Abstract
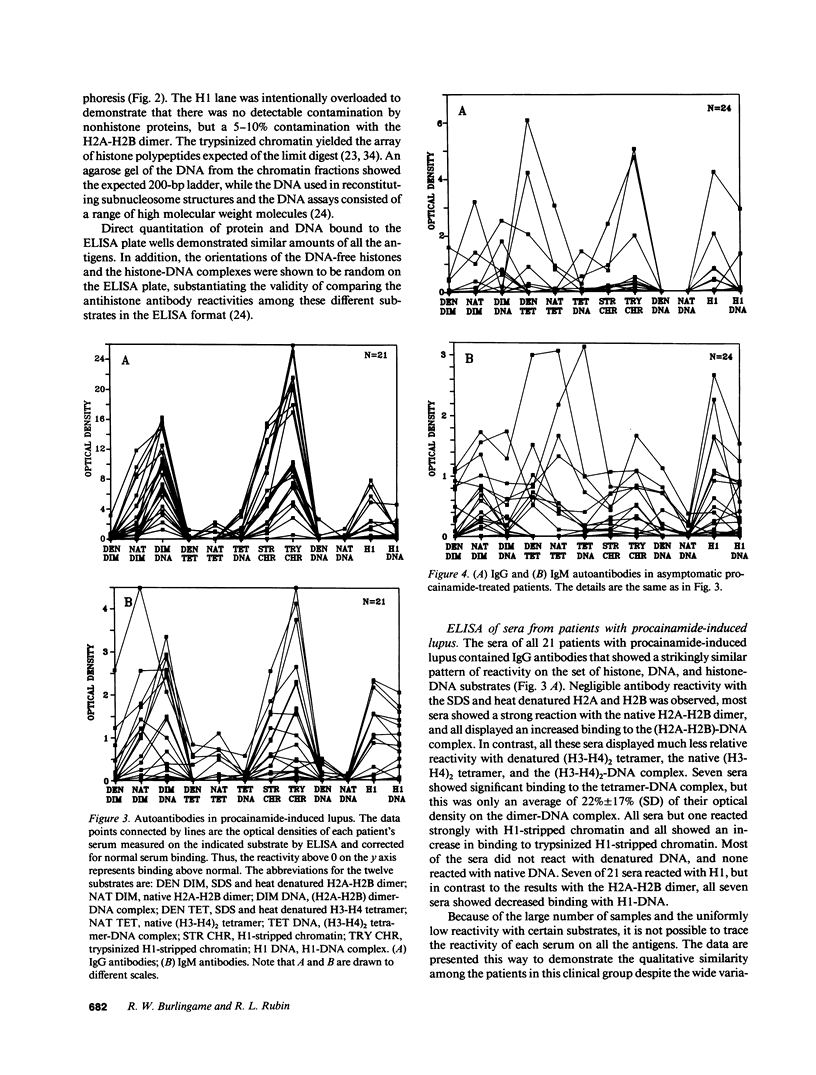
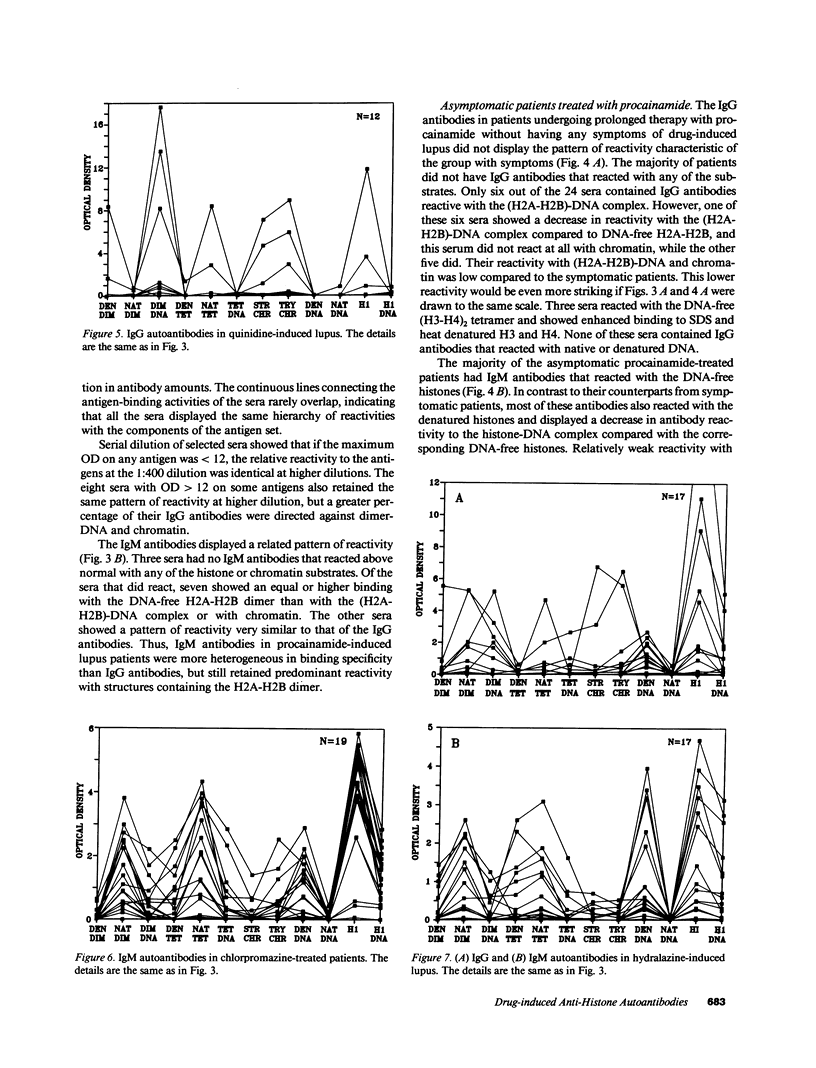
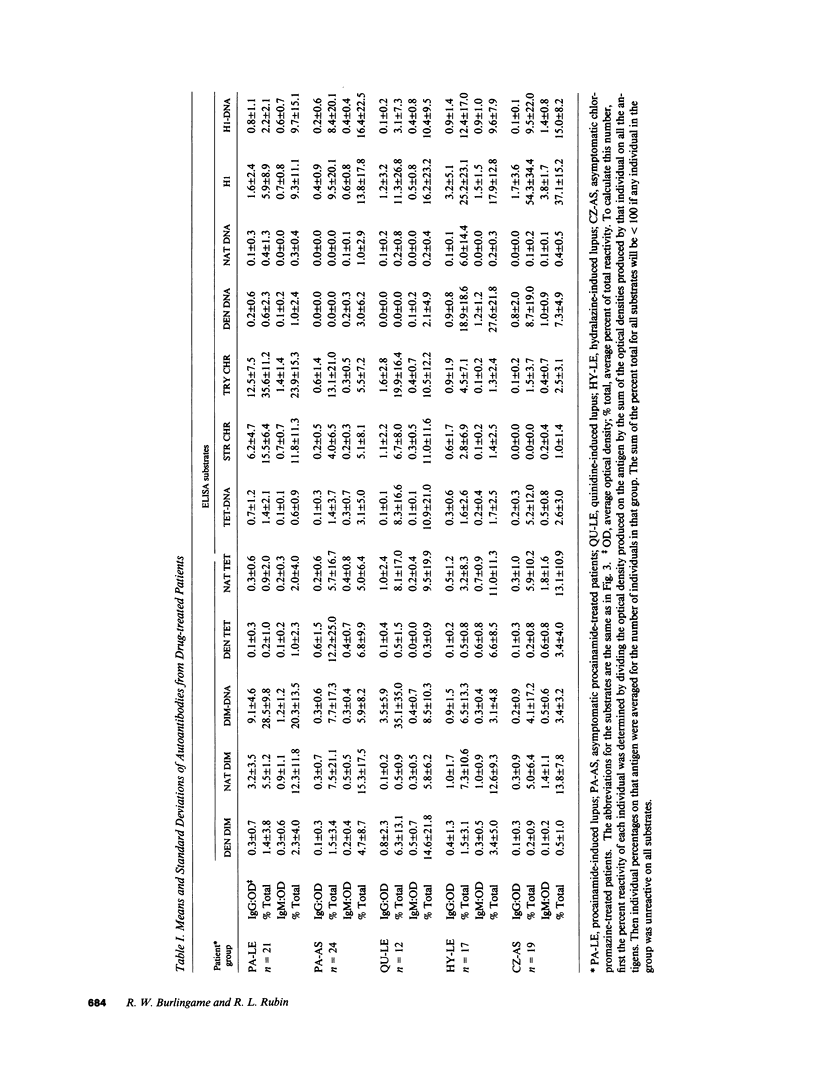
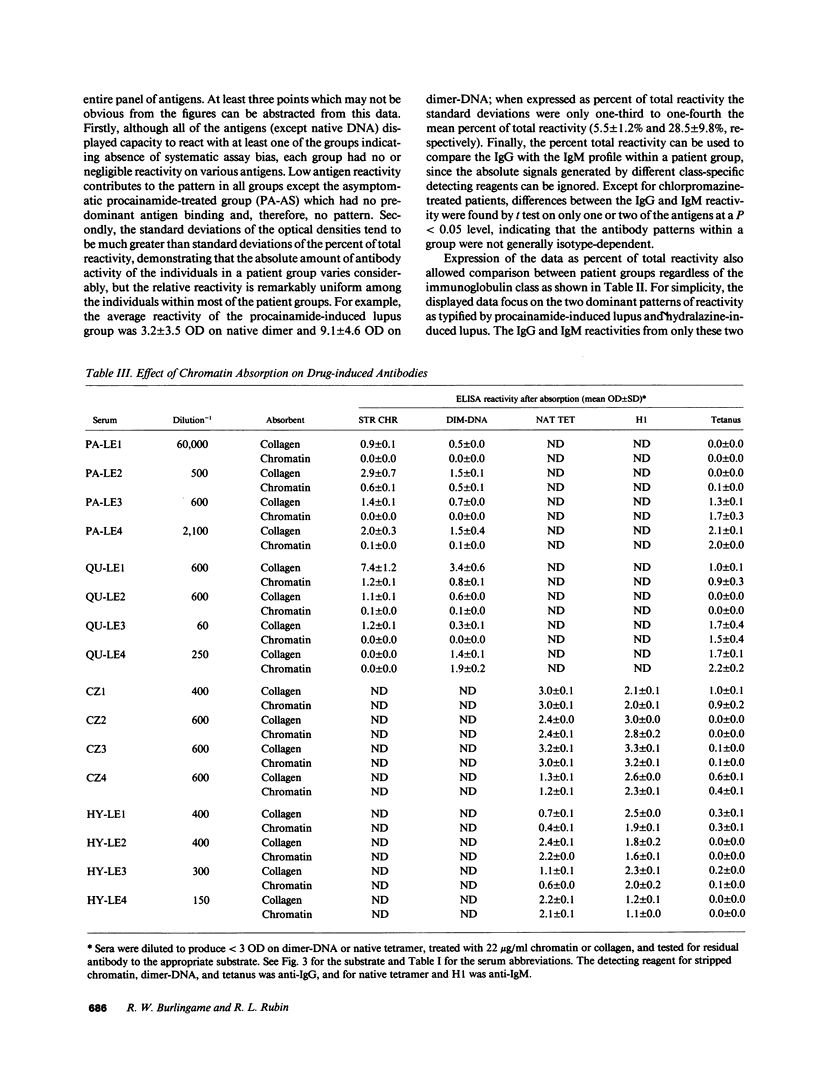
Increasing evidence suggests that autoantibodies in the rheumatic diseases are a consequence of immune selection by self-material, but the nature of the in vivo immunogen is unknown. Insight into this problem may be obtained by measuring autoantibody binding to various forms of a target antigen. Antihistone antibodies arising as a side effect of therapy with various drugs offer an opportunity to explore this premise because many forms of histone have been characterized and adapted to ELISA formats. Two patterns of antibody reactivity were observed. All 21 patients with symptomatic procainamide-induced lupus and 7 of 12 patients with quinidine-induced lupus had IgG antibodies reacting predominantly with the (H2A-H2B)-DNA complex and with chromatin. In contrast, antibodies in 19 of 24 patients taking procainamide without accompanying lupus-like symptoms did not show any pattern. The second pattern was observed in 18/19 chlorpromazine-treated patients and 14/17 patients with hydralazine-induced lupus in which IgM antibodies displayed more reactivity with DNA-free histones than with the corresponding histone-DNA complexes and almost no binding to H1-stripped chromatin. Absorption studies were entirely consistent with these results. Thus, the two patterns of reactivity with nucleosomal components reflect the molecular substructure of chromatin, suggesting that two processes underlie antihistone antibody induction by drugs. In one, IgG autoantibodies appear to be elicited by chromatin, whereas in the other, autoimmune tolerance to native chromatin appears largely intact, and IgM antibodies may be driven by DNA-free histone.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. E., Sanders C. E., Jr, Budinsky R. A., Donovan-Brand R., Roberts S. M., Hess E. V. Immunomodulatory effects of procainamide metabolites: their implications in drug-related lupus. J Lab Clin Med. 1989 Apr;113(4):482–492. [PubMed] [Google Scholar]
- Burlingame R. W., Love W. E., Wang B. C., Hamlin R., Nguyen H. X., Moudrianakis E. N. Crystallographic structure of the octameric histone core of the nucleosome at a resolution of 3.3 A. Science. 1985 May 3;228(4699):546–553. doi: 10.1126/science.3983639. [DOI] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J Immunol Methods. 1990 Dec 5;134(2):187–199. doi: 10.1016/0022-1759(90)90380-e. [DOI] [PubMed] [Google Scholar]
- Böhm L., Crane-Robinson C. Proteases as structural probes for chromatin: the domain structure of histones. Biosci Rep. 1984 May;4(5):365–386. doi: 10.1007/BF01122502. [DOI] [PubMed] [Google Scholar]
- Canoso R. T., de Oliveira R. M. Characterization and antigenic specificity of chlorpromazine-induced antinuclear antibodies. J Lab Clin Med. 1986 Sep;108(3):213–216. [PubMed] [Google Scholar]
- Cohen M. G., Kevat S., Prowse M. V., Ahern M. J. Two distinct quinidine-induced rheumatic syndromes. Ann Intern Med. 1988 Mar;108(3):369–371. doi: 10.7326/0003-4819-108-3-369. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Radding J. A., Harding M. W., Bernstein R. M., Hardin J. A. Autoantigenic histone epitopes: a comparison between procainamide- and hydralazine-induced lupus. Arthritis Rheum. 1987 Jun;30(6):689–694. doi: 10.1002/art.1780300612. [DOI] [PubMed] [Google Scholar]
- Draper L. R., Hirata A. A. Antibody responses in rabbits to soluble and particulate forms of bovine serum albumin. Immunology. 1968 Jul;15(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Watson D. K., Moudrianakis E. N. A chromatin-bound proteolytic activity with unique specificity for histone H2A. Cell. 1976 Dec;9(4 Pt 2):785–792. doi: 10.1016/0092-8674(76)90141-0. [DOI] [PubMed] [Google Scholar]
- Gleichmann E., Van Elven E. H., Van der Veen J. P. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982 Feb;12(2):152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- Gohill J., Cary P. D., Couppez M., Fritzler M. J. Antibodies from patients with drug-induced and idiopathic lupus erythematosus react with epitopes restricted to the amino and carboxyl termini of histone. J Immunol. 1985 Nov;135(5):3116–3121. [PubMed] [Google Scholar]
- Gonzalez-Redondo J. M., Stoming T. A., Lanclos K. D., Gu Y. C., Kutlar A., Kutlar F., Nakatsuji T., Deng B., Han I. S., McKie V. C. Clinical and genetic heterogeneity in black patients with homozygous beta-thalassemia from the southeastern United States. Blood. 1988 Sep;72(3):1007–1014. [PubMed] [Google Scholar]
- Harmon C. E., Portanova J. P. Drug-induced lupus: clinical and serological studies. Clin Rheum Dis. 1982 Apr;8(1):121–135. [PubMed] [Google Scholar]
- Hess E. Drug-related lupus. N Engl J Med. 1988 Jun 2;318(22):1460–1462. doi: 10.1056/NEJM198806023182209. [DOI] [PubMed] [Google Scholar]
- Hobbs R. N., Clayton A. L., Bernstein R. M. Antibodies to the five histones and poly(adenosine diphosphate-ribose) in drug induced lupus: implications for pathogenesis. Ann Rheum Dis. 1987 May;46(5):408–416. doi: 10.1136/ard.46.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmerson R. Polypeptide fragments of horse cytochrome c activate a small subset of secondary B lymphocytes primed against the native protein. J Immunol. 1987 Sep 15;139(6):1939–1945. [PubMed] [Google Scholar]
- Kotzin B. L., Lafferty J. A., Portanova J. P., Rubin R. L., Tan E. M. Monoclonal anti-histone autoantibodies derived from murine models of lupus. J Immunol. 1984 Nov;133(5):2554–2559. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litwin A., Adams L. E., Zimmer H., Foad B., Loggie J. H., Hess E. V. Prospective study of immunologic effects of hydralazine in hypertensive patients. Clin Pharmacol Ther. 1981 Apr;29(4):447–456. doi: 10.1038/clpt.1981.62. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Mencke A. J., Rill R. L. Circular dichroism and thermal denaturation studies of subnucleosomes and their relationships to nucleosome structure. Biochemistry. 1982 Aug 31;21(18):4362–4370. doi: 10.1021/bi00261a027. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Mencke A. J., Chambers S. A., Oosterhof D. K., Rill R. L. Subnucleosomes and their relationships to the arrangement of histone binding sites along nucleosome deoxyribonucleic acid. Biochemistry. 1982 Aug 31;21(18):4350–4362. doi: 10.1021/bi00261a026. [DOI] [PubMed] [Google Scholar]
- Oohara I., Wada A. Spectroscopic studies on histone-DNA interactions. I. The interaction of histone (H2A, H2B) dimer with DNA: DNA sequence dependence. J Mol Biol. 1987 Jul 20;196(2):389–397. doi: 10.1016/0022-2836(87)90699-1. [DOI] [PubMed] [Google Scholar]
- Oohara I., Wada A. Spectroscopic studies on histone-DNA interactions. II. Three transitions in nucleosomes resolved by salt-titration. J Mol Biol. 1987 Jul 20;196(2):399–411. doi: 10.1016/0022-2836(87)90700-5. [DOI] [PubMed] [Google Scholar]
- Parker C. W. Hapten immunology and allergic reactions in humans. Arthritis Rheum. 1981 Aug;24(8):1024–1036. doi: 10.1002/art.1780240808. [DOI] [PubMed] [Google Scholar]
- Pollard K. M., Chan E. K., Rubin R. L., Tan E. M. Monoclonal autoantibodies to nuclear antigens from murine graft-versus-host disease. Clin Immunol Immunopathol. 1987 Jul;44(1):31–40. doi: 10.1016/0090-1229(87)90049-3. [DOI] [PubMed] [Google Scholar]
- Portanova J. P., Arndt R. E., Tan E. M., Kotzin B. L. Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J Immunol. 1987 Jan 15;138(2):446–451. [PubMed] [Google Scholar]
- Read C. M., Baldwin J. P., Crane-Robinson C. Structure of subnucleosomal particles. Tetrameric (H3/H4)2 146 base pair DNA and hexameric (H3/H4)2(H2A/H2B)1 146 base pair DNA complexes. Biochemistry. 1985 Jul 30;24(16):4435–4450. doi: 10.1021/bi00337a027. [DOI] [PubMed] [Google Scholar]
- Richardson B., Cornacchia E., Golbus J., Maybaum J., Strahler J., Hanash S. N-acetylprocainamide is a less potent inducer of T cell autoreactivity than procainamide. Arthritis Rheum. 1988 Aug;31(8):995–999. doi: 10.1002/art.1780310809. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., McNally E. M., Nusinow S. R., Robinson C. A., Tan E. M. IgG antibodies to the histone complex H2A-H2B characterize procainamide-induced lupus. Clin Immunol Immunopathol. 1985 Jul;36(1):49–59. doi: 10.1016/0090-1229(85)90038-8. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Reimer G., McNally E. M., Nusinow S. R., Searles R. P., Tan E. M. Procainamide elicits a selective autoantibody immune response. Clin Exp Immunol. 1986 Jan;63(1):58–67. [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Lucas A. H., Spiegelberg H. L., Tan E. M. IgG subclasses of anti-tetanus toxoid antibodies in adult and newborn normal subjects and in patients with systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986 Oct 15;137(8):2522–2527. [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Tsay G., Pollard K. M. Pseudoautoimmunity in normal mice: anti-histone antibodies elicited by immunization versus induction during graft-versus-host reaction. Clin Immunol Immunopathol. 1990 Feb;54(2):320–332. doi: 10.1016/0090-1229(90)90093-6. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- TORRIGIANI G., ROITT I. M. THE ENHANCEMENT OF 19S ANTIBODY PRODUCTION BY PARTICULATE ANTIGEN. J Exp Med. 1965 Jul 1;122:181–193. doi: 10.1084/jem.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Chan E. K., Sullivan K. F., Rubin R. L. Antinuclear antibodies (ANAs): diagnostically specific immune markers and clues toward the understanding of systemic autoimmunity. Clin Immunol Immunopathol. 1988 May;47(2):121–141. doi: 10.1016/0090-1229(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Totoritis M. C., Tan E. M., McNally E. M., Rubin R. L. Association of antibody to histone complex H2A-H2B with symptomatic procainamide-induced lupus. N Engl J Med. 1988 Jun 2;318(22):1431–1436. doi: 10.1056/NEJM198806023182204. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woosley R. L., Drayer D. E., Reidenberg M. M., Nies A. S., Carr K., Oates J. A. Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med. 1978 May 25;298(21):1157–1159. doi: 10.1056/NEJM197805252982101. [DOI] [PubMed] [Google Scholar]