Synopsis
Cholangiocarcinoma are rare malignant tumors whose incidence is increasing worldwide. Risk factors for this malignancy include biliary diseases characterized by chronic inflammation of the bile duct epithelia. Diagnosis of these cancers remains difficult due to the lack of sensitive diagnostic tests. The prognosis is poor likely due to the lack of effective treatments for unresectable cancer.
Keywords: Biliary tract cancers, liver flukes, Liver cancers
Overview
Recent studies have shown an increased global incidence of cholangiocarcinoma, rare malignant tumors with morphological features of biliary tract epithelia. This aggressive and poorly understood malignancy remains largely incurable. Biliary tract inflammation resulting from liver fluke infection or other causes is a well-defined risk factor for cholangiocarcinoma. Recent studies have explored the relationship between inflammatory mediators and biliary tract carcinogenesis, and provided insight into the molecular and genetic perturbations involved in the pathogenesis of cholangiocarcinoma.
CLASSIFICATION
Cholangiocarcinomas can be classified into two major categories, based on their anatomic location. Intrahepatic cholangiocarcinoma (ICC) arise within the hepatic parenchyma, and most often present as a mass lesion without major bile duct obstruction or jaundice. Ductal cholangiocarcinoma arises within the large bile ducts, namely common bile duct, common hepatic duct, and right or left hepatic ducts up to the secondary bifurcation. Perihilar cholangiocarcinoma, or Klatskin’s tumors, are often considered separately but should be classified as ductal cholangiocarcinoma based on their location and presentation. These lesions can extend into the hepatic parenchyma and have also been classified as intrahepatic or extrahepatic. The lack of a consistent convention has led to inaccurate reporting of the epidemiology, natural history and prognosis. Amongst the hilar lesions, the Bismuth classification has been widely used as a guide to surgical intervention. Intrahepatic and ductal cholangiocarcinoma can be further classified based on pathological basis. The staging system used by the Liver Cancer Study Group of Japan classifies intrahepatic cholangiocarcinomas as mass-forming, periductal infiltrating, intraductal or mixed. Similarly extrahepatic ductal cholangiocarcinoma can be further described as sclerosing, nodular or papillary (1). However, this clinical and pathological classification requires modification to incorporate hilar lesions. The tumor-node-metastasis TNM classification for biliary tract cancers has not been useful in clinical practice as the T classification does not differentiate prognosis e.g. in T2 and T3 tumors (2). In a recent study, the number of lesions and presence of vascular invasion were important prognostic factors, whereas tumor size was not (2).
EPIDEMIOLOGY
The incidence of these cancers shows a marked variation worldwide. In regions where liver flukes are endemic, the rates of intrahepatic cholangiocarcinoma are extremely high, for example, in the Khon Kaen region in Thailand, cholangiocarcinoma accounts for >85% of all cancers. In the United States the incidence ranges from 3–8 per 100,000. In most other parts of the world, the incidence of intrahepatic cholangiocarcinoma has increased over the last few decades, with an annual percent change of about 9% (3;4). However, observations over the past decade indicate that the rate of increase may be leveling off. The grouping together of intrahepatic cholangiocarcinoma and hepatocellular cancer in epidemiological reports has confounded an analysis of the true incidence of these cancers in the United States. The incidence of ductal cholangiocarcinoma is less variable. The reported incidence of extrahepatic cholangiocarcinoma, for example, is twice as high in Manitoba, Canada compared to the United Kingdom (5).
There are racial and gender differences in the incidence of these cancers. The incidence of intrahepatic cholangiocarcinoma is higher in men compared to women (6;7), while the incidence of extrahepatic cholangiocarcinoma is comparable between the two sexes (6). The incidence is increased in African-Americans (1.5-fold), American Indian and Hispanic (1.8-fold) and in Asian American and Pacific Islanders (2.5-fold) compared to Whites in the United States. Overall, the prevalence is greater in males, except in Hispanics, in whom the prevalence is greater in females (7). The overall age-adjusted mortality rates for intrahepatic cholangiocarcinoma in the USA are highest for American Indian/Alaska Native and Asian Pacific groups. However, the rate of increase in mortality is similar for all racial groups with an annual percent change of greater that 3.5%, except for Asian/Pacific islander women for whom mortality rates has been slightly decreasing (7). According to American Cancer Society, in the USA the estimated deaths for extrahepatic CCA in 2008 were 3340 per year with 62% of these occurring in males (6).
RISK FACTORS
Several risk factors have been identified for cholangiocarcinoma (Table 1). Although the risk factors vary geographically, there is a strong association of cholangiocarcinoma with chronic biliary tract infection and inflammation. Well characterized risk factors include liver fluke infestations, chronic viral hepatitis, hepatolithiasis, choledochal cysts, and primary sclerosing cholangitis (PSC).
Table 1.
Clinical scenarios in which a suspicion of cholangiocarcinoma should be raised
| • Jaundice, systemic illness, weight loss in patient with known risk factor such as liver fluke infection, PSC, hepatolithiasis |
| • Jaundice or systemic illness in patient from areas with liver fluke endemicity |
| • Bile duct stricture causing jaundice |
| • Intrahepatic liver mass |
Liver fluke infestation
Liver fluke infestation with either Clonorchis sinensis or Opisthorchis viverrini lead to an increased risk of cholangiocarcinoma. Both of these liver flukes are now considered as grade I carcinogens by the World Health Organization and the International Agency for Research on Cancer.
Clonorchis Sinensis infection is endemic in south China, Japan, Korea and Taiwan due to a long tradition of consuming raw freshwater fish or shellfish. Chronic infection with heavy parasite loads has been associated with various hepatobiliary diseases. Dwelling in the bile ducts, Clonorchis induces an inflammatory reaction involved in the malignant transformation of cholangiocytes. Patients affected by clonorchiasis have a higher risk to develop cholangiocarcinoma. In a recent analysis of more than 3000 Korean patients the incidence of cholangiocarcinoma was 8.6% (8). Since the introduction of Praziquantel the incidence of clonorchiasis has been reduced in the endemic areas. However it has not been eradicated and chronic infestation remains the main cause of cholangiocarcinoma in these areas. The diagnosis of Clonorchis sinensis is problematic, since fecal examination for eggs has low sensitivity and the intradermal test with diluted antigens of clonorchis can cross-react with other parasites such as Paragonimus Westermani (9). Thus, the diagnosis can be missed. Adult worms can remain in the peripheral intrahepatic bile ducts for 20–30 years causing chronic persistent infection (10) and subsequently resulting in cholangiocarcinoma.
Opistorchis viverrini is highly prevalent in Thailand and Laos. As with Clonorchis sinensis, humans become infected by ingesting undercooked fish containing infective metacercariae. This infection is associated with a number of benign hepatobiliary diseases, including cholangitis, obstructive jaundice, hepatomegaly and cholecystitis. The risk of cholangiocarcinoma depends on the intensity of the infection (11), previous or current exposure to infection and host genetic polymorphisms (12). Less than 10% of people infected with O. viverrini may develop cholangiocarcinoma (13). However, other than for Thailand, cancer registration and statistics are not available for many other countries in the region. Thus, an accurate estimate of the risk of developing cholangiocarcinoma is difficult (14). Conversely >80% of cholangiocarcinoma in Thailand are positive for the detection of O.viverrini by real time PCR (15). Repeated infection with O. viverrini induces oxidative DNA lesions, such as 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), in the bile duct epithelium which is carcinogenetic. The urinary level of 8-oxodG was found significantly higher in cholangiocarcinoma patients than in O. viverrini–infected patients and healthy subjects. Praziquantel is effective against O.viverrini and can significantly decrease the urinary level of 8-oxodG. Thus, urinary 8-oxodG may be a useful biomarker to monitor infection and the efficacy of treatment as well as for surveillance for tumors (16). Repeated infections with O.viverrini may accelerate DNA damage (17). Thus, a preventive strategy to reduce infection such as by decreased consumption of infected fish may be a more rationale strategy to reduce the incidence of cholangiocarcinoma than one that is focused on treating established infections.
Primary sclerosing cholangitis
In the Western world, the incidence of liver fluke infestation is quite low and PSC is the main risk factor for cholangiocarcinoma. PSC is a chronic idiopathic inflammatory disorder characterized by fibrosis and bile duct strictures. The risk of cholangiocarcinoma varies from 7 – 40% (18;19). The duration of PSC does not correlate with the development of cholangiocarcinoma, and the estimated risk of cholangiocarcinoma is 9% after 10 or 20 years (19). In a recent study the median interval between the diagnosis of PSC and cholangiocarcinoma was 2.5 years, with all cases developing within 3 years. Thus, it is unclear whether or not cholangiocarcinoma occurs as a synchronous condition or as a consequence of PSC in these patients (20).
Congenital abnormalities of the biliary tract
Patients with choledochal cysts, congenital cystic dilations of the biliary tract are at risk of malignancy. Similarly, patients with Caroli’s disease or congenital hepatic fibrosis, which are developmental abnormalities resulting in multiple intrahepatic cysts are also at risk of developing cholangiocarcinoma.
Chronic viral hepatitis
Hepatitis C virus (HCV) infection has been recently described as a risk factor for cholangiocarcinoma. In a retrospective US cohort study HCV infection was significantly associated with an increased risk of cholangiocarcinoma. Although it remained low (4 per 100,000 person-years), the risk in the HCV-infected cohort was more than doubled than in the uninfected cohort. Interestingly, the risk for extrahepatic cholangiocarcinoma was not increased (21). A prospective cohort study from Japan showed that 2.3% of 600 patients with HCV-related cirrhosis developed ICC during a mean follow up of 7 years, with a risk of developing cholangiocarcinoma significantly higher than general population (22). Although growing evidence is supporting the carcinogenic effects of HCV proteins in hepatocellular carcinoma, the involvement of HCV in cholangiocarcinogenesis is less clear. The HCV core protein can alter cellular proliferation and apoptosis in hilar cholangiocarcinoma cells (23). Since intrahepatic cholangiocarcinoma and hepatocellular carcinoma may arise from the same progenitor cells, common mechanisms may account for malignant transformation (24).
Hepatitis B is also a recognized risk factor. In China, where HBV infection is endemic, the main risk factors are Hepatitis B virus (HBV) infection and hepatolithiasis. The prevalence of HBsAg seropositivity and hepatolithiasis was increased from 9 to 48 % and from 1 to 5% respectively in intrahepatic cholangiocarcinoma patients compared to controls (25). In studies conducted in other regions such as Korea, Italy and USA, HBsAg positivity in ICC patients ranged from 0.2 to 13% (26–29). It is hoped that screening and vaccination strategies for HBV in regions of high endemicity may lead to a reduction of cholangiocarcinoma, similar to the dramatic effects of these strategies in reducing hepatocellular cancer.
Hepatolithiasis
The relationship between hepatolithiasis and cholangiocarcinoma has been long recognized (30). In cholangiocarcinoma associated with hepatolithiasis, the stones are closely situated within or adjacent to the tumor foci. Carcinomatous cells spread along the lumenal surface of the stone-containing bile ducts and invade the ductal walls. Features of “chronic proliferative cholangitis” are usually found within these bile ducts (31). A wide range of molecular alterations have been described in this setting such as inactivation of p16, increased expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2), over-expression of the proto-oncogene c-met and lack of the tumor suppressor caudal-related homeobox gene 2 (CDX2). These have been noted in precursor lesions or in established cholangiocarcinoma (32). Most primary hepatolithiasis is calcium bilirubinate, although cases have also been described in cholesterol hepatolithiasis which are very rare (33).
Alcohol and toxins
Certain exposures may lead to increased risk of cholangiocarcinoma. Multiple case-control analyses have reported an association between cholangiocarcinoma and alcohol use (34;35). Several cases of cholangiocarcinoma have been described following the iatrogenic exposure to thorotrast (thorium dioxide), a radiocontrast agent used in the past (36). Toxin exposures may be linked to outbreaks of cholangiocarcinoma that have been noted in Italy, West Virginia, and British Columbia, although convincing evidence for any likely culprits is lacking.
Cirrhosis and other causes
Cirrhosis has been previously associated with a higher incidence of cholangiocarcinoma in a large cohort study in Denmark (37). The risk of cholangiocarcinoma might be mediated by the HCV-induced liver cirrhosis. Analysis of the Surveillance, Epidemiology and End Results-Medicare database established the relationship between several risk factors in the US population. Cirrhosis as well as primary biliary cirrhosis (PBC) were significantly more common among extrahepatic and intrahepatic cholangiocarcinoma cases than in controls (38). Alcoholic liver disease, type II diabetes, obesity and HIV infection are also significantly associated with cholangiocarcinoma.
Screening for cholangiocarcinoma
The lifetime risk for cholangiocarcinoma varies according to the specific risk factors involved. These tumors are usually silent or associated with non-specific symptoms and diagnosis is frequently late (Fig.1). Although early detection is needed to improve survival rates from these devastating tumors, there are no proven effective screening tests to date. Serum or stool tests for liver flukes as well as urinary level of 8-oxodG might be promising strategies in endemic areas. Societal approaches that target population behavior might be more useful. Several tumor markers may support a diagnosis of cholangiocarcinoma, but none are sensitive enough to be used for screening purposes. The most commonly used markers are carbohydrate antigen (CA 19-9) and carcinoembryonic antigen (CEA). However, they can be elevated in the presence of other malignancies as well as with benign conditions such as cholangitis and hepatolithiasis. Moreover, the value of screening for cholangiocarcinoma is debatable given the poor response to treatment.
Figure 1.
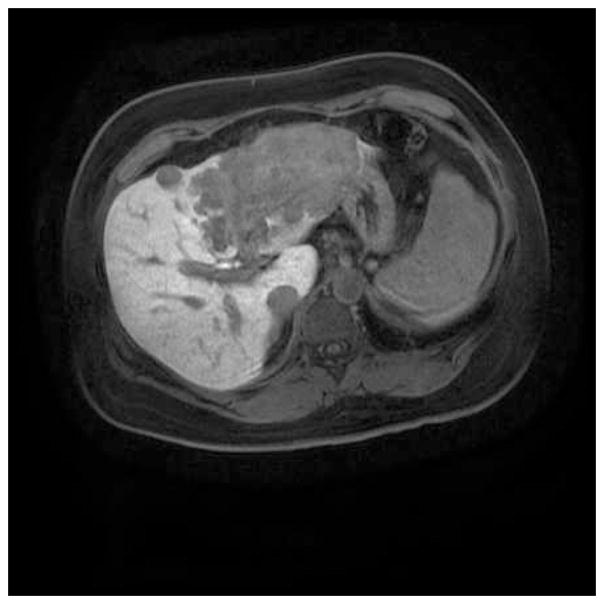
MRI liver showing a multifocal, poorly differentiated intrahepatic cholangiocarcinoma with vascular invasion.
PATHOGENESIS
Study of the pathogenesis of cholangiocarcinoma provides a paradigm for the study of the role of infection and chronic epithelial inflammation in malignancy. The pathogenic mechanisms involved in biliary fluke-associated cholangiocarcinoma are likely to be multifactorial. Biliary tract injury arises as a consequence of mechanical injury due to the migration of the flukes, metabolic toxins and immunopathological processes (39).
Metabolic products released by liver flukes may be directly toxic, or may promote an immunological response that results in proliferation of fibroblasts, and over-expression of transforming growth factors (40) and metalloproteinases (41). Evidence for fluke-induced host inflammatory response in mediating biliary damage is further provided by the expression of Opistorchis antigens in peripheral macrophages and epithelioid cells (42). Subsequent cytokine-dependent activation of effector cells results in oxidative stress which causes cytotoxicity and enhances mutagenesis. This results in DNA damage with malfunction of mismatch repair systems and aberrant regulation of cellular apoptosis. N-nitroso compounds are primary carcinogens leading to cholangiocarcinoma. Humans infected with Opistorchis seem to have a higher endogenous nitrosation potential than uninfected people as result of the production of nitric oxide (NO) and the stimulation of NO synthase (NOS). Population-based variations in the expression of genes involved in the detoxification of carcinogens may also contribute to the high incidence in endemic areas such as Thailand compared to non endemic areas (43).
Biliary constituents
Carcinogenesis of biliary epithelia is a multistep process that involves the transformation from hyperplasia to dysplasia and eventually to carcinoma. Chronic inflammation and cellular injury together with partial obstruction of bile flow results in bile-stasis and in chronic exposure of biliary cells to the carcinogenetic action of bile components. The bile from patients with inflammatory biliary injuries contains increased levels of oxysterols, oxygenated derivatives of cholesterol. Oysterols and bile acids, such as deoxycholic acid, may promote carcinogenesis by inducing the expression of COX-2, transactivating the Epidermal Growth Factor Receptor (EGFR), repressing E-Cadherin and blocking the degradation of the antiapoptotic myeloid cell leukemia protein 1 (Mcl-1) (44;45). In experimentally induced cholestasis, reduced glutathione (GSH), as well as the enzymes indispensable for GSH synthesis, are decreased in the bile. GSH participates in the detoxification of many molecules and in the defense against oxidative stress. Therefore, the alteration in GSH content may lead to DNA damage and deregulation of apoptosis in cells of patients with chronic biliary disorders (46).
Genetic and epigenetic abnormalities
Neoplastic transformation of biliary epithelia is accompanied by a number of molecular and genetic alterations. Activation of autonomous growth signaling, such as hepatocyte growth factor (HGF)/met, Interleukin-6 (IL-6), ErbB-2, K-ras (20–50%), BRAF and COX-2 are responsible for abnormal cell proliferation and survival. Abnormalities of DNA mismatch repair, such as microsatellite instability, increase the risk of genetic damages. Immortalization of biliary cells is mediated by the modulation of telomerase activity (47). Several tumor suppressor genes are inactivated in cholangiocarcinoma. P53 is usually lost in cholangiocarcinoma cells (20–70%) due to loss of heterozygosity or inactivating mutations. p16 INK4a is frequently silenced by promoter hypermethylation. Inactivation of p16 together with lack of p21Waf1/cip1,p27 Kip1, p57 kip2 and increased cyclin D1 are responsible for cell cycle dysregulation. Regulation of apoptosis is aberrant in cholangiocarcinoma cells due to the over-expression of antiapoptotic proteins, such as Bcl-2, Bcl-xl and Mcl-1. Specific mucin antigen expression profiles have been associated with predictive value. Invasion and metastasis are favored by the loss of E-cadherin and catenins. Cholangiocarcinoma growth may be facilitated by increased angiogenesis mediated by over-expression of Vascular Endothelial Growth Factor (VEGF), COX-2, Transforming Growth Factor (TGF) beta1 (48;49).
Cytokine mediated signaling pathways
Several cytokines or growth factors are known to have mitogenic or proliferative effects on biliary cells, such as IL-6, HGF, TGF-α, EGF, cerbB-2, heterogeneous immunoglubulin A (IgA), and leukocyte inhibitory factor (LIF) via an autocrine or paracrine effect. Neoplastic transformation of biliary cells is associated with constitutive production of IL-6, as demonstrated by positive cytoplasmic immunohisotchemical staining and over-expression of IL-6 mRNA and protein in cholangiocarcinoma cells (50). This production is significantly enhanced by other inflammatory cytokines, such as Tumor Necrosis Factor (TNF)-alpha and Interleukin-1, as result of the complex paracrine-autocrine stimulation that takes place in the inflammatory-mediated carcinogenesis (51). In addition, expression of IL-6 receptor on tumor cells make them hyper-sensitive to the exogenous IL-6 from environmental sources such as stromal and immune cells (51;52). The response to IL-6 stimulation differs between normal and malignant cholangiocytes with aberrant expression of p38 MAPK in the latter (51;53–55). IL-6-signaling modulates gene expression and sustains mitogenic signals that promote cholangiocarcinoma cell growth and survival through different mechanisms (55). Some of these effects are exerted through the modulation of microRNAs, small non coding RNAs that regulate gene expression. In in vitro and in animal models, IL-6 over-expression was shown to reduce the expression of miR-370 resulting in the enhancement of p38MAPK activation (56). Interestingly in others disease models IL-6 expression is regulated by microRNAs, suggesting that a circulatory loop might be responsible for the sustained expression of IL-6 in cholangiocarcinoma. IL-6 may also alter the methylation status of cholangiocarcinoma cells by increasing the expression of DNA methyltransferase (DNMT) 1. This results in methylation-mediated silencing of oncosuppressor genes and promotion of cellular proliferation and survival (57). Furthermore, IL-6 mediates chemo-resistance of cholangiocarcinoma cells by the p38 and STAT3-dependent modulation of the anti-apoptotic protein Mcl-1 (54;55). All together these data suggest that IL-6 signaling plays a central role in colangiocarcinogenesis and provide the rationale for the evaluation of IL-6 targeting agents as sensitizers or cytotoxic agents useful in cholangiocarcinoma treatment.
Oxidative injury and DNA damage
Biliary injury of diverse causes results in the recruitment of inflammatory cells and the release of pro-inflammatory cytokines which increase the generation of NO by inducing iNOS. NO may favor the possibility of oncogenic mutations, may inhibit apoptosis through the nitrosylation of caspase 9 and may cause bile ductular cholestasis by inhibiting ion transporters of the biliary cells (58).
All this evidence confirms that the pathogenesis of cholangiocarcinoma is enhanced by inflammatory mediators that mediate oncogenic signaling. The relevance of inflammation in cholangiocarcinoma is also supported by recent finding showing that patients with a neutrophil to lymphocyte ratio (NLR) > 5 have larger tumors with intrahepatic satellite lesions, micro-vascular invasion and lymoh node involvement and predicts poorer overall and disease free survival in patients undergoing radical surgery. This index may reflect a weaker lymphocyte mediated immune response to the tumor and the enrichment of cytokines produced by circulating and intra-tumoral neutrophils that can enhance tumor cell growth (59).
DIAGNOSIS
Clinical presentation
Suspicion of cholangiocarcinoma may be raised in several different clinical scenarios (Table 2). Intrahepatic cholangiocarcinoma usually present as a mass-lesion within the liver and may be asymptomatic until they are quite advanced. Ductal cancers are more likely to be symptomatic due to biliary obstruction and often present with jaundice. Because of the tendency of these cancers to spread along the biliary tract they may also be more extensive at time of diagnosis.
Table 2.
Risk factors for cholangiocarcinoma
| • Liver flukes (clonorchis sinensis, opistorchis viverrini) |
| • Primary Sclerosing Cholangitis |
| • Choledochal cysts |
| • Viral hepatitis B and C |
| • HIV |
| • Cirrhosis |
| • Hepatolithiasis |
| • Toxins |
| • Lynch syndrome |
| • Diabetes |
| • Obesity |
Risk factor analysis
In liver fluke endemic regions, an assessment of liver fluke infection can be performed using stool studies to look for eggs. PCR-based techniques capable of amplifying species of flukes directly from eggs have a better sensitivity and specificity than the standard microscopic examination of stool samples (60). Use of these or other similar non-invasive assays may be helpful for diagnosis as well as for epidemiological surveys of the distribution of liver flukes and may enable individual or population based targeted approaches. In patients with known ulcerative colitis, evaluation for serum markers of cholestasis, abdominal imaging, ultrasonography or cholangiography may be useful to diagnose PSC.
Diagnostic studies
A combination of tumor markers, imaging and cytological studies may be used to diagnose cholangiocarcinoma (Table 3). The diagnosis is difficult to make, especially in patients with underlying PSC who may have biliary stictures. For patients with intrahepatic cholangiocarcinoma, biopsy may reveal adenocarcinoma, which may prompt evaluation and exclusion of potential other primary sites.
Table 3.
Diagnostic tests for cholangiocarcinoma
| Diagnostic test | Sensitivity |
|---|---|
| Tumor markers | |
| CA19.9 | 80–90% |
| CEA | 30% |
| Radiology | |
| Computational Tomography (CT) | 50–60% |
| Magnetic Resonance Imaging (MRI) | 50–90% |
| Intraductal ultrasound | 89–95% |
| Endoscopy | |
| Cholangiography and sampling | 45–75% |
| Nuclear medicine | |
| PET | 85% (18% in infiltrative CCA) |
| Cytology analysis | |
| Routine cytology | 16–68% |
| Digital Imaging Analysis (DIA) | 44% |
| Fluorescence In Situ Hybridization (FISH) | 43% |
TREATMENT
Surgery
Survival from cholangiocarcinoma is extremely poor with an average 5-year survival rate of ~5%. Surgical resection or transplantation could be curative in selected patients who have no evidence of distant spread, vascular invasion or extrahepatic spread with limited resectable disease. For ductal carcinomas, exploration to assess resectabiliaty may be appropriate to consider in such patients. For intrahepatic cholangiocarcinoma, hepatic resection may be appropriate. Reported outcomes show a wide variation. Although recent series suggest that outcomes have improved in recent years, variable classifications and differing patient populations make interpretation difficult. Liver transplantation is appropriate for very few individuals with localized, early stage disease and is only offered at a handful of centers worldwide. Neither transplantation nor resection are appropriate for individuals with evidence of portal vein or hepatic artery invasion on pre-operative imaging, or endoscopic ultrasound. There is no data on the benefit of using adjuvant therapy after a margin-negative resection.
Photodynamic therapy
This involves the intravenous administration of a photosensitizer followed by endoscopic delivery of light at a specific wavelength which will cause tumor cell death due to oxidative injury. Use of this approach with stenting can improve survival compared to stenting alone. It should be considerd for patients with locally advanced disease but it is not widely available.
Medical
Cholangiocarcinoma is highly resistant to chemotherapy. However, systemic chemotherapy may be considered in patients with advanced cholangiocarcinoma as there may be some benefit compared to best supportive care (61). The literature is quite limited and consists of several small series involving multiple tumors only. Various chemotherapeutic agents, dosing regimens and combinations have been tested with overall poor survival improvement. 5-FU, gemcitabine, oxaliplatin and docetaxel have shown the most activity as single agents (62). A recent phase III trial evaluated the combination of gemcitabine and cisplatin and showed this combination to be superior to single agent chemotherapy with a higher response rate and a prolonged overall survival that reached 11. 7 months in the combination arm. According to these data gemcitabine and cisplatin should be considered as the standard therapy for locally advanced and metastatic cholangiocarcinomas (63). Several new molecular targeted agents and biological agents are being explored for the treatment of cholangiocarcinoma.
Palliative therapy
In patients with biliary tract obstruction, decompression may be helpful. Cholestatic liver dysfunction and biliary cirrhosis may rapidly occur in patients with unrelieved obstruction. However, placement of stents in patients who are candidates for surgery may interfere with preoperative evaluation for resectability and intraoperative determination of tumor extent.
PREVENTION
In regions of high liver fluke endemicity, the optimal approach to chemoprevention involves appropriate public health measures and education to reduce the impact and incidence of liver fluke infestation. However, an estimated 40–50 million people in Southeast Asia may have fluke infection and may be candidates for eradication efforts. Although reducing the consumption of uncooked fish may be an effective strategy, these cultural and traditional practices are deeply entrenched and difficult to change. Active surveillance for fluke infection and potential interventions for eradication using chemotherapy may reduce the development of cholangiocarcinoma but such approaches have not been evaluated for their chemopreventive efficacy in reducing the incidence of cholangiocarcinoma.
In the West, chemopreventive strategies may be considered for patients with primary sclerosing cholangitis, who have a higher frequency of cholangiocarcinoma and may be associated with inflammatory bowel disease or colorectal cancer. Ursodeoxycholic acid (UDCA) is a hydrophilic bile salt that may protect the biliary tree by stabilizing bile duct epithelium and hepatocyte cell membranes and by increasing bile flow from the liver, thereby reducing intrahepatic bile stasis and exposure time to toxic bile salts (64). It also seems to exert a protective effect on colic mucosa by reducing faecal concentrations of deoxycholic acid (65). UDCA has been evaluated as a potential chemopreventive measure for PSC but there is no clear benefit of efficacy in reducing the rate of tumor formation (20;66). Further evaluation in randomized trials is necessary.
Novel approaches to the chemoprevention may be based on new insights from molecular pathogenesis. The epidermal growth factor receptor gene, for example, is located on the short arm of chromosome 7. Patients with PSC who show trisomy 7 and or EGFR expression in biliary tract epithelia may be appropriate to consider for chemoprevention with EGFR blockers if they have a higher risk of cholangiocarcinoma. Over-expression of COX-2 has been reported in extra-hepatic cholangiocarcinoma, and COX-2 inhibitors could be potentially useful chemopreventive agents in such patients (67).
CHALLENGES IN CHOLANGIOCARCINOMA
Cholangiocarcinoma has a dismal prognosis and is almost always incurable as it is refractory to most currently used surgical or medical interventions. A major limitation in the management of cholangiocarcinoma has been that the nosology and definitions of disease are poorly defined. This has limited the information that can be obtained from epidemiological studies, and has made it difficult to compare outcomes of different management strategies. Oncological trials for biliary cancers, for example, have often included both intrahepatic and extrahepatic malignancies. A clinically useful classification system that separates these two very different diseases with different risk factors, pathogenetic mechanisms and clinical behavior is needed. Risk factors for cholangiocarcinoma vary considerably, being primarily infectious in the East, and non-infectious in the West. Thus, the clinical behavior and management of this tumor shows regional variations despite shared pathogenetic mechanisms. Investigation on the role of inflammatory mediators and processes on cholangiocarcinoma pathogenesis is likely to be beneficial given the common involvement of chronic biliary tract inflammation in cholangiocarcinoma. The epidemiogical trends are concerning in that they show a global increase in incidence and mortality, but yet the disease has received little interest amongst research funding bodies, and public health administrators, and it continues to be regarded as a rare disease of little importance in the West. There are many challenges in the current management and approach to cholangiocarcinoma, both from the perspective of the individual as well as from a public health perspective.
Acknowledgments
Funding support: Supported in part by the National Institutes of Health, DK 06378
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chiara Braconi, Email: chiara.braconi@osumc.edu, The Ohio State University Medical Center, 420 west 12thavenue, Columbus, OH 43210. Tel: 614 866 5870.
Tushar Patel, Email: tushar.patel@osumc.edu, Professor of Medicine, The Ohio State University Medical Center, 395 West 12th Avenue, Suite 210F, Columbus, OH 43210, Tel: 614 293 6255, Fax: 614 293 0861.
Reference List
- 1.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288–91. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 2.Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009 Jan;16(1):14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001 Jun;33(6):1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002 May 3;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004 Jun;126(7):1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006 Nov;26(9):1047–53. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim HG, Han J, Kim MH, Cho KH, Shin IH, Kim GH, et al. Prevalence of clonorchiasis in patients with gastrointestinal disease: a Korean nationwide multicenter survey. World J Gastroenterol. 2009 Jan 7;15(1):86–94. doi: 10.3748/wjg.15.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SI. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol. 1998 Mar;36(1):37–45. doi: 10.3347/kjp.1998.36.1.37. [DOI] [PubMed] [Google Scholar]
- 10.HOU PC. The pathology of Clonorchis sinensis infestation of the liver. J Pathol Bacteriol. 1955 Jul;70(1):53–64. doi: 10.1002/path.1700700106. [DOI] [PubMed] [Google Scholar]
- 11.Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, et al. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994 Mar 1;305(2):241–52. doi: 10.1016/0027-5107(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 12.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005 Dec 10;117(5):854–60. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 13.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004 May;9(5):588–94. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008 Nov;24(11):497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suksumek N, Leelawat K, Leelawat S, Russell B, Lek-Uthai U. TaqMan real-time PCR assay for specific detection of Opisthorchis viverrini DNA in Thai patients with hepatocellular carcinoma and cholangiocarcinoma. Exp Parasitol. 2008 Jun;119(2):217–24. doi: 10.1016/j.exppara.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Thanan R, Murata M, Pinlaor S, Sithithaworn P, Khuntikeo N, Tangkanakul W, et al. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol Biomarkers Prev. 2008 Mar;17(3):518–24. doi: 10.1158/1055-9965.EPI-07-2717. [DOI] [PubMed] [Google Scholar]
- 17.Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004 Aug;25(8):1535–42. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 18.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004 Mar;99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 19.Claessen MM, Lutgens MW, van Buuren HR, Oldenburg B, Stokkers PC, van der Woude CJ, et al. More right-sided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis. 2009 Sep;15(9):1331–6. doi: 10.1002/ibd.20886. [DOI] [PubMed] [Google Scholar]
- 20.Kitiyakara T, Chapman RW. Chemoprevention and screening in primary sclerosing cholangitis. Postgrad Med J. 2008 May;84(991):228–37. doi: 10.1136/pgmj.2007.064592. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009 Jan;49(1):116–23. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000 Jun 1;88(11):2471–7. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Chen RF, Li ZH, Chen JS, Kong XH, Zou SQ. Human normal biliary epithelial cells transformation and tumor development induced by hepatitis C virus core protein. Zhonghua Wai Ke Za Zhi. 2005 Feb 1;43(3):153–6. [PubMed] [Google Scholar]
- 24.Roskams T. Different types of liver progenitor cells and their niches. J Hepatol. 2006 Jul;45(1):1–4. doi: 10.1016/j.jhep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008 Jan 28;14(4):632–5. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008 Jul;103(7):1716–20. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 27.Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996 Oct;25(5):933–40. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 28.Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001 Dec;12(10):959–64. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 29.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005 Mar;128(3):620–6. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Falchuk KR, Lesser PB, Galdabini JJ, Isselbacher KJ. Cholangiocarcinoma as related to chronic intrahepatic cholangitis and hepatolithiasis. Case report and review of the literature. Am J Gastroenterol. 1976 Jul;66(1):57–61. [PubMed] [Google Scholar]
- 31.Nakanuma Y, Terada T, Tanaka Y, Ohta G. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch A Pathol Anat Histopathol. 1985;406(1):45–58. doi: 10.1007/BF00710556. [DOI] [PubMed] [Google Scholar]
- 32.Kuroki T, Tajima Y, Kanematsu T. Hepatolithiasis and intrahepatic cholangiocarcinoma: carcinogenesis based on molecular mechanisms. J Hepatobiliary Pancreat Surg. 2005;12(6):463–6. doi: 10.1007/s00534-005-1004-1. [DOI] [PubMed] [Google Scholar]
- 33.Kawakami H, Kuwatani M, Onodera M, Hirano S, Kondo S, Nakanishi Y, et al. Primary cholesterol hepatolithiasis associated with cholangiocellular carcinoma: a case report and literature review. Intern Med. 2007;46(15):1191–6. doi: 10.2169/internalmedicine.46.0151. [DOI] [PubMed] [Google Scholar]
- 34.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002 Feb 15;155(4):323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 35.Torbenson M, Yeh MM, Abraham SC. Bile duct dysplasia in the setting of chronic hepatitis C and alcohol cirrhosis. Am J Surg Pathol. 2007 Sep;31(9):1410–3. doi: 10.1097/PAS.0b013e318053d122. [DOI] [PubMed] [Google Scholar]
- 36.Zhu AX, Lauwers GY, Tanabe KK. Cholangiocarcinoma in association with Thorotrast exposure. J Hepatobiliary Pancreat Surg. 2004;11(6):430–3. doi: 10.1007/s00534-004-0924-5. [DOI] [PubMed] [Google Scholar]
- 37.Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998 Oct;28(4):921–5. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 38.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007 Oct;5(10):1221–8. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007 Jul;4(7):e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thuwajit C, Thuwajit P, Uchida K, Daorueang D, Kaewkes S, Wongkham S, et al. Gene expression profiling defined pathways correlated with fibroblast cell proliferation induced by Opisthorchis viverrini excretory/secretory product. World J Gastroenterol. 2006 Jun 14;12(22):3585–92. doi: 10.3748/wjg.v12.i22.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakobwong S, Pinlaor S, Yongvanit P, Sithithaworn P, Pairojkul C, Hiraku Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with Opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int J Parasitol. 2009 Jun;39(7):825–35. doi: 10.1016/j.ijpara.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000 May;30(6):735–40. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 43.Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, et al. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006 Oct;44(4):1025–38. doi: 10.1002/hep.21330. [DOI] [PubMed] [Google Scholar]
- 44.Yoon JH, Werneburg NW, Higuchi H, Canbay AE, Kaufmann SH, Akgul C, et al. Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer Res. 2002 Nov 15;62(22):6500–5. [PubMed] [Google Scholar]
- 45.Fukase K, Ohtsuka H, Onogawa T, Oshio H, Ii T, Mutoh M, et al. Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci. 2008 Sep;99(9):1785–92. doi: 10.1111/j.1349-7006.2008.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol. 1998 Oct;275(4 Pt 1):G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- 47.Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytes. Life Sci. 2006 Apr 18;78(21):2494–502. doi: 10.1016/j.lfs.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005 Jan;41(1):5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 49.Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 2: molecular pathology and treatment. J Gastroenterol Hepatol. 2002 Oct;17(10):1056–63. doi: 10.1046/j.1440-1746.2002.02780.x. [DOI] [PubMed] [Google Scholar]
- 50.Sugawara H, Yasoshima M, Katayanagi K, Kono N, Watanabe Y, Harada K, et al. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998 Aug;33(2):145–53. doi: 10.1046/j.1365-2559.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 51.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999 Nov;30(5):1128–33. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 52.Okada K, Shimizu Y, Nambu S, Higuchi K, Watanabe A. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol. 1994 Sep;9(5):462–7. doi: 10.1111/j.1440-1746.1994.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 53.Yokomuro S, Tsuji H, Lunz JG, III, Sakamoto T, Ezure T, Murase N, et al. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000 Jul;32(1):26–35. doi: 10.1053/jhep.2000.8535. [DOI] [PubMed] [Google Scholar]
- 54.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005 Dec;42(6):1329–38. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 55.Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006 Jun;44(6):1055–65. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008 Jan 10;27(3):378–86. doi: 10.1038/sj.onc.1210648. [DOI] [PubMed] [Google Scholar]
- 57.Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006 Nov 1;66(21):10517–24. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 58.Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003 Mar;124(3):737–53. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 59.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008 May 1;97(6):513–8. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 60.Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, et al. A New PCR-Based Approach Indicates the Range of Clonorchis sinensis Now Extends to Central Thailand. PLoS Negl Trop Dis. 2009;3(1):e367. doi: 10.1371/journal.pntd.0000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996 Aug;7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 62.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008 Apr;13(4):415–23. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 63.Valle J, Harpreet W, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary tract cacner. New Engl J Med. 2010 Apr;362:1273–8. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 64.Colombo C, Crosignani A, Assaisso M, Battezzati PM, Podda M, Giunta A, et al. Ursodeoxycholic acid therapy in cystic fibrosis-associated liver disease: a dose-response study. Hepatology. 1992 Oct;16(4):924–30. doi: 10.1002/hep.1840160412. [DOI] [PubMed] [Google Scholar]
- 65.Pardi DS, Loftus EV, Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003 Apr;124(4):889–93. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 66.Forsmo HM, Horn A, Viste A, Hoem D, Ovrebo K. Survival and an overview of decision-making in patients with cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008 Aug;7(4):412–7. [PubMed] [Google Scholar]
- 67.Wu GS, Wang JH, Liu ZR, Zou SQ. Expression of cyclooxygenase-1 and -2 in extra- hepatic cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2002 Aug;1(3):429–33. [PubMed] [Google Scholar]


