G., Montagnoli A., Di Marcotullio L., Coulombe P., Draetta G.F., Pagano M., Meloche S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–6682. doi: 10.1093/emboj/20.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R.J., Groudine M., Gordon M., Roberts J.M., Clurman B.E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shukla V., Zimmermann H., Wang L., Kettenmann H., Raab S., Hammer K., Sévigny J., Robson S.C, Braun N. Functional expression of the ecto-atpase ntpdase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci. Res. 2005;80:600–610. doi: 10.1002/jnr.20508. [DOI] [PubMed] [Google Scholar]
- Tang D., Yeung J., Lee K. Y., Matsushita M., Matsui H., Tomizawa K., Hatase O., Wang J. H. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Delalle I., Caviness V. S., Jr, Chae T., Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Tsvetkov L.M., Yeh K.H., Lee S.J., Sun H., Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- Vernon A. E., Devine C., Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development. 2003;130:85–92. doi: 10.1242/dev.00193. [DOI] [PubMed] [Google Scholar]
- Vernon A. E., Philpott A. A single cdk inhibitor, p27Xic1, functions beyond cell cycle regulation to promote muscle differentiation in Xenopus. Development. 2003;130:71–83. doi: 10.1242/dev.00180. [DOI] [PubMed] [Google Scholar]
- Vlach J., Hennecke S., Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. A., Lavik E. B. Engineering the CNS stem cell microenvironment. Regen. Med. 2009;4:865–877. doi: 10.2217/rme.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li H., Yabut O., Fitzpatrick H., D'Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7:3487–3490. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.L., Li B.S., Kanungo J., Kesavapany S., Amin N., Grant P, Pant H.C. Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol. Biol. Cell. 2007;18:404–413. doi: 10.1091/mbc.E06-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. L., Kesavapany S., Gravell M., Hamilton R. S., Schubert M., Amin N., Albers W., Grant P., Pant H. C. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur. J. Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.