Abstract
Purpose
Combining tumor antigens with an immunostimulant can induce the immune system to specifically eliminate cancer cells. Generally, this combination is accomplished in an ex vivo, customized manner. In a preclinical lymphoma model, intratumoral injection of a Toll-like receptor 9 (TLR9) agonist induced systemic antitumor immunity and cured large, disseminated tumors.
Patients and Methods
We treated 15 patients with low-grade B-cell lymphoma using low-dose radiotherapy to a single tumor site and—at that same site—injected the C-G enriched, synthetic oligodeoxynucleotide (also referred to as CpG) TLR9 agonist PF-3512676. Clinical responses were assessed at distant, untreated tumor sites. Immune responses were evaluated by measuring T-cell activation after in vitro restimulation with autologous tumor cells.
Results
This in situ vaccination maneuver was well-tolerated with only grade 1 to 2 local or systemic reactions and no treatment-limiting adverse events. One patient had a complete clinical response, three others had partial responses, and two patients had stable but continually regressing disease for periods significantly longer than that achieved with prior therapies. Vaccination induced tumor-reactive memory CD8 T cells. Some patients' tumors were able to induce a suppressive, regulatory phenotype in autologous T cells in vitro; these patients tended to have a shorter time to disease progression. One clinically responding patient received a second course of vaccination after relapse resulting in a second, more rapid clinical response.
Conclusion
In situ tumor vaccination with a TLR9 agonist induces systemic antilymphoma clinical responses. This maneuver is clinically feasible and does not require the production of a customized vaccine product.
INTRODUCTION
Passive immunotherapy with monoclonal antibodies has been an important development in the treatment of several malignancies including lymphoma; however, patients' clinical course is still characterized by relapse and progressive decrease in response to therapy.1 Therefore, numerous efforts have been made to develop a therapeutic vaccine that induces a patient's immune system to eliminate his/her own tumor. Recently, this goal was achieved for a prostate cancer vaccine with a randomized clinical trial that demonstrated a benefit in overall survival.2 In that example, a customized product was made from the patient's dendritic cells loaded ex vivo with a standardized tumor antigen. A similar approach has recently been reported for patients with lymphoma with some success.3 Another customized vaccine—lymphoma idiotype protein—despite initially promising results,4 has not demonstrated clinical benefit in randomized, phase III trials.5–7 If possible, it would be more practical to trigger an immune response without having to manufacture a customized vaccine product.
CpG refers to a class of immunostimulatory oligonucleotides that are ligands for Toll-like receptor 9 (TLR9). These compounds can activate both lymphoma B-cells as well as nearby antigen-presenting cells. In a murine lymphoma model, combining intratumoral CpG with cytotoxic therapy effectively provides tumor antigens to antigen-presenting cells and activates them to present engulfed antigens to T cells. We found that only the combination induces tumor-reactive CD8 T cells and cures animals of a large systemic tumor burden. In that model, TLR expression on either the tumor cells or the host dendritic cells was sufficient for the maneuver to be effective.8 The intratumoral CpG approach appears to target nonidiotype antigens, as idiotype escape variants are still effectively eliminated.9
Most non-Hodgkin's lymphomas are derived from B cells, which express TLR910 and are sensitive to radiotherapy.11 Therefore, we hypothesized that by combining intratumoral CpG with local low-dose radiotherapy we could both treat the irradiated tumor site and induce immune-mediated regression of distant, nonirradiated sites of lymphoma.
PATIENTS AND METHODS
Patient Selection
Eligible patients had biopsy-confirmed low-grade B-cell lymphoma and had relapsed after at least one standard therapy. Patients had at least three sites of disease, for: (1) pretreatment excisional biopsy, (2) intratumoral CpG injection, and (3) response assessment.
Inclusion criteria included: Eastern Cooperative Oncology Group performance status of 0 to 1, WBC ≥ 2,000/uL; platelet count ≥ 75,000/mm3; absolute neutrophil count ≥ 1,000, serum creatinine ≤ 2.0 mg/dL, and bilirubin ≤ 1.5 mg/dL. Wash out periods for prior therapy: chemotherapy −4 weeks, radiotherapy −4 weeks, rituximab −12 weeks. An institutional review board approved the protocol, and all patients gave written informed consent before undergoing treatment. The study was registered at Clinicaltrials.gov as NCT00185965.
Safety Monitoring
Patients were assessed for toxicity before and after each injection of PF-3512676 injection using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. All safety issues of the trial were monitored by an institutional data safety monitoring board and a guideline for dose reduction and early stopping rules were established before initiation.
Treatment Schema: In Situ CpG Vaccination
Low-dose radiotherapy was administered to a solitary tumor site totaling 4 Gy over 2 consecutive days. Patients received the CpG-enriched oligodeoxynucleotide TLR9 agonist PF-351267612 (Pfizer, New York, NY) 6 mg by intratumoral injection at the same tumor site immediately before the first radiation dose, after the second radiation dose, and weekly for 8 consecutive weeks thereafter. This is a class B CpG molecule, 23 nucleotides in length, which has been studied previously in patients with non-Hodgkin's lymphoma by intravenous and subcutaneous routes of administration.12,13
Clinical Response Measurement
Imaging of involved sites including neck, chest, abdomen, and pelvis was performed within 30 days before initial vaccination and repeated 3 weeks after final vaccination; follow-up imaging was performed every 3 months until progression.
Clinical responses were measured by a central reviewer per the International Working Group criteria14 modified to exclude the irradiated site of disease. The results were displayed as the fold-change of the cross-products of up to six nonirradiated index lesions. Lesions were tracked using the image Physician Annotation Device (iPad; http://bimm.stanford.edu/main/ipad) software tool15 and each patient was scored per their best response time point. One patient with skin-only disease was observed per volumetric measurement of index lesions and with supportive photographic documentation.
Immune Response Measurement
Single-cell suspensions of patient tumor B cells were activated with PF-3512676 and soluble CD40L (Seattle Genetics, Bothell, WA) then irradiated to 50 Gy. Peripheral blood lymphocytes were coincubated with activated tumor cells for 120 hours, then, restimulated with activated tumor cells for an additional 24 hours. The resulting cells were stained with fluorochrome-conjugated antibodies against CD4, CD8, CD137, and CD45RO (BD Biosciences, San Jose, CA), and analyzed by flow cytometry. For intracellular cytokine measurement, cells were treated with Golgi-Plug (BD Biosciences) for the final 5 hours of coincubation, stained for CD4, CD8, interferon-γ, interleukin (IL) -2, tumor necrosis factor α, and tumor necrosis factor β (BD Biosciences).
Regulatory T-Cell Induction
Peripheral blood lymphocytes obtained before vaccination were cultured with or without activated tumor B cells (as above) for 120 hours in the absence of other stimulants. The resulting cells were stained for surface CD4, CD25, and intracellular forkhead box protein P3 (FOXP3; eBioscience, San Diego, CA).
Statistical Analysis
Demographic characteristics and baseline clinical characteristics were summarized with the use of descriptive statistics. All enrolled patients were included in analyses of the predefined primary end point of clinical response. Secondary end points included progression-free and overall survival as well as the immune response measurements described. One patient with primary cutaneous marginal zone lymphoma was excluded from immune response assays because of insufficient biopsy specimen.
Logistic regression and Cox's proportional hazards models were used to determine whether clinical end points were associated with baseline clinical characteristics, treatment-induced flu-like symptoms, or immune response end points. The clinical characteristics tested were: age, Follicular Lymphoma International Prognostic Index score, pretreatment tumor bulk, cross-product of the treated (irradiated) site, and number of prior therapies.
RESULTS
Baseline Patient Characteristics
A total of 15 patients with relapsed, low-grade lymphoma were treated per protocol (Table 1). Most patients had follicular (grade 1 to 2) histology, with one patient each having nodal or primary cutaneous marginal zone lymphoma. On enrollment, patients had a median age of 62 years (range, 38 to 65 years), had experienced treatment failure with a median of three standard therapies (range, 1 to 6), and all had advanced stage (III/IV). The single, superficial site of disease, which was irradiated and injected with CpG was either cervical, axillary, inguinal, or subcutaneous and were of median short axis measurement 2.0 cm (range, 1.0 to 8.5 cm).
Table 1.
Patient Characteristics and Adverse Reactions
| Patient | Age (years) | Stage | Disease | No. of Prior Therapies | Most Recent Prior Therapy | Prior TTP (weeks) | Systemic Flu-Like Reaction (grade) | Injection Site Reaction (grade) | Clinical Response | TTP (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | IV | FL | 4 | RTX | 18 | — | — | PD | 8 |
| 2 | 62 | III | FL | 3 | RTX | 14 | — | — | SD | 45 |
| 3 | 38 | IV | FL | 1 | CVP | 120 | 1 | — | CR | 61 |
| 4 | 59 | IV | FL | 3 | RIT | 313 | — | — | SD | 77 |
| 5 | 63 | IV | FL | 1 | CVP | 95 | — | — | SD | 21 |
| 6 | 63 | IV | FL | 6 | VP-16 | 5 | — | — | SD | 12 |
| 7 | 65 | III | MZL | 4 | CVP | 87 | — | — | PD | 12 |
| 8 | 55 | III | FL | 1 | RTX | 24 | 1 | — | SD | 131 |
| 9 | 51 | IV | FL | 3 | RIT | 13 | — | — | SD | 11 |
| 10 | 62 | IV | PCMZL | 5 | R-CVP | 16 | 1 | 2 | PR | 29 |
| 11 | 63 | IV | FL | 1 | RTX | 56 | 2 | — | PR | 111 |
| 12 | 64 | III | FL | 1 | CVP | 64 | — | — | PR | 64 |
| 13 | 65 | IV | FL | 3 | RIT | 56 | — | — | PD | 10 |
| 14 | 61 | IV | FL | 3 | RIT | 28 | — | — | SD | 23 |
| 15 | 59 | III | FL | 1 | RTX | 28 | 1 | — | SD | 73 |
Abbreviations: TTP, time to progression; FL, follicular lymphoma; RTX, rituximab; PD, progressive disease; SD, stable disease; CVP, cyclophosphamide, vincristine, prednisone; CR, complete remission; RIT, radioimmunotherapy; VP-16, etoposide; MZL, marginal zone lymphoma; PCMZL, primary cutaneous marginal zone lymphoma; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; PR, partial remission.
Safety and Adverse Effects
Therapy was well-tolerated. The only observed adverse events were a grade 2 injection site reaction consisting of erythema, induration, and tenderness and flu-like reactions consisting of grade 1 to 2 fevers, arthralgias, or myalgias, lasting 24 to 72 hours after injection in a minority of patients (Table 1) and resulted in no dose modifications or delays. All patients completed the full course of therapy. No signs or symptoms of auto-immune phenomena (eg, colitis, arthritis, inflammation of skin or liver) were observed either during or after therapy.
Clinical Responses
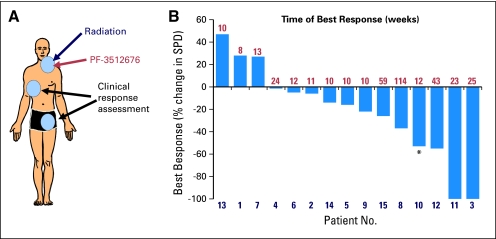
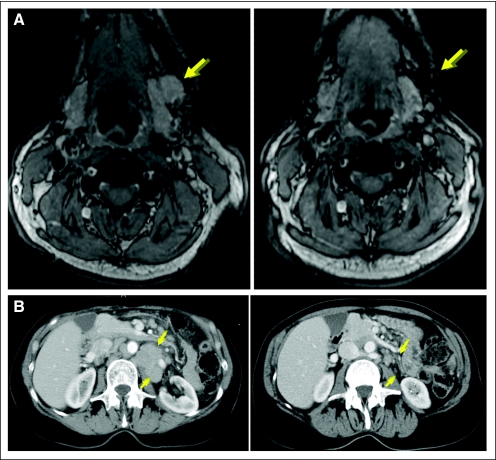
All 15 enrolled patients were evaluable for clinical response with a median follow-up of 33.7 months. At the treated site, there were seven complete regressions, six partial regressions, and two patients with stable disease, consistent with prior reports of low-dose radiotherapy.11 Clinical responses (excluding the treated site; Fig 1A) yielded an overall objective response rate of 27% with one complete response, three partial responses (PR), and eight patients with stable disease. Patient 3 (Fig 2A) obtained a complete response lasting 61 weeks; patient 10 (Fig 2B) obtained a PR lasting 20 weeks; patient 11 (Fig 3A) obtained a PR, ongoing at 111 weeks; and patient 12 (Fig 3B) had a PR remarkable for regression of bulky retroperitoneal lymph nodes measuring 21 cm2 before vaccination and 6 cm2 at best response and lasting 64 weeks. For complete imaging for these patients see Figures 2 to 4 and Appendix Figure A1 (online only).
Fig 1.
Intratumoral vaccination induces objective clinical responses. (A) Patients received 2 Gy × 2 radiation combined with intratumoral injection of PF-3512676 to a single disease site and disease was measured at up to six distant sites. (B) Waterfall plot showing percent change in the cross-product sum at the time of best response (indicated above ordinate) versus pretreatment. (*) Refers to patient 10, whose primary cutaneous disease was instead measured as a three-dimensional sum. SPD, sum of products of greatest diameter.
Fig 2.
Intratumoral vaccination induces objective clinical responses [extended]. (A) Complete response in patient 3, treated site: occipital; visualized site: bilateral axillae. (B) Partial response in patient 10, treated site: suprasternal cutaneous; visualized site: supra-orbital cutaneous.
Fig 3.
Intratumoral vaccination induces objective clinical responses [extended]. (A) Partial response (PR) in patient 11, treated site: right supraclavicular; visualized site: left submandibular. (B) PR in patient 12, treated site: left inguinal; visualized site: retroperitoneal lymph node conglomerate.
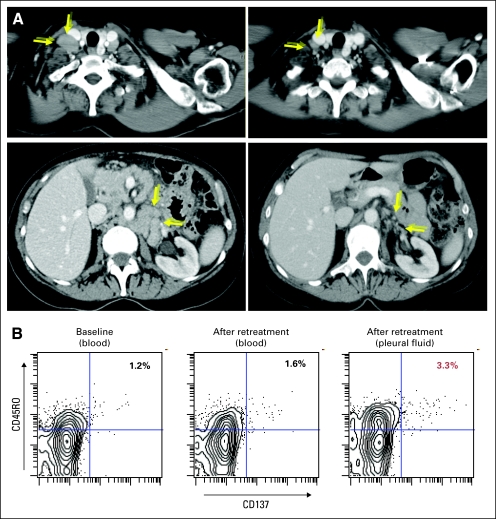
Fig 4.
Re-treatment of a responding patient. Patient 12 received re-treatment as in Figure 1 with intratumoral injection of PF-3512676 18 mg. (A) Partial response in patient 12, treated site: right inguinal; visualized sites: right supraclavicular and retroperitoneal lymph node conglomerate. (B) Increased proportion of tumor-reactive memory CD8 T cells per CD137 upregulation in pleural fluid after re-treatment; data shown are gated on live, CD8+ cells, statistics shown are percentage of CD45RO+CD137+ cells among that gate.
Two patients had subclinical systemic tumor regressions (Fig 1B) of surprising duration given the rapidity of prior recurrences. Patient 8 has had a 37% cross-product reduction, ongoing at 131 weeks; patient 15 has had a 26% reduction, ongoing at 73 weeks. In all, five of these six objective or subclinical responses were of equal or greater duration than that of their prior therapy (Table 1). The kinetics of the responses were also notable as they generally were greatest at delayed time points ≥ 24 weeks after therapy with continued regressions even 2 years after therapy, as reported in other studies of active immunotherapy.6,16
We examined baseline characteristics, including follicular lymphoma international prognostic indices, pretreatment tumor bulk, and number of prior therapies, as well as the development of flu-like symptoms during therapy, for correlation with clinical response. Greater magnitude of clinical response correlated only with fewer prior therapies (1 v > 1; two-sided t-test, P = .0072) and with treatment-induced flu-like symptoms (two-sided t-test, P = .003). These predictors were not entirely independent of each other, multiple linear regression modeling yielded P = .11 and P = .05, respectively.
Immune Responses
Induction of tumor cell immunogenicity.
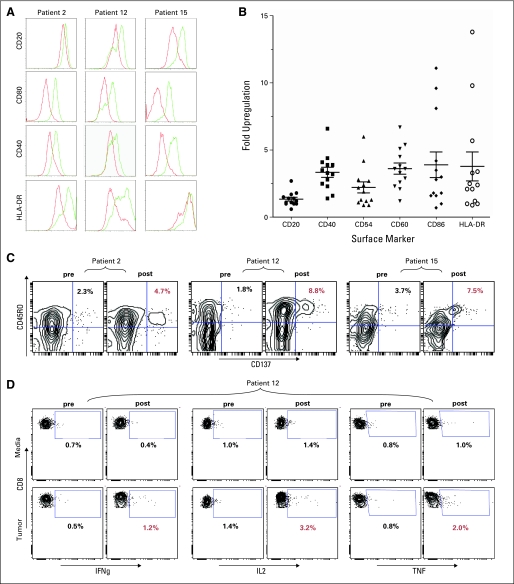
Prior studies have shown that CpG can induce an immunogenic phenotype in B-cell derived malignancies.10,17,18 We therefore tested the direct effect of CpG on the phenotype of tumor cells in this cohort of patients. Pretreatment biopsy cells were cultured with PF-3512676 and assessed for surface expression of antigen presentation and costimulatory molecules. All samples tested (n = 13) demonstrated upregulation of multiple markers (Appendix Figs A1A and A1B, online only), though there was significant variability in degree and in which molecules were affected. No correlation between induction of tumor cell phenotype and clinical responses was observed.
Induction of tumor-reactive immune responses.
The preclinical model demonstrated that antitumor, memory CD8 T cells are both necessary and sufficient for tumor protection.8,19 Therefore, peripheral blood lymphocytes from before and after treatment were cocultured with CpG-activated, autologous tumor cells and assessed for expression of an activated phenotype. Measurement of T-cell activation included expression of the activation marker CD137 as well as intracellular levels of the cytokines interferon-γ, IL-2, and tumor necrosis factor. Of these, CD137 appeared to be the most sensitive readout, specifically among the memory (CD45RO+) CD8 T cells (Appendix Fig A1C, online only) although cytokine-response induction was also seen after vaccination (Appendix Fig A1D, online only). In some instances, continued disease regression was paralleled by improving immune response (data not shown) with peak T-cell response occurring up to 1 year after vaccination. Despite this temporal correlation of immune and clinical response in some patients, not all clinically responding patients demonstrated tumor-reactive CD8 T cells at the time points measured and the correlation between immune and clinical responses across the cohort was not statistically significant.
Regulatory T-Cell Induction: Correlation With Clinical Response
We, and others, have previously reported that follicular lymphoma tumors are enriched for regulatory T cells (Treg) and that follicular lymphoma tumor cells can induce a Treg phenotype and suppressive function in conventional CD4 T cells.20,21 We therefore measured the degree to which CpG-activated tumor B cells from the study cohort could induce Treg phenotype in autologous CD4 cells.
Prevaccination peripheral blood lymphocytes were cultured either with media, or with autologous, irradiated, CpG-activated tumor B cells and assessed for CD4, CD25, and intracellular FOXP3 expression by flow cytometry. Baseline levels of CD25+ FOXP3+ Treg among peripheral blood CD4 cells was low (range, 2.7% to 16%; mean 7.3%), but on culture with autologous tumor, the proportion was variably increased (range, 2.7% to 52.8%; mean 19.7%). Notably, Treg induction was greater with CpG-activated tumor cells than with untreated tumor cells (data not shown).
The fold-induction of the Treg phenotype appeared dichotomous (Fig 5A), with some patients' tumor cells (n = 5) inducing a several-fold increase in Treg (range, 4.5- to 12.8-fold; mean, 6.6-fold) and others (n = 9) having minimal effect (range, 0.9- to 2.1-fold; mean, 1.5-fold). We compared the magnitude of clinical response between the two groups and observed that the non-Treg inducers trended toward better responses (two-sided t-test P = .056). In support of this finding, there was significantly longer progression-free survival in the Treg noninducers versus Treg inducers (log-rank test, P = .0058; Fig 5B). This correlation also appeared significant when Treg fold induction was treated as a continuous variable (Cox proportional hazard model, P = .0049; Fig 5C). In contrast, the baseline proportion of Treg in each patient's tumor and peripheral blood did not appear to correlate with clinical outcome measures (data not shown), although such a relationship has been observed by others in an ex vivo lymphoma vaccine study.3
Fig 5.
Regulatory T-cell (Treg) induction by CpG-activated tumors. (A) Prevaccination peripheral blood lymphocytes were cultured with media or with autologous, CpG-activated tumor B-cells for 120 hours, then assessed per flow cytometry for CD4, CD25, and forkhead box protein P3 (FOXP3). Data shown are gated on live CD4+ cells; Treg fold-induction statistics shown are CD25+FOXP3+tumor/CD25+FOXP3+media. Progression-free survival (PFS) for each patient was correlated to Treg fold-induction using the latter as a (B) dichotomous variable (≤ 3-fold v > 3-fold) and comparing PFS by log-rank test, or (C) as a continuous variable and relating to PFS by Cox proportional hazard model.
Re-Treatment With In Situ CpG Vaccine
Patient 12 achieved a PR lasting 64 weeks but eventually recurred and was re-treated using a higher dose of PF-3512676. This patient again tolerated treatment with no symptomatic adverse effects, and achieved a second PR with total reduction in the six index lesions from 39 to 15 cm2 (Fig 4A). The magnitude of this response was slightly greater than that achieved after initial vaccination (60% v 55%) and significantly more rapid (12 v 43 weeks).
Although the patient was asymptomatic, follow-up imaging demonstrated a grade 2 left pleural effusion consisting of a sterile exudate, containing 94% T cells and no evidence of lymphoma. A number of these cells were tumor-reactive memory CD8 T cells, at significantly higher proportions than that found in blood (Fig 4B). Notably the patient's nearby mediastinal adenopathy improved significantly (7.50 v 2.98 cm2) and the effusion resolved over the following 2 months.
DISCUSSION
Here, we demonstrate that in situ vaccination is feasible, safe, and sufficiently powerful to induce objective clinical and immune responses even in patients with significant lymphoma burden. Several studies of ex vivo, custom-manufactured lymphoma vaccines using patient-specific idiotype,22,23 idiotype-pulsed dendritic cells,24 and tumor lysate–pulsed dendritic cells3 have also demonstrated objective clinical responses and these prior results prompted our pursuit of the practicable in situ approach. This approach—by its nature—must be studied in patients with clinically evident disease, in contrast to the described randomized studies5–7 performed in the post-therapy adjuvant setting. As compared with other comparably practical off-the-shelf vaccines—such as allogeneic cell lines25—in situ vaccination has the potential advantage of more accurately encompassing each individuals' relevant tumor antigens. As compared with other approaches to increase the potency of immunotherapy with systemic immunostimulation, such as high-dose IL-226, vaccination appears relatively nontoxic.
Whereas intratumoral injection of immunostimulants has been studied previously,27,28 our preclinical model indicated that the combination of immunostimulation and cytotoxic therapy was required to induce sufficiently powerful antitumor immunity. The use of low-dose, local irradiation rather than chemotherapy allowed for the measurement of systemic immunity at untreated sites of disease and is supported by data showing that cells killed by radiation are especially “immunogenic.”29 Conversely, the need for the intratumoral route of CpG administration was seen in the preclinical model and consistent with its lack of clinical efficacy when administered systemically.13
Tumor-reactive, memory CD8 T cells were shown to mediate antitumor immunity in the preclinical model, and we have demonstrated their induction in several of our clinically responding patients; however, we did not find a strict correlation between T-cell immunity and clinical responses as has generally been the case in cancer vaccine trials.2,3,24,30,31 Approaches to improve the detection of tumor-reactive T cells could include sampling of biologic compartments other than peripheral blood, such as tumor sites, as suggested by the pleural effusion findings of patient 12. In addition, several recent immune-response studies showing that vaccine-induced T cells peak at day 14 and decline sharply thereafter,32–34 have prompted earlier immune-response measurements in an ongoing follow-up study.
We observed that patients with Treg-inducing tumors had poorer clinical outcomes after vaccination. This biomarker could be either a specific predictor of response to in situ vaccination or a general prognosticator of poor outcomes regardless of therapy. Interestingly, patients with highly Treg-infiltrated tumors have shown favorable clinical outcomes after standard therapy.35,36 If Treg induction predicts good response to standard therapy, but a poor response to the in situ vaccine, then it would be a powerful clinical tool for selecting appropriate patients for vaccination. This interesting finding is still preliminary and is being evaluated prospectively in an ongoing follow-study (ClinicalTrials.gov-ID: NCT00880581).
Despite numerous effective therapies for indolent lymphoma, the disease is characterized by relapse and diminished response to re-treatment.1 An effective vaccine could break from this paradigm, as reimmunization characteristically boosts pre-existing immune memory. Herein, one patient who achieved a PR from vaccination and later relapsed was re-treated on a follow-up vaccine study. Re-treatment yielded a more rapid clinical response of greater magnitude. The improved response may have resulted from the boosting of immune memory or from the higher PF-3512676 dose, although either alternative would have implications for improving in situ vaccination. This second response suggests that those patients who initially respond and subsequently progress might do so because of loss of the initial immune response rather than the tumor's development of inherent resistance to the therapy.
This study's findings have clear implications for future development of the in situ vaccination approach. The use of low-dose PF-3512676 was necessary given the lack of prior safety data with intratumoral administration. This study suggests that the safety of this route is comparable to subcutaneous administration, which has been well-tolerated at doses up to five-fold higher than used here12 and with higher doses inducing greater Type 1 helper T cells type cytokine responses.37 This study's correlation of clinical response with fewer prior therapies suggests that vaccination might be even more effective in previously untreated patients. These considerations have prompted the aforementioned follow-up study using a higher PF-3512676 dose in patients with either recurrent or previously untreated lymphoma.
The induction of tumor-reactive CD8 T cells and the inferior outcomes of patients with Treg-inducing tumors suggest that enhancing T-effector cells or inhibiting Treg might further increase the power of vaccination. We have recently described two such approaches preclinically. The first uses combinations of monoclonal antibodies targeting T-cell costimulatory molecules,38 several of which are currently being studied clinically. A second approach, which we refer to as immunotransplant, harvests and reinfuses vaccine-primed T cells to the patient after lymphodepletive conditioning19 is being investigated in a phase I/II study (ClinicalTrials.gov-ID:NCT00490529).
These encouraging preliminary results suggest that CpG-based in situ vaccination warrants further study as a novel therapy for patients with lymphoma. The combination of cytotoxic therapy and intratumoral immunostimulation has been studied preclinically for a variety of common tumor types39–41 and might also be directly translated to the clinic.
Supplementary Material
Acknowledgment
We thank Ash Alizadeh, MD, PhD, Holbrook Kohrt, MD, and Eric Berlin for helpful discussion in preparation of this article.
Appendix
Fig A1.
Immune responses. (A, B) CpG induction of immunogenic phenotype in patient tumor cells. Histograms represent coculture with PF-3512676 (green line) or media alone (red line). (C) Induction of tumor-reactive memory CD8 T cells per upregulation of CD137; data are gated on live CD8+ cells, statistics are percentages of CD45RO+CD137+ cells among that gate. (D) Induction of tumor-reactive CD8 T cells per interferon-γ (IFNg), interleukin-2 (IL2), and tumor necrosis factor (TNF) upregulation; data are gated on live CD8+ cells, statistics are percentages of cytokine(+) cells among that gate.
Footnotes
See accompanying editorial on page 4295
Supported by Grant No. P01-CA34233 from the National Institutes of Health (R.L.), a Lymphoma Research Foundation Career Development Award (J.D.B.), an American Society of Clinical Oncology Young Investigator Award (W.Z.A), and a Lymphoma Research Foundation Fellowship (W.Z.A). R.L. is an American Cancer Society Clinical Research Professor.
Presented in part in oral format at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00185965.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joshua D. Brody, Weiyun Z. Ai, Ronald Levy
Administrative support: Joshua D. Brody, Weiyun Z. Ai, Ronald Levy
Provision of study materials or patients: Joshua D. Brody, Weiyun Z. Ai, Ranjana H. Advani, Youn H. Kim, Richard T. Hoppe, Irene Wapnir, Ronald Levy
Collection and assembly of data: Joshua D. Brody, Weiyun Z. Ai, Debra K. Czerwinski, James A. Torchia, Mia Levy, Susan J. Knox, Lewis K. Shin, Robert J. Tibshirani, Ronald Levy
Data analysis and interpretation: Joshua D. Brody, Weiyun Z. Ai, Debra K. Czerwinski, James A. Torchia, Mia Levy, Lewis K. Shin, Robert J. Tibshirani, Ronald Levy
Manuscript writing: Joshua D. Brody, Weiyun Z. Ai, Debra K. Czerwinski, James A. Torchia, Mia Levy, Ranjana H. Advani, Youn H. Kim, Richard T. Hoppe, Susan J. Knox, Lewis K. Shin, Irene Wapnir, Robert J. Tibshirani, Ronald Levy
Final approval of manuscript: Joshua D. Brody, Weiyun Z. Ai, Debra K. Czerwinski, James A. Torchia, Mia Levy, Ranjana H. Advani, Youn H. Kim, Richard T. Hoppe, Susan J. Knox, Lewis K. Shin, Irene Wapnir, Robert J. Tibshirani, Ronald Levy
REFERENCES
- 1.Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol. 2005;18:1–10. doi: 10.1016/j.beha.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 3.Di Nicola M, Zappasodi R, Carlo-Stella C, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: A pilot study. Blood. 2009;113:18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 4.Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 5.Freedman A, Neelapu SS, Nichols C, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27:3036–3043. doi: 10.1200/JCO.2008.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy R, Robertson M, Ganjoo K, et al. Results of a phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared to non-specific immunotherapy, KLH with GM-CSF, in patients with follicular non-Hodgkin's lymphoma (fNHL) AACR Meeting Abstracts. 2008;(abstr LB-204) [Google Scholar]
- 7.Schuster SN, Gause SS, Muggia BL, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J Clin Oncol. 2009;27(suppl; abstr 2):5s. [Google Scholar]
- 8.Li J, Song W, Czerwinski DK, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 9.Varghese B, Widman A, Do J, et al. Generation of CD8+ T cell-mediated immunity against idiotype-negative lymphoma escapees. Blood. 2009;114:4477–4485. doi: 10.1182/blood-2009-05-223263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourke E, Bosisio D, Golay J, et al. The toll-like receptor repertoire of human B lymphocytes: Inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 11.Haas RL, Poortmans P, de Jong D, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21:2474–2480. doi: 10.1200/JCO.2003.09.542. [DOI] [PubMed] [Google Scholar]
- 12.Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 13.Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Levy MA, Garg A, Tam A, et al. LesionViewer: A tool for tracking cancer lesions over time. AMIA Annu Symp Proc. 2007;2007:443–447. [PMC free article] [PubMed] [Google Scholar]
- 16.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 17.Jahrsdorfer B, Mühlenhoff L, Blackwell SE, et al. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11:1490–1499. doi: 10.1158/1078-0432.CCR-04-1890. [DOI] [PubMed] [Google Scholar]
- 18.Decker T, Schneller F, Sparwasser T, et al. Immunostimulatory CpG-oligonucleotides cause proliferation, cytokine production, and an immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood. 2000;95:999–1006. [PubMed] [Google Scholar]
- 19.Brody JD, Goldstein MJ, Czerwinski DK, et al. Immunotransplantation preferentially expands T-effector cells over T-regulatory cells and cures large lymphoma tumors. Blood. 2009;113:85–94. doi: 10.1182/blood-2008-05-155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai WZ, Hou JZ, Zeiser R, et al. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer. 2009;124:239–244. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang ZZ, Novak AJ, Stenson MJ, et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoges S, Rodrìguez-Calvillo M, Zabalegui N, et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 23.Redfern CH, Guthrie TH, Bessudo A, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman JM, Czerwinski DK, Davis TA, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: Clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 25.Fournier P, Schirrmacher V. Randomized clinical studies of anti-tumor vaccination: State of the art in 2008. Expert Rev Vaccines. 2009;8:51–66. doi: 10.1586/14760584.8.1.51. [DOI] [PubMed] [Google Scholar]
- 26.Sosman JA, Carrillo C, Urba WJ, et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol. 2008;26:2292–2298. doi: 10.1200/JCO.2007.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann MA, Kors C, Audring H, et al. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 28.Molenkamp BG, Sluijter BJ, van Leeuwen PA, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 29.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 30.Neelapu SS, Kwak LW, Kobrin CB, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11:986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 31.Ribas A, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng Y, Jing Y, Campbell AE, et al. Age-related impaired type 1 T cell responses to influenza: Reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Choi SJ, Park WB, et al. Detailed kinetics of immune responses to a new cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine. 2007;25:6287–6291. doi: 10.1016/j.vaccine.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 34.Treanor J, Wu H, Liang H, et al. Immune responses to vaccinia and influenza elicited during primary versus recent or distant secondary smallpox vaccination of adults. Vaccine. 2006;24:6913–6923. doi: 10.1016/j.vaccine.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 36.Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 37.Krieg AM, Efler SM, Wittpoth M, et al. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Houot R, Levy R. Idiotype vaccination for lymphoma: Moving towards optimisation. Leuk Lymphoma. 2009;50:1–2. doi: 10.1080/10428190802517807. [DOI] [PubMed] [Google Scholar]
- 39.Najar HM, Dutz JP. Topical CpG enhances the response of murine malignant melanoma to dacarbazine. J Invest Dermatol. 2008;128:2204–2210. doi: 10.1038/jid.2008.59. [DOI] [PubMed] [Google Scholar]
- 40.VanOosten RL, Griffith TS. Activation of tumor-specific CD8+ T cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- 41.Meng Y, Carpentier AF, Chen L, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116:992–997. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.