Abstract
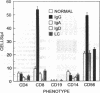
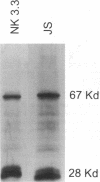
BACKGROUND. Multiple myeloma remains an incurable malignancy due to marked resistance of the tumor to standard doses of chemotherapy. Treatment approaches, using chemotherapeutic dose escalation and hematopoietic stem cell support have resulted in significant augmentation of tumor mass reduction such that complete remissions are effected in approximately 50% of patients. These remissions are however, often not durable. In the setting of minimal residual disease, therefore, adjunctive immunotherapy may be useful. METHODS. Peripheral blood mononuclear cells were studied from 28 untreated patients with multiple myeloma (MM). Mononuclear cell CD16 (FcR gamma III) expression was determined by flow cytometry. The effect of lymphocyte-derived soluble CD16, isolated by affinity chromatography, on MM cell growth and differentiation was assessed. MM cell proliferation, viability, immunoglobulin production and gene expression was studied. RESULTS. Data are presented indicating that cells expressing CD16 are increased in untreated patients with IgG-secreting myeloma. The predominant phenotype of these cells is CD8+ or CD56+. These CD16+ cells can produce a soluble form of the Fc receptor (sFcR, sCD16) that can bind to surface Ig on cultured human IgG-secreting myeloma cells and effect suppression of tumor cell growth and Ig secretion. This effector function is accompanied by concomitant suppression of c-myc as well as IgH and IgL gene transcription. Finally, prolonged exposure to sCD16 causes myeloma tumor cell cytolysis. CONCLUSIONS. sCD16 and possibly other soluble FcR are candidate molecules for adjunctive immunotherapy of myeloma, once complete responses have been effected by intensive cytotoxic therapy, now possible in up to 50% of newly diagnosed patients.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avvisati G., Mandelli F. The role of interferon-alpha in the management of myelomatosis. Hematol Oncol Clin North Am. 1992 Apr;6(2):395–405. [PubMed] [Google Scholar]
- Barlogie B., Alexanian R., Dicke K. A., Zagars G., Spitzer G., Jagannath S., Horwitz L. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood. 1987 Sep;70(3):869–872. [PubMed] [Google Scholar]
- Barlogie B., Epstein J., Selvanayagam P., Alexanian R. Plasma cell myeloma--new biological insights and advances in therapy. Blood. 1989 Mar;73(4):865–879. [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Broome H. E., Reed J. C., Godillot E. P., Hoover R. G. Differential promoter utilization by the c-myc gene in mitogen- and interleukin-2-stimulated human lymphocytes. Mol Cell Biol. 1987 Aug;7(8):2988–2993. doi: 10.1128/mcb.7.8.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield S. M., Morrison S. L. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991 Jun 1;173(6):1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A., Silvian I., Tatarsky I., Spira G. Impaired immunoglobulin synthesis in multiple myeloma: a B-cell dysfunction. Am J Hematol. 1986 Jun;22(2):143–154. doi: 10.1002/ajh.2830220205. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Tamma S. L., Bessler M., Wei C. F., Thorbecke G. J. IgD-receptor-positive human T lymphocytes. I. Modulation of receptor expression by oligomeric IgD and lymphokines. J Immunol. 1990 Dec 1;145(11):3556–3561. [PubMed] [Google Scholar]
- Conrad D. H. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu Rev Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Nadler L. M. Cell surface markers in hematologic malignancies. Semin Oncol. 1987 Jun;14(2):193–212. [PubMed] [Google Scholar]
- Gould J., Alexanian R., Goodacre A., Pathak S., Hecht B., Barlogie B. Plasma cell karyotype in multiple myeloma. Blood. 1988 Feb;71(2):453–456. [PubMed] [Google Scholar]
- Greil R., Fasching B., Loidl P., Huber H. Expression of the c-myc proto-oncogene in multiple myeloma and chronic lymphocytic leukemia: an in situ analysis. Blood. 1991 Jul 1;78(1):180–191. [PubMed] [Google Scholar]
- Harrison D., Phillips J. H., Lanier L. L. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II). J Immunol. 1991 Nov 15;147(10):3459–3465. [PubMed] [Google Scholar]
- Hoover R. G., Dieckgraefe B. K., Lake J., Kemp J. D., Lynch R. G. Lymphocyte surface membrane immunoglobulin in myeloma. III. IgA plasmacytomas induce large numbers of circulating, adult-thymectomy-sensitive, theta +, Lyt-1-2+ lymphocytes with IgA-Fc receptors. J Immunol. 1982 Dec;129(6):2329–2331. [PubMed] [Google Scholar]
- Hoover R. G., Hickman S., Gebel H. M., Rebbe N., Lynch R. G. Expansion of Fc receptor-bearing T lymphocytes in patients with immunoglobulin G and immunoglobulin A myeloma. J Clin Invest. 1981 Jan;67(1):308–311. doi: 10.1172/JCI110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. G., Kornbluth J. Immunoregulation of murine and human myeloma. Hematol Oncol Clin North Am. 1992 Apr;6(2):407–424. [PubMed] [Google Scholar]
- Hoover R. G., Lynch R. G. Isotype-specific suppression of IgA: suppression of IgA responses in BALB/c mice by T alpha cells. J Immunol. 1983 Feb;130(2):521–523. [PubMed] [Google Scholar]
- Hoover R. G., Lynch R. G. Lymphocyte surface membrane immunoglobulin in myeloma. II. T cells with IgA-Fc receptors are markedly increased in mice with IgA plasmacytomas. J Immunol. 1980 Sep;125(3):1280–1288. [PubMed] [Google Scholar]
- Ishizaka K. Regulation of IgE synthesis. Annu Rev Immunol. 1984;2:159–182. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Parham P., Punt J., Herzenberg L. A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985 Jan 1;161(1):1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., Cooper M. D., Kearney J. F., Mosteller L. M., Michalek S. M., Koopman W. J., McGhee J. R. Isotype specificity of helper T cell clones. Peyer's patch Th cells preferentially collaborate with mature IgA B cells for IgA responses. J Exp Med. 1984 Mar 1;159(3):798–811. doi: 10.1084/jem.159.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth J., Flomenberg N., Dupont B. Cell surface phenotype of a cloned line of human natural killer cells. J Immunol. 1982 Dec;129(6):2831–2837. [PubMed] [Google Scholar]
- Kyle R. A. Diagnostic criteria of multiple myeloma. Hematol Oncol Clin North Am. 1992 Apr;6(2):347–358. [PubMed] [Google Scholar]
- Lauria F., Foa R., Cavo M., Gobbi M., Raspadori D., Giubellino M. C., Tazzari P. L., Tura S. Membrane phenotype and functional behaviour of T lymphocytes in multiple myeloma: correlation with clinical stages of the disease. Clin Exp Immunol. 1984 Jun;56(3):653–658. [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R., March C. J., Schoenborn M. A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990 Dec 1;172(6):1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaia M., Bianchi A., Dianzani U., Camponi A., Attisano C., Boccadoro M., Pileri A. Defective interleukin-2 induction of lymphokine-activated killer (LAK) activity in peripheral blood T lymphocytes of patients with monoclonal gammopathies. Clin Exp Immunol. 1990 Jan;79(1):100–104. doi: 10.1111/j.1365-2249.1990.tb05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaia M., Dianzani U., Pioppo P., Boccadoro M., Redoglia V., Omede P., Pileri A. Emergence of activated lymphocytes in CD4 and CD8 subpopulations of multiple myeloma: correlation with the expansion of suppressor T-cells (CD8+ OKM1+) and ecto-5'nucleotidase deficiency. J Clin Lab Immunol. 1988 Jun;26(2):89–95. [PubMed] [Google Scholar]
- Massaia M., Dianzani U., Pioppo P., Sibilla E., Boccadoro M., Pileri A. Multiple myeloma: ecto-5' nucleotidase deficiency of suppressor/cytotoxic (CD8) lymphocytes is a marker for the expansion of suppressor T cells. Clin Exp Immunol. 1987 Aug;69(2):426–432. [PMC free article] [PubMed] [Google Scholar]
- Mathiot C., Teillaud J. L., Elmalek M., Mosseri V., Euller-Ziegler L., Daragon A., Grosbois B., Michaux J. L., Facon T., Bernard J. F. Correlation between soluble serum CD16 (sCD16) levels and disease stage in patients with multiple myeloma. J Clin Immunol. 1993 Jan;13(1):41–48. doi: 10.1007/BF00920634. [DOI] [PubMed] [Google Scholar]
- Mathur A., Lynch R. G. Increased T gamma and T mu cells in BALB/c mice with IgG and IgM plasmacytomas and hybridomas. J Immunol. 1986 Jan;136(2):521–525. [PubMed] [Google Scholar]
- Mathur A., Maekawa S., Ovary Z., Lynch R. G. Increased T epsilon cells in BALB/c mice with an IgE-secreting hybridoma. Mol Immunol. 1986 Nov;23(11):1193–1201. doi: 10.1016/0161-5890(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Mathur A., Van Ness B. G., Lynch R. G. In vivo and in vitro regulation of IgE production in murine hybridomas. J Immunol. 1990 Dec 1;145(11):3610–3617. [PubMed] [Google Scholar]
- Mellman I. S., Plutner H., Steinman R. M., Unkeless J. C., Cohn Z. A. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J Cell Biol. 1983 Mar;96(3):887–895. doi: 10.1083/jcb.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. S., Hoover R. G. Defective isotype-specific regulation of IgA anti-erythrocyte autoantibody-forming cells in NZB mice. J Immunol. 1989 Jun 15;142(12):4282–4288. [PubMed] [Google Scholar]
- Müller S., Hoover R. G. T cells with FC receptors in myeloma; suppression of growth and secretion of MOPC-315 by T alpha cells. J Immunol. 1985 Jan;134(1):644–647. [PubMed] [Google Scholar]
- Nathan C., Cohn Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med. 1980 Jul 1;152(1):198–208. doi: 10.1084/jem.152.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Kubagawa H., Sanders S. K., Cooper M. D. Biochemical nature of an Fc mu receptor on human B-lineage cells. J Exp Med. 1990 Oct 1;172(4):1165–1175. doi: 10.1084/jem.172.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken M. M., Kay N. E. T-cell subpopulations in multiple myeloma: correlation with clinical disease status. Br J Haematol. 1981 Dec;49(4):629–634. doi: 10.1111/j.1365-2141.1981.tb07273.x. [DOI] [PubMed] [Google Scholar]
- Osterborg A., Nilsson B., Björkholm M., Holm G., Mellstedt H. Natural killer cell activity in monoclonal gammopathies: relation to disease activity. Eur J Haematol. 1990 Sep;45(3):153–157. doi: 10.1111/j.1600-0609.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Ozer H., Han T., Henderson E. S., Nussbaum A., Sheedy D. Immunoregulatory T cell function in multiple myeloma. J Clin Invest. 1981 Mar;67(3):779–789. doi: 10.1172/JCI110095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglieroni T., MacKenzie M. R. Studies on the pathogenesis of an immune defect in multiple myeloma. J Clin Invest. 1977 Jun;59(6):1120–1133. doi: 10.1172/JCI108736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Pilarski L. M., Andrews E. J., Mant M. J., Ruether B. A. Humoral immune deficiency in multiple myeloma patients due to compromised B-cell function. J Clin Immunol. 1986 Nov;6(6):491–501. doi: 10.1007/BF00915255. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M., Jensen G. S. Monoclonal circulating B cells in multiple myeloma. A continuously differentiating, possibly invasive, population as defined by expression of CD45 isoforms and adhesion molecules. Hematol Oncol Clin North Am. 1992 Apr;6(2):297–322. [PubMed] [Google Scholar]
- Pilarski L. M., Ruether B. A., Mant M. J. Abnormal function of B lymphocytes from peripheral blood of multiple myeloma patients. Lack of correlation between the number of cells potentially able to secrete immunoglobulin M and serum immunoglobulin M levels. J Clin Invest. 1985 Jun;75(6):2024–2029. doi: 10.1172/JCI111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989 Aug 1;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. W., Foon K. A. T gamma-lymphoproliferative disease and related disorders in humans and experimental animals: a review of the clinical, cellular, and functional characteristics. Blood. 1984 Dec;64(6):1146–1158. [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Roman S., Moore J. S., Darby C., Müller S., Hoover R. G. Modulation of Ig gene expression by Ig binding factors. Suppression of alpha-H chain and lambda-2-L chain mRNA accumulation in MOPC-315 by IgA-binding factor. J Immunol. 1988 May 15;140(10):3622–3630. [PubMed] [Google Scholar]
- Schneider C., Gustincich S., Del Sal G. The complexity of cell proliferation control in mammalian cells. Curr Opin Cell Biol. 1991 Apr;3(2):276–281. doi: 10.1016/0955-0674(91)90152-o. [DOI] [PubMed] [Google Scholar]
- Selvanayagam P., Blick M., Narni F., van Tuinen P., Ledbetter D. H., Alexanian R., Saunders G. F., Barlogie B. Alteration and abnormal expression of the c-myc oncogene in human multiple myeloma. Blood. 1988 Jan;71(1):30–35. [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Teillaud J. L., Brunati S., Elmalek M., Astier A., Nicaise P., Moncuit J., Mathiot C., Job-Deslandre C., Fridman W. H. Involvement of FcR+ T cells and of IgG-BF in the control of myeloma cells. Mol Immunol. 1990 Dec;27(12):1209–1217. doi: 10.1016/0161-5890(90)90024-t. [DOI] [PubMed] [Google Scholar]
- Uchida A., Yagita M., Sugiyama H., Hoshino T., Moore M. Strong natural killer (NK) cell activity in bone marrow of myeloma patients: accelerated maturation of bone marrow NK cells and their interaction with other bone marrow cells. Int J Cancer. 1984 Sep 15;34(3):375–381. doi: 10.1002/ijc.2910340314. [DOI] [PubMed] [Google Scholar]