Abstract
Purpose
Tumor antigen (TA) –targeted monoclonal antibodies (mAb), rituximab, trastuzumab, and cetuximab, are clinically effective for some advanced malignancies, especially in conjunction with chemotherapy and/or radiotherapy. However, these results are only seen in a subset (20% to 30%) of patients. We discuss the immunologic mechanism(s) underlying these clinical findings and their potential role in the variability in patients' clinical response.
Methods
We reviewed the evidence indicating that the effects of TA-targeted mAb-based immunotherapy are mediated not only by inhibition of signaling pathways, but also by cell-mediated cytotoxicity triggered by the infused TA-targeted mAb. We analyzed the immunologic variables that can influence the outcome of antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro and in animal model systems. We also analyzed the correlation reported between these variables and the clinical response to mAb-based immunotherapy.
Results
Of the variables that influence ADCC mediated by TA-targeted mAb, only polymorphisms of Fcγ receptors (FcγR) expressed by patients' lymphocytes were correlated with clinical efficacy. However, this correlation is not absolute and is not observed in all malignancies. Thus other variables may be responsible for the antitumor effects seen in mAb-treated patients. We discuss the evidence that triggering of TA-specific cellular immunity by TA-targeted mAb, in conjunction with immune escape mechanisms used by tumor cells, may contribute to the differential clinical responses to mAb-based immunotherapy.
Conclusion
Identification of the mechanism(s) underlying the clinical response of patients with cancer treated with TA-targeted mAb is crucial to optimizing their application in the clinic and to selecting the patients most likely to benefit from their use.
INTRODUCTION
Convincing evidence indicates that tumor antigen (TA) –targeted monoclonal antibody (mAb) –based immunotherapy, using rituximab (anti-CD20), trastuzumab (anti–human epidermal growth factor 2 [HER2]), and cetuximab (anti–human epidermal growth factor 1 [HER1]/epidermal growth factor receptor [EGFR]), is clinically effective in lymphoma, breast cancer, and head and neck (HNC) and colorectal carcinomas (CRC), respectively.1–9 Despite the disparate etiologies leading to the development of these malignancies, mAb therapy provides clinical response rates and a survival advantage in each of them,10 and their therapeutic efficacy is often enhanced by combination with radiotherapy or chemotherapy11–14 These findings have restored confidence among clinical oncologists in the value of biologic therapy for the treatment of malignant disease and have facilitated enrollment in clinical trials with TA-targeted mAb. As a result, during the last few years, a large number of patients have been treated with TA-targeted mAb-based immunotherapy. Two findings are noteworthy. First, although the antigens used as targets are expressed by a large number of normal cells, administration of TA-targeted mAb causes adverse effects, including allergic reactions to the introduced foreign proteins, only in a limited number of patients. Second, as single agents, TA-targeted mAbs yield response rates of 8% to 10% in advanced, heavily pretreated and recurrent disease10; their therapeutic efficacy is often enhanced by combination with radiotherapy or chemotherapy, with the response rate increasing up to 30%. A related observation is that efficacy is seen in only some of the malignant diseases expressing the targeted TA on tumor cells.
These findings raise the question of which mechanism(s) underlie(s) the therapeutic efficacy of TA-targeted mAb-based immunotherapy. Answers to this question have both theoretical and practical implications. On one hand, it will contribute to our understanding of why TA-targeted mAb-based immunotherapy has a differential clinical effect on patients with a given type of malignant disease and why it works in only some of the diseases that express the targeted TA on tumor cells. On the other hand, it will likely define criteria to select patients to be treated with TA-targeted mAb-based immunotherapy, to monitor their clinical response, and to optimize the immunotherapy schedule.
We first review the evidence indicating that not only inhibition of signal transduction pathways, but also immunologic mechanisms, underlie the antitumor activity of currently used TA-targeted mAbs. Then we describe the variables that influence the extent of cell-dependent lysis of target cells mediated by TA-targeted mAb in vitro and in animal models. Finally, we discuss the clinical relevance of these variables as well as the experimental15 and clinical evidence16 that argues for a role of TA-targeted cellular immunity triggered by TA-targeted mAb, and immunoescape mechanisms, in the clinical outcome of TA-targeted mAb-based immunotherapy.
CLINICAL ACTIVITY OF TA-TARGETED mAbs
A large number of reviews describe the clinical efficacy of the therapeutic, TA-targeted mAbs, rituximab, trastuzumab, and cetuximab, in lymphoid and epithelial malignancies (Table 1).7,17–21 Although TA-targeted mAbs may be used as single agents, most clinical scenarios use these mAbs in conjunction with radiotherapy and/or chemotherapy and demonstrate enhancement of clinical activity as compared with conventional therapy when given without the mAb.4,7,22,23 The clinical efficacy of mAb-based immunotherapy is manifested by higher cure rates in previously untreated patients with cancer and prolongation of overall survival in patients with recurrent/metastatic disease.8,9,24 Clinical response is observed in mAb-treated patients over ≥ 1 weeks, a time frame consistent with a T-lymphocyte–mediated lytic effect, and these kinetics coincide with the transport of TA to the draining lymph node in vivo within 48 hours.25–27 In mAb-treated patients with cancer, tumor lysis syndrome is rarely observed over the course of hours or days,28 as would be expected for purely natural killer cell (NK cell) –mediated lytic effects. In addition, blockade of Erb-B receptor activation (phosphorylation) occurs within 10 to 20 minutes of mAb treatment in vitro,29,30 arguing for additional mechanisms in the clinical responses observed in these patients in vivo. The reduction in relative risk of death or recurrence is between 20% and 30% with the addition of cetuximab, trastuzumab, or rituximab to radiotherapy4 or chemotherapy.5,31,32 Recently, administration of trastuzumab has been combined with HER2 peptide vaccines to augment the clinical activity of the mAb,12 and adjuvant therapies used in breast carcinoma do not seem to reduce the activity of TA-specific T cells.12,33 Recurrence of disease in patients demonstrating initial clinical response is likely to represent escape of tumor cells from the antitumor effect exerted by the TA-targeted mAb used.1–6,34 Because the clinical efficacy of these TA-targeted mAbs has been well-described elsewhere,7,17–21 we will focus on the potential role that immunologic and immune escape mechanisms play in the differential clinical response to mAb-based immunotherapy.
Table 1.
Selected FDA-Approved TA-Targeted mAbs for Human Cancers
| mAb | Target | Isotype | FDA-Approved Diseases |
|---|---|---|---|
| Cetuximab | EGFR/HER1 | Chimeric IgG1 | EGFR-positive colon cancer, HNC |
| Panitumumab | EGFR/HER1 | Fully human IgG2 | EGFR-positive colon cancer, HNC |
| Rituximab | CD20 | Chimeric IgG1 | CD20+ low-grade lymphoma, diffuse large B-cell lymphoma, follicular lymphoma |
| Trastuzumab | HER2/neu | Humanized IgG1 | HER2/neu-positive breast cancer |
Abbreviations: FDA, US Food and Drug Administration; mAbs, monoclonal antibodies; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; Ig, immunoglobulin; HNC, head and neck cancer.
MECHANISMS UNDERLYING THE ANTITUMOR ACTIVITY OF TA-TARGETED mAbs
TA Expression and Signaling Blockade Mediated by TA-Targeted mAbs
CD20, EGFR (ErbB1, HER1), and HER2 (ErbB2) are the molecules targeted by rituximab, cetuximab, and trastuzumab, respectively, which are clinically effective in the treatment of lymphoma, HNC and CRC, and breast cancer, respectively. These targets share several features. First, they are expressed on both normal and malignant cells, although the latter express much higher levels of TA, with mutations occurring only in rare cases.22 These quantitative differences appear to play a role in the pathogenesis of the disease, because an association has been found between increased levels of these antigens and prognosis. An additional similarity among these clinically efficacious mAbs as therapeutic agents seems to be their targeting of surface receptors involved in downstream signal transduction. EGFR and HER2 are tyrosine kinase receptors that belong to the Erb-B/HER receptor family. HER1 and HER2 initiate signaling through several pathways, including the phosphatidylinositol 3-kinase(PI3K)/AKT and Ras/mitogen-activated protein (MAP) kinase pathway, which promote cell survival and proliferation.35 CD20, the targeted antigen of rituximab, has been suggested to trigger antiapoptotic pathways in B cells through Bcl-2.34,36 In addition, in CRC, cetuximab clinical efficacy is significantly predicted by the absence of activating k-RAS mutations, in addition to some predictive power of Fcγ receptor (FcγR) polymorphisms for clinical response in these patients.8,37 This mechanism(s) is (are) not likely to play a major role in HNC, which also benefits from the therapeutic activity of cetuximab, because this disease does not manifest appreciable mutations in k-RAS (or EGFR38,39). These findings support the use of receptor tyrosine kinase inhibitors (TKI) for the treatment of EGFR-overexpressing diseases. However, lack of target specificity of TKIs for ErbB family kinases, and their generally lower clinical activity than mAbs recognizing these targets, have focused attention on mAb-based therapies directed at this family of growth factor receptors. In addition, the kinetics of clinical response observed in mAb-treated patients results in tumor shrinkage over weeks, consistent with a T-lymphocyte–mediated lytic effect, not usually over the course of hours, as would be expected for purely NK cell–mediated effects. Furthermore, the nearly complete reduction in Erb-B receptor activation (phosphorylation) within 10 to 20 minutes of mAb blockade of ligand binding in vitro38,40,41 also argues for additional mechanisms besides signaling inhibition in the clinical responses observed in these patients in vivo.
IMMUNOLOGIC VARIABLES MODULATING THE EXTENT OF ANTITUMOR ACTIVITY OF TA-TARGETED mAbs
Several lines of evidence suggest that blockade of signal transduction may not be the only mechanism of action mediating clinical benefit of mAb-treated patients with cancer.35,42,43 Indeed, the potential role of immunologic mechanisms in the therapeutic efficacy of ErbB-targeted mAb (as opposed to TKI) is supported by several lines of evidence. First, tumor cell apoptosis is not observed in vitro without the addition of lymphocytes to the culture system.40 Second, correlation of clinical response is observed in patients expressing certain polymorphisms of mAb-binding receptors on NK cells, monocytes, and granulocytes known to have lytic activity. Last, biomarkers of clinical response, such as level of expression, activation, or genomic amplification of EGFR, have not been consistently correlated with clinical response to mAb therapy targeting these receptors,44 indicating that other mechanisms may explain the observed clinical activity.
Among the variables known to play a role in the antitumor activity of TA-targeted mAbs is their ability to mediate lysis of tumor cells in vitro by NK cells, monocytes, and granulocytes in an antibody-dependent cell-mediated cytotoxicity (ADCC) assay. The extent of lysis in this assay is in turn influenced by several variables, and they, or at least some of them, may contribute to the differential clinical response of patients treated with mAb-based immunotherapy. Many of these variables have been characterized preclinically in vitro and in animal model systems. In particular, the role of B cells and complement has been investigated, but is not the primary focus of this review.45–47 The available information will be reviewed regarding patients' clinical responses to TA-targeted mAb-based immunotherapy.
Expression and Density of the Targeted TA on Tumor Cell Surface
In a number of tumor types, the expression level of the targeted TA (CD20, HER2, or EGFR) on target cells has been shown to influence the extent of their mAb-mediated lysis in vitro, especially when the effector cells (ie, NK cells and monocytes) display low lytic efficiency.40,48 However, in most studies, allogeneic tumor cells with different levels of the targeted TA have been used as targets.49 Therefore, the potential interference of confounding variables cannot be excluded. At any rate, the available data are at variance with the conflicting results about the relationship between the expression level of the targeted TA (CD20, HER2, or EGFR) on tumors and clinical response. Although clinical responses to EGFR-targeted mAb, cetuximab, are generally not correlated with level of EGFR expression on tumor cells,43 Burtness et al50 showed an inverse correlation with EGFR expression, and Chung et al51 even showed clinical activity in EGFR-negative tumors. One might argue that the discrepancy between the results of the in vitro studies and the clinical findings reflects the lack of sensitivity of the method used, generally standard immunohistochemical staining, to measure target antigen expression in malignant lesions. However, an alternative possibility we favor is the role of additional immunologic variables,41,52–54 as described below.
Influence of mAb Isotype and Dose on the Induction of TA-Targeted Cellular Immunity and Antitumor Activity
In addition to FcγR polymorphism, mAb concentration, association constant, and, most importantly, isotype subclass play an important role in the extent of cell-dependent lysis of target cells, with human immunoglobulin (Ig) G1 and IgG3 being more efficient at mediating lysis of target cells than IgG2 and IgG4 isotypes (Table 2).48,55 A dose-response relationship has been identified for mAb antitumor activity in vitro.48,49 Although the dose relationship plateaus above 10 μg/mL, plasma levels are 50 to 150 μg/mL, indicating sufficient circulating mAb available for maximal tumor cell binding in patients. When a single TA is targeted by mAbs of different IgG isotype, variability in immune activation may be mediated by differential FcγR binding affinity. In this regard, a unique example is the two mAbs targeting EGFR currently approved by the US Food and Drug Administration, cetuximab (IgG1 isotype) and panitumumab (IgG2 isotype). These two mAbs compete for the same ligand binding site(s) on the ecto-domain of EGFR, but the IgG2 isotype of the latter is predicted to result in lower ability to induce cellular immune reactions (Table 2).40,56 A recent preclinical study did demonstrate, unexpectedly, the ability of panitumumab to mediate ADCC through myeloid-derived granulocytes, including neutrophils.55 Further clinical studies using panitumumab in CRC and HNC are underway to clarify whether this represents an actual mechanism of antitumor activity in patients.
Table 2.
mAb Isotype Binding Affinity to Polymorphic FcγR Subtypes
| Characteristic | CD64, FcγRI | CD32 |
CD16 |
|||
|---|---|---|---|---|---|---|
| FcγRIIa | FcγRIIb | FcγRIIc | FcγRIIIa | FcγRIV | ||
| Affinity | High | Medium | Low | Low | Medium | Medium |
| Specificity | High | Low | Medium | Low | Medium | High |
| Isotype that binds preferably | IgG1, IgG3 | IgG1, IgG3 | IgG1, IgG2 | IgG2a, IgG2b | IgG1 | IgG2a, IgG2b |
| Comments | 131-R/R genotype has lower affinity for IgG1 | Inhibitory receptor | 176-F/F most common genotype, associated with lower affinity for IgG1 | |||
Abbreviations: mAb, monoclonal antibody; FcγR, Fcγ receptor; Ig, immunoglobulin.
Complement-dependent lysis (CDC) may also be observed in vitro using IgG1 isotype TA-targeted mAb57 and has been proposed to mediate effects of rituximab, trastuzumab, and more recently cetuximab in vitro and in murine systems, particularly in association with B cells.45–47 However, the rapid effects of CDC question its major role in clinical responses to mAb-based immunotherapy, which are usually observed over ≥ 1 weeks. CDC has also been suggested to play a major role in some of the adverse effects observed.57–64 However, this mechanism of action does not provide an explanation for variability in patient responses to the mAb used (Table 3).
Table 3.
Molecular Mechanisms Underlying Therapeutic Efficacy of TA-Targeted mAbs
| Triggering of antibody-dependent cellular cytotoxicity |
| Activation of: |
| Phagocytosis |
| Complement-dependent cytotoxicity |
| DC maturation and TA uptake |
| Induction of cellular immunity leading to: |
| Presentation of TA by antigen-presenting cell (ie, DC) |
| Activation of CD4+ T-cell–mediated killing |
| Activation of B cells and eosinophils |
| Activation of TA-targeted cytotoxic T lymphocytes |
Abbreviations: TA, tumor antigen; mAbs, monoclonal antibodies; DC, dendritic cell.
FcγR Polymorphisms and Disease Status
Interactions between tumor cells coated with TA-targeted mAbs and effector cells such as NK cells are mediated by FcγR.65 These receptors are expressed by monocytes and NK cells, the major effector cells in mAb-mediated lysis of tumor cells.1–3,37,66 The functional significance of FcγR polymorphisms is highlighted by its association with the extent of in vitro lysis of target cells in ADCC and with the control of growth of human tumors grafted in immunodeficient mice.2,3, 43,67 Among the activating receptors, FcγR IIIa is expressed on NK cells and FcγR IIa on monocyte-derived dendritic cells (DC), B cells, and granulocytic cells. Little clinical information is available regarding the role of inhibitory (IIb) FcγR in mAb-treated patients with cancer; this topic is an intriguing area of potential investigation. Clinical activity of TA-targeted mAb seems to be associated with patients who harbor particular so-called high-responder FcγR IIa/IIIa H- and V- encoding polymorphisms, arguing for a role of FcγR in the clinical efficacy of at least some of these malignancies.11 The molecular basis for the differential binding of the Fc portion of Abs to polymorphic FcγR reflects the substitution of histidine (H) with arginine (R) in codon 131 of FcγRIIa and of valine (V) with phenylalanine (F) in codon 158 of FcγR IIIa.68,69 In a murine xenograft model of breast cancer70 and HNC (R.L. Ferris, unpublished data), the antitumor effects of trastuzumab and cetuximab, respectively, depend in part on the presence of FcγR-bearing immune cells, including NK cells.2,3 Only limited information is available about the effect of disease status on function of effector cells, but in vitro comparison of the lytic activity of peripheral-blood mononuclear cells from healthy donors and from patients with cancer suggests that the latter have reduced ability to lyse mAb-coated tumor cells. These defects can be corrected in vitro with cytokines such as interleukin (IL) -2, IL-15, and IL-21, which are known to enhance NK cell expression of FcγR.48,49,71
It should be stressed that polymorphic genotypes of FcγR do not seem to be associated with improved clinical outcome in every patient and every disease. For example, the FcγRIIa-131 H polymorphism seems to be indicative of improved response rate in patients with breast carcinoma, CRC, and follicular lymphoma treated with trastuzumab, cetuximab, and rituximab, respectively. In contrast, the FcγRIIIa-158F polymorphism is correlated with improved response rates in patients with CRC72 treated with cetuximab, but is also linked to poor response rates in patients with breast carcinoma treated with trastuzumab,1 CRC treated with cetuximab,37 and hematologic malignancies treated with rituximab.3,73 Moreover, FcγRIIa/IIIa polymorphisms are not associated with clinical outcome in patients with chronic lymphocytic leukemia treated with rituximab or alemtuzumab, in patients with diffuse large B-cell lymphoma treated with rituximab, and in patients with follicular lymphoma treated with sequential cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy and rituximab. Moreover, relevance of this immunologic mechanism of action to clinical efficacy is correlative, and NK cell-mediated lysis occurs in vitro over 4 to 6 hours, a time frame that would be consistent with a more rapid tumor lysis and clinical response than that observed in patients with cancer (a week or more). Lastly, even when clinical responses are observed, this is not seen in every patient whose lymphocytes express favorable FcγR genotype and whose tumor expresses the targeted TA.3,37,73 In addition, as mentioned, conflicting data have been published in CRC37,72 for the allele with best predictive ability for cetuximab clinical response. These results argue in favor of a role for other variables, in addition to the FcγR polymorphism, in the clinical response to mAb-based immunotherapy.
Potential Role of TA-Targeted Cellular Immunity in the Clinical Efficacy of mAb-Based Immunotherapy
Most of the variables found to influence ADCC of tumor cells by FcγR-bearing effector cells in vitro and in animal model systems are not associated with clinical responses.74,75 In addition, the association of patients' FcγR genotype with clinical outcome to mAb therapy is significant, but not absolute. These clinical findings support the role of additional mechanisms in the clinical responses observed in patients with cancer treated with these agents. As mentioned above, generation of TA-mediated cellular immunity in vivo is consistent with the kinetics of clinical responses observed in treated patients with cancer. An increasing number of results in animal model systems and in clinical settings indicates that TA-targeted antibodies trigger or enhance TA-targeted cellular immune responses,15,16 involving cytotoxic T lymphocytes (CTL) and helper (Th) T cells.16,74,76–79 This evidence is reviewed below.
FcγR and Antigen Presentation
Therapeutic mAbs are effective in enhancing antigen cross-presentation by DC to T cells in vitro and in vivo, resulting in augmentation of TA-targeted CTL generation. A growing body of evidence suggests that the uptake, internalization, and presentation of apoptotic cell-derived or soluble TA to CD8+ T cells by DC are enhanced by various receptor(s) on DC that have endocytic activity80 and by activating FcγR such as FcγRI, IIa, and III.16,74,78,81 Antigen uptake, in the form of immune complexes or opsonized tumor cells, is associated with enhanced antigen presentation by DC,74,78 as well as by B cells, which express FcγR. Depending on the associated costimulatory signals, these effects can be stimulatory or suppressive of TA-specific activation, leading to cellular immunity or plasma cell induction (humoral immunity). Thus treatment of patient with cancer with mAbs may trigger a TA-targeted CD8+ T-cell response by enhancing the antigen uptake through FcγR on DC in the microenvironment or draining nodes. The detection of TA-targeted CTL in mice after immunotherapy with mAb78,81 and of a CD4+ T-cell response in patients with breast cancer treated with trastuzumab16 support the hypothesis that TA-targeted mAb induce TA-specific T-cell responses in vivo. It has also been suggested that the FcγRIIa polymorphism plays a role in mAb-mediated TA cross-presentation by DC.27,74, 76,77
NK Cell–DC Crosstalk Enhancement of Cellular Immunity Triggered by TA-Targeted mAbs
Potential mechanisms of enhanced cross-presentation induced by mAb-based immunotherapy include facilitation of TA:mAb complex uptake by DC, enhancement of FcγR ligation and stimulation of DC,74,76 induction of costimulatory and adhesion molecules on the DC surface, and upregulation of antigen processing machinery (APM) components known to be crucial for optimal TA processing and presentation.82,83
Although most innate immune cells express both inhibitory and activating FcγR, NK cells constitutively express only a low-affinity, activating FcγRIIIa (CD16), which initiates lytic activity on encountering mAb-coated targets. In addition to mediating ADCC, the subpopulation of NK cells84 characterized by high CD56 expression, referred to as CD56bright, secretes T helper type 1 (Th1) cytokines, such as interferon γ, tumor necrosis factor α, and chemokines, such as macrophage inflammatory protein-(MIP)-1α, MIP-1β, and RANTES, that inhibit tumor cell proliferation, enhance antigen presentation, and aid in the chemotaxis of T cells.48,85–87 NK cell–DC cross-talk follows the recruitment of both NK cells and DC to sites of inflammation,87,88 potentially reducing the activity and number of immunosuppressive, regulatory T cells (known as suppressor T cells; Tregs).52,89 The resulting potent activating bidirectional signaling can shape both the innate immune response within inflamed peripheral tissues and the adaptive immune response in secondary lymphoid organs. Additionally, NK cells in the presence of cytokines released by DC become activated, regulating both the quality and the intensity of innate immune responses. In turn, DC in the presence of cytokines released by activated NK cells enhance cross-presentation and priming of T cells. In conclusion, through direct interactions and secretion of cytokines/chemokines,48,86,87 NK cells may function as helper cells90 and enhance and broaden T-cell priming against multiple TA,91 including the targeted TA as well as “private” TA. The latter type of TA has been suggested by results obtained in animal model systems to be more efficient than shared TA in mediating tumor rejection by T-cell immunity. Their clinical application, however, is hampered by the limited progress made in their identification and by the practical difficulties to implement immunotherapeutic therapies individualized for each patient.
Enhancement of TA Cross-Presentation by DC to T Cells in the Presence of TA-Targeted mAbs
Although many investigators have explored the induction of ADCC, a growing body of evidence, as well as our preliminary results, indicate that TA-targeted mAb, including cetuximab, rituximab, and trastuzumab, can effectively trigger TA-specific CTL responses. In preclinical studies,25 mAbs recognizing a TA have been shown to activate targeted TA-specific CD8+ T-cell responses. This activation is thought to occur via the processing of exogenously acquired antigens by antigen-presenting cells followed by presentation on major histocompatibility complex (MHC) class I antigens to CD8+ T cells, termed cross-presentation. DCs in particular efficiently process externally acquired antigens and present them via enhanced cross-priming on MHC class I molecules on their cell surface to enhance CD8+ T-cell activation (the effector cells that ultimately recognize and lyse antigen-expressing tumors). It has been reported that a mAb recognizing the rat HER2/neu antigen expressed by murine mammary tumor cells can induce TA uptake and cross-priming that correlated with improved in vivo tumor rejection.25
Interestingly, in mice the CD8– population of DC does not typically cross-prime unless specifically activated via its Fcγ receptor,27 a finding consistent with the in vitro data presented above. DC express the low-affinity FcγR III, the high-affinity FcγR I, and the complement receptor 3 and mannose receptor, and these receptor-mediated phagocytic mechanisms require formation of immune complexes. These immune complexes bind directly to FcγR to initiate phagocytic signal transduction,92 activation of FcγR through the binding of immune complexes, and enhanced antigen presentation in DC.16,76–78,93,94 By enhancing the uptake of TA by DC and their presentation to T cells,74,77,78,81 these emerging new data suggest that the generation of HLA class I restricted, TA-targeted T cells triggered by therapeutic mAbs may be influenced by the FcγR expressed by NK cells and monocyte-derived DCs.76–78 In addition, mAb:TA complexes may enhance the induction of DC cross-presentation by inducing or upregulating the expression of APM components and costimulatory molecules associated with maturation phenotype of DC.82,95,96 Enhancement of DC maturation programs and upregulation of APM components known to be highly correlated with optimal TA cross-presentation,82 such as TAP1/2, are strongly induced by incubation with cetuximab and activated NK cells.76
POTENTIAL ROLE OF IMMUNE ESCAPE IN THE LACK OF CLINICAL RESPONSE TO mAb-BASED IMMUNOTHERAPY
Clinical responses to TA-targeted mAb-based immunotherapy are correlated with the patients' particular FcγR genotypes72; however, tumor progression often occurs in these patients. Escape mechanisms used by tumor cells to evade mAb-induced antitumor immunity may play a role in patients' differential clinical response to TA-targeted mAb-based immunotherapy.97 For instance, mAb-mediated tumor cell lysis may be influenced by tumor cell expression of NK cell inhibitory proteins, such as HLA-E98 and HLA-G.99,100 It has been shown that NK cell dysfunction is frequently observed in patients with cancer and especially in those with advanced disease.101,102 This variable could influence the extent of lysis in ADCC independently of the FcγRIIIa polymorphism. Inhibitory signals transmitted to NK cells and CTL may provide a mechanism of immune escape by tumor cells, such as through Treg cells, as shown in multiple cancer types.99,100,103,104 In addition, rituximab-mediated NK cell lysis has been recently shown to be inhibited by HLA-G mediated interference with NK cell activation and tumor cell killing.105 Thus analysis of classical and nonclassical HLA class I106 antigen expression by tumor cells, which can influence NK cell lysis,107,108 should contribute to define the mechanisms underlying differential responses to mAb-mediated antitumor immune effects in vitro and in vivo.
We note that nonimmune escape from TA-targeted mAb therapy may occur, mediated by accessory signaling pathways that compensate for blockade of the pathways downstream of the targeted TA. Indeed, recent evidence from cetuximab-resistant tumor systems indicates that treatment escape may be mediated by upregulation of G-protein coupled receptors that can bypass the inhibited Erb-B receptor targeted by the mAb30,109–111 or expression of other HER family receptors, such as HER2 or HER3.112,113 This is corroborated by the value of elevated levels of ErbB family ligands as predictors of clinical response to trastuzumab and cetuximab.10,114
Impact of APM Component Defects on In Vivo Tumor Cell Recognition by HLA Class I–Restricted,TA-Targeted CTL
APM plays a crucial role in the generation of HLA class I-TA–derived peptide complexes expressed by antigen-presenting cells. In tumor cells or in DCs cross-presenting TA, the APM generates peptides from mostly, although not exclusively, endogenous TA, which are presented by surface MHC molecules to cognate CTL. Abnormalities in the expression and/or function of APM components have been found in malignant cells with a frequency of 50% to 70%115,116; these defects result in scrambled or altered expression of trimolecular complexes on the surface of tumor cells, thereby allowing for tumor cell escape from T-cell recognition. The recently16 described induction of TA-specific T cells in mAb-treated patients with cancer provides the rationale for suggesting that APM defects in tumor lesions play a role in the differential clinical response to cetuximab-based immunotherapy.77,78,107,116–118
ROLE OF CELLULAR NETWORKS IN mAb-MEDIATED ANTITUMOR ACTIVITIES
In summary, mAb immunotherapy is clinically effective, but there is significant variability among patients' responses. Tumor signaling pathways do not adequately explain the variability in clinical response observed nor exclude other potential mechanisms of antitumor activity. Immunologic mechanisms, such as ADCC, are modulated by mAb-binding, polymorphic FcγR on immune cells, level of TA expression by tumor cells, concentration of mAb used, and frequency and reactivity of immune cells in the tumor microenvironment, including TA-targeted CTL and Tregs. In addition, various potential mechanisms of escape from TA-targeted mAb therapy have been detected to enable avoidance of TA recognition or immune cell-dependent tumor lysis. A model we propose for cellular cascades initiated by mAb immunotherapy is shown in Figure 1. Further work must investigate the balance of stimulatory and immunosuppressive networks in conjunction with conventional chemo- and radiotherapeutic strategies. Incorporation of TA-targeted mAb should also be considered to a much greater extent in cancer vaccine-based strategies.12,16,78
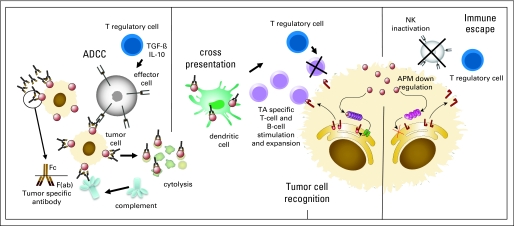
Fig 1.
Immune cellular network mediated by tumor antigen (TA) –targeted monoclonal antibodies (mAbs) in the tumor microenvironment to induce antitumor activity. Direct cell lysis of mAb-bound tumor cells overexpressing the targeted TA may occur. First, exposure of TA-positive tumor cells to TA-targeted mAbs leads to their opsonization through the binding of cetuximab to the TA epitopes expressed on tumor targets. Recognition of tumor cells opsonized with mAbs is mediated via the FcγRIII (CD16) expressed on natural killer (NK) cells and FcγRIIa on monocytes, dendritic cells (DCs), and other granulocytes.40,48 These effectors of innate immunity are activated in the presence of mAb-coated tumor cell targets and proceed to release perforin and granzymes, thus inducing tumor cell death (antibody-dependent cell-mediated cytotoxicity [ADCC]). The mAb-coated TAs released by dying cells in the form of immune complexes are avidly taken up by DCs, processed, and presented to T cells. This recruitment of NK cells and other FcγR-bearing immune cells occurs, liberating tumor cell products and TAs in the setting of inflammatory cytokines and chemokines. Infiltration of DCs and lymphocytes into the microenvironment may lead to uptake and processing of TAs by DCs and induction of TA-specific cellular immune responses. TGF-β, transforming growth factor β; IL-10, interleukin 10; APM, antigen processing machinery.
A number of intriguing questions are raised by these data. For instance, if our hypothesis is correct, that TA-specific T-cell immunity plays an important role in the clinical responses to mAb-based immunotherapy, why are mAbs more effective than vaccines that elicit T cells? This difference may reflect the induction by TA-targeted mAbs of a cellular immune response to a broader range of TA, including private antigens and cytokines in the tumor microenvironment. In addition, Th1 type cytokines may upregulate APM components in tumor cells and/or downregulate HLA-G, therefore counteracting escape mechanisms. If induction of a T-cell response is important, the combination of TA-specific mAbs with vaccination or immunomodulators such as anti-CTLA4 mAb garners greater rationale. The latter may be more effective because we have not yet identified the TAs that are clinically relevant. Patients treated with TA-targeted mAbs may also be a useful source of T cells to identify new, clinically relevant TAs. Downstream immunologic effects, such as the triggering of the idiotypic cascade, which we have observed in cetuximab-treated patients with HNC (unpublished data), is an area for further investigation. Finally, passive administration of mAb might be replaced by vaccination with peptide mimics to induce TA-targeted antibodies, as recently described in the HER2 system.119
Acknowledgment
We thank Charmaine Wallace and Karen Seisek for help with manuscript preparation.
Glossary Terms
- ADCC (antibody-dependent cell-mediated cytotoxicity):
a mechanism of cell-mediated immunity whereby an effector cell of the immune system actively lyses a target cell that has been bound by specific antibodies.
- Antigen processing machinery:
a pathway of degradative and chaperone proteins in the cytoplasm and endoplasmic reticulum (ER) that process and transport antigen, degrading whole protein in the cytoplasm into short peptide fragments, which are transported into the ER and then bound to HLA antigens. The trimolecular HLA-β2 microglobulin-antigenic peptide complex is then transported to the cell surface for presentation to T lymphocytes.
- CDC (complement-dependent lysis):
process of target cell lysis by a cascade of soluble proteins activated by cells coated with immunoglobulin G or immunoglobulin GM antibodies.
- Cross-presentation:
a process of antigen-specific T-cell stimulation by dendritic cells and other antigen-presenting cells, which take up exogenous antigen and process it for recognition by HLA class I-restricted cytotoxic T lymphocytes, and in some cases HLA class II-restricted antigen presentation to CD4+ T lymphocytes.
- Cytokines:
Cell communication molecules that are secreted in response to external stimuli.
- Cytotoxic T lymphocyte:
a T lymphocyte (a type of white blood cell) that is capable of inducing the death of tumor cells; they also kill cells that are infected with viruses.
- Immunoescape:
a general term referring to the many efforts by tumor cells to suppress or evade antitumor immunity. This may lead to upregulation of inhibitory proteins, or downregulation of required proteins necessary for efficient antitumor immunity.
- Microenvironment:
the unique complex of tumor cells, stromal, and immune infiltrate that can promote or reject tumors, as well as shape their phenotype through contact-dependent or soluble mediators.
- NK cells (natural killer cells):
NK cells belong to the innate immune system and are specialized to kill target cells that are either infected with viruses or host cells that have become cancerous. CD56 is a surface marker specific to NK cells.
- Regulatory T cells (known as suppressor T cells):
are a specialized subpopulation of T cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self-antigens. This is an important “self-check” built into the immune system so that responses do not go haywire. Regulatory T cells come in many forms, including those that express the CD8 transmembrane glycoprotein (CD8+ T cells), those that express CD4, CD25 and Foxp3 (CD4+CD25+ regulatory T cells or “Tregs”) and other T cell types that have suppressive function. These cells are involved in closing down immune responses after they have successfully tackled invading organisms and also in keeping in check immune responses that may potentially attack one's own tissues (autoimmunity).
Footnotes
Supported by National Institutes of Health Grants No. R01 DE19727 and 1P50 CA097190.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Robert L. Ferris, Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: None Research Funding: Robert L. Ferris, Amgen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Robert L. Ferris, Elizabeth M. Jaffee,Soldano Ferrone
Financial support: Robert L. Ferris
Administrative support: Robert L. Ferris
Provision of study materials or patients: Robert L. Ferris
Collection and assembly of data: Robert L. Ferris
Data analysis and interpretation: Robert L. Ferris, Elizabeth M. Jaffee, Soldano Ferrone
Manuscript writing: Robert L. Ferris, Elizabeth M. Jaffee,Soldano Ferrone
Final approval of manuscript: Robert L. Ferris, Elizabeth M. Jaffee, Soldano Ferrone
REFERENCES
- 1.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 2.Weng WK, Czerwinski D, Timmerman J, et al. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Beers SA, French RR, et al. Anti-CD20 monoclonal antibodies - historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert Opin Drug Saf. 2009;8:223–235. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 8.Gill S, Goldberg RM. Cetuximab, chemotherapy and KRAS status in mCRC. Nat Rev Clin Oncol. 2009;6:379–380. doi: 10.1038/nrclinonc.2009.83. [DOI] [PubMed] [Google Scholar]
- 9.Spiro H. Cetuximab for metastatic colorectal cancer. N Engl J Med. 2009;361:95. doi: 10.1056/NEJMc090927. [DOI] [PubMed] [Google Scholar]
- 10.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 11.Kowalczyk A, Gil M, Horwacik I, et al. The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Lett. 2009;281:171–182. doi: 10.1016/j.canlet.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Armstrong D, Biedrzycki B, et al. A phase I vaccine safety and chemotherapy dose-finding trial of an allogeneic GM-CSF-secreting breast cancer vaccine given in a specifically timed sequence with immunomodulatory doses of cyclophosphamide and doxorubicin. Hum Gene Ther. 2004;15:313–337. doi: 10.1089/104303404322886165. [DOI] [PubMed] [Google Scholar]
- 14.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: A chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abes R, Gelize E, Fridman WH, et al. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. doi: 10.1182/blood-2009-10-248609. [epub ahead of print on May 3, 2010] [DOI] [PubMed] [Google Scholar]
- 16.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 17.Hall PS, Cameron DA. Current perspective: trastuzumab. Eur J Cancer. 2009;45:12–18. doi: 10.1016/j.ejca.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Mariani G, Fasolo A, De Benedictis E, et al. Trastuzumab as adjuvant systemic therapy for HER2-positive breast cancer. Nat Clin Pract Oncol. 2009;6:93–104. doi: 10.1038/ncponc1298. [DOI] [PubMed] [Google Scholar]
- 19.Rasul KI, Kerr DJ. Targeted therapies: Cetuximab plus chemotherapy in patients with advanced NSCLC. Nat Rev Clin Oncol. 2009;6:499–500. doi: 10.1038/nrclinonc.2009.108. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez J, Viudez A, Ponz-Sarvise M, et al. Improving disease control in advanced colorectal cancer: Panitumumab and cetuximab. Crit Rev Oncol Hematol. 2010;74:193–202. doi: 10.1016/j.critrevonc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.William WN, Jr, Kim ES, Herbst RS. Cetuximab therapy for patients with advanced squamous cell carcinomas of the head and neck. Nat Clin Pract Oncol. 2009;6:132–133. doi: 10.1038/ncponc1321. [DOI] [PubMed] [Google Scholar]
- 22.Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsoutsou PG, Koukourakis MI, Azria D, et al. Optimal timing for adjuvant radiation therapy in breast cancer: A comprehensive review and perspectives. Crit Rev Oncol Hematol. 2009;71:102–116. doi: 10.1016/j.critrevonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Vermorken JB, Mesia R, Vega-Villegas ME, et al. Cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil (5-FU) in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (R&M SCCHN) (EXTREME) J Clin Oncol. 2006;24(suppl):289s. doi: 10.1200/JCO.2005.04.3547. abstr 5537. [DOI] [PubMed] [Google Scholar]
- 25.Kim PS, Armstrong TD, Song H, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–1711. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reilly RT, Machiels JP, Emens LA, et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61:880–883. [PubMed] [Google Scholar]
- 27.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan G, D'Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406–2408. doi: 10.1200/JCO.2007.14.7603. [DOI] [PubMed] [Google Scholar]
- 29.Lango MN, Shin DM, Grandis JR. Targeting growth factor receptors: Integration of novel therapeutics in the management of head and neck cancer. Curr Opin Oncol. 2001;13:168–175. doi: 10.1097/00001622-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Carlisle DL, Swick MC, et al. Gastrin-releasing peptide activates Akt through the epidermal growth factor receptor pathway and abrogates the effect of gefitinib. Exp Cell Res. 2007;313:1361–1372. doi: 10.1016/j.yexcr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Chumsri S, Jeter S, Jacobs LK, et al. Pathologic complete response to preoperative sequential doxorubicin/cyclophosphamide and single-agent taxane with or without trastuzumab in stage II/III HER2-positive breast cancer. Clin Breast Cancer. 2010;10:40–45. doi: 10.3816/CBC.2010.n.005. [DOI] [PubMed] [Google Scholar]
- 32.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 33.Coveler AL, Goodell V, Webster DJ, et al. Common adjuvant breast cancer therapies do not inhibit cancer vaccine induced T cell immunity. Breast Cancer Res Treat. 2009;113:95–100. doi: 10.1007/s10549-008-9910-y. [DOI] [PubMed] [Google Scholar]
- 34.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–3636. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 35.Harari PM, Allen GW, Bonner JA. Biology of interactions: Antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 36.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 37.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 38.Sharafanski M, Ferris RL, Ferrone S, et al. Epidermal growth factor receptor targeted therapy of squamous cell carcinoma of the head and neck. Head and Neck. doi: 10.1002/hed.21365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- 40.López-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1277–1281. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 41.Valentini AM, Pirrelli M, Caruso ML. EGFR-targeted therapy in colorectal cancer: Does immunohistochemistry deserve a role in predicting the response to cetuximab? Curr Opin Mol Ther. 2008;10:124–131. [PubMed] [Google Scholar]
- 42.Harari PM, Huang S. Radiation combined with EGFR signal inhibitors: Head and neck cancer focus. Semin Radiat Oncol. 2006;16:38–44. doi: 10.1016/j.semradonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Grandis JR, Rinaldo A, et al. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30:667–674. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- 44.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 45.Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 46.Beum PV, Mack DA, Pawluczkowycz AW, et al. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol. 2008;181:8120–8132. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- 47.Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): Considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 48.López-Albaitero A, Lee SC, Morgan S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1853–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 50.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 51.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 52.Toi M, Sperinde J, Huang W, et al. Differential survival following trastuzumab treatment based on quantitative HER2 expression and HER2 homodimers in a clinic-based cohort of patients with metastatic breast cancer. BMC Cancer. 2010;10:56. doi: 10.1186/1471-2407-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esteva FJ, Yu D, Hung MC, et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 54.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 56.Andrade Filho PA, Lopez-Albaitero A, Gooding W, et al. Novel Immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 33:83–91. doi: 10.1097/CJI.0b013e3181b8f421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dechant M, Weisner W, Berger S, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 58.Mineo JF, Bordron A, Quintin-Roue I, et al. Recombinant humanised anti-HER2/neu antibody (Herceptin) induces cellular death of glioblastomas. Br J Cancer. 2004;91:1195–1199. doi: 10.1038/sj.bjc.6602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10:3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 60.Zhao WP, Zhu B, Duan YZ, et al. Neutralization of complement regulatory proteins CD55 and CD59 augments therapeutic effect of herceptin against lung carcinoma cells. Oncol Rep. 2009;21:1405–1411. doi: 10.3892/or_00000368. [DOI] [PubMed] [Google Scholar]
- 61.Lee SC, Lopez-Albaitero A, Ferris RL. Immunotherapy of head and neck cancer using tumor antigen-specific monoclonal antibodies. Curr Oncol Rep. 2009;11:156–162. doi: 10.1007/s11912-009-0023-5. [DOI] [PubMed] [Google Scholar]
- 62.Bellosillo B, Villamor N, Lopez-Guillermo A, et al. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–2777. doi: 10.1182/blood.v98.9.2771. [DOI] [PubMed] [Google Scholar]
- 63.Cragg MS, Morgan SM, Chan HT, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–1052. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- 64.van der Kolk LE, Grillo-Lopez AJ, Baars JW, et al. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 65.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 66.Dall'Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: Influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 67.Varchetta S, Gibelli N, Oliviero B, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 68.Tax WJ, Tamboer WP, Jacobs CW, et al. Role of polymorphic Fc receptor Fc gammaRIIa in cytokine release and adverse effects of murine IgG1 anti-CD3/T cell receptor antibody (WT31) Transplantation. 1997;63:106–112. doi: 10.1097/00007890-199701150-00020. [DOI] [PubMed] [Google Scholar]
- 69.Warmerdam PA, van den Herik-Oudijk IE, Parren PW, et al. Interaction of a human Fc gamma RIIb1 (CD32) isoform with murine and human IgG subclasses. Int Immunol. 1993;5:239–247. doi: 10.1093/intimm/5.3.239. [DOI] [PubMed] [Google Scholar]
- 70.Curcio C, Di Carlo E, Clynes R, et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her-2/neu carcinomas. J Clin Invest. 2003;111:1161–1170. doi: 10.1172/JCI17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roda JM, Joshi T, Butchar JP, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 73.Weng WK, Negrin RS, Lavori P, et al. Immunoglobulin G Fc receptor FcgammaRIIIa 158 V/F polymorphism correlates with rituximab-induced neutropenia after autologous transplantation in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:279–284. doi: 10.1200/JCO.2009.25.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhodapkar KM, Krasovsky J, Williamson B, et al. Antitumor monoclonal antibodies enhance cross-presentation of Cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dittmer D, Pati S, Zambetti G, et al. Gain of function mutations in p53. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 76.Amigorena S, Bonnerot C. Fc receptor signaling and trafficking: A connection for antigen processing. Immunol Rev. 1999;172:279–284. doi: 10.1111/j.1600-065x.1999.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 77.Clynes R. Antitumor antibodies in the treatment of cancer: Fc receptors link opsonic antibody with cellular immunity. Hematol Oncol Clin North Am. 2006;20:585–612. doi: 10.1016/j.hoc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Harbers SO, Crocker A, Catalano G, et al. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 81.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.López-Albaitero A, Mailliard R, Hackman T, et al. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. 2009;32:465–473. doi: 10.1097/CJI.0b013e3181a1c24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whiteside TL, Stanson J, Shurin MR, et al. Antigen-processing machinery in human dendritic cells: Up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173:1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 84.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 85.Roda JM, Parihar R, Magro C, et al. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 86.Kalinski P, Mailliard RB, Giermasz A, et al. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 87.Mailliard RB, Son YI, Redlinger R, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: Two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 88.Lucas M, Schachterle W, Oberle K, et al. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mailliard RB, Alber SM, Shen H, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.el-Shami K, Tirosh B, Bar-Haim E, et al. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 92.Shaw DR, Griffin FM., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981;289:409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- 93.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Machy P, Serre K, Leserman L. Class I-restricted presentation of exogenous antigen acquired by Fcgamma receptor-mediated endocytosis is regulated in dendritic cells. Eur J Immunol. 2000;30:848–857. doi: 10.1002/1521-4141(200003)30:3<848::AID-IMMU848>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 95.Lin W, Voskens CJ, Zhang X, et al. Fc-dependent expression of CD137 on human NK cells: Insights into “agonistic” effects of anti-CD137 monoclonal antibodies. Blood. 2008;112:699–707. doi: 10.1182/blood-2007-11-122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilcox RA, Tamada K, Strome SE, et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 97.Leibowitz MS, Nayak JV, Ferris RL. Head and neck cancer immunotherapy: Clinical evaluation. Curr Oncol Rep. 2008;10:162–169. doi: 10.1007/s11912-008-0025-8. [DOI] [PubMed] [Google Scholar]
- 98.Levy EM, Sycz G, Arriaga JM, et al. Cetuximab-mediated cellular cytotoxicity is inhibited by HLA-E membrane expression in colon cancer cells. Innate Immun. 2009;15:91–100. doi: 10.1177/1753425908101404. [DOI] [PubMed] [Google Scholar]
- 99.Diepstra A, Poppema S, Boot M, et al. HLA-G protein expression as a potential immune escape mechanism in classical Hodgkin's lymphoma. Tissue Antigens. 2008;71:219–226. doi: 10.1111/j.1399-0039.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 100.Lin A, Yan WH, Xu HH, et al. HLA-G expression in human ovarian carcinoma counteracts NK cell function. Ann Oncol. 2007;18:1804–1809. doi: 10.1093/annonc/mdm356. [DOI] [PubMed] [Google Scholar]
- 101.Whiteside TL, Chikamatsu K, Nagashima S, et al. Antitumor effects of cytolytic T lymphocytes (CTL) and natural killer (NK) cells in head and neck cancer. Anticancer Res. 1996;16:2357–2364. [PubMed] [Google Scholar]
- 102.Kuss I, Donnenberg AD, Gooding W, et al. Effector CD8+CD45RO-CD27-T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Cancer. 2003;88:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inagaki A, Ishida T, Yano H, et al. Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC. Int J Cancer. 2009;125:212–221. doi: 10.1002/ijc.24351. [DOI] [PubMed] [Google Scholar]
- 104.Ugurel S, Reinhold U, Tilgen W. HLA-G in melanoma: A new strategy to escape from immunosurveillance? Onkologie. 2002;25:129–134. doi: 10.1159/000055222. [DOI] [PubMed] [Google Scholar]
- 105.Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902–3907. doi: 10.1158/1078-0432.CCR-05-2376. [DOI] [PubMed] [Google Scholar]
- 106.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: Molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–133. doi: 10.1385/IR:33:2:113. [DOI] [PubMed] [Google Scholar]
- 107.Li D, Ronson B, Guo M, et al. Interleukin 2 gene transfer prevents NKG2D suppression and enhances antitumor efficacy in combination with cisplatin for head and neck squamous cell cancer. Cancer Res. 2002;62:4023–4028. [PubMed] [Google Scholar]
- 108.Melioli G, Semino C, Margarino G, et al. Expansion of natural killer cells in patients with head and neck cancer: Detection of “noninhibitory” (activating) killer Ig-like receptors on circulating natural killer cells. Head Neck. 2003;25:297–305. doi: 10.1002/hed.10198. [DOI] [PubMed] [Google Scholar]
- 109.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas SM, Bhola NE, Zhang Q, et al. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res. 2006;66:11831–11839. doi: 10.1158/0008-5472.CAN-06-2876. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Q, Bhola NE, Lui VW, et al. Antitumor mechanisms of combined gastrin-releasing peptide receptor and epidermal growth factor receptor targeting in head and neck cancer. Mol Cancer Ther. 2007;6:1414–1424. doi: 10.1158/1535-7163.MCT-06-0678. [DOI] [PubMed] [Google Scholar]
- 112.Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoel MJ, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: Rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15:5445–5456. doi: 10.1158/1078-0432.CCR-08-2980. [DOI] [PubMed] [Google Scholar]
- 113.Koutras AK, Fountzilas G, Kalogeras KT, et al. The upgraded role of HER3 and HER4 receptors in breast cancer. Crit Rev Oncol Hematol. 2010;74:73–78. doi: 10.1016/j.critrevonc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316:1083–1100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 115.Ferris R, Whiteside TL, Ferrone S. Clinical significance of downregulated antigen processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 116.López-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 117.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–3895. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 118.Banerjee D, Matthews P, Matayeva E, et al. Enhanced T-cell responses to glioma cells coated with the anti-EGF receptor antibody and targeted to activating FcgammaRs on human dendritic cells. J Immunother. 2008;31:113–120. doi: 10.1097/CJI.0b013e31815a5892. [DOI] [PubMed] [Google Scholar]
- 119.Kaumaya PT, Foy KC, Garrett J, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol. 2009;27:5270–5277. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]