Abstract
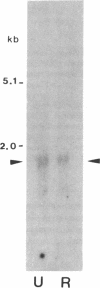
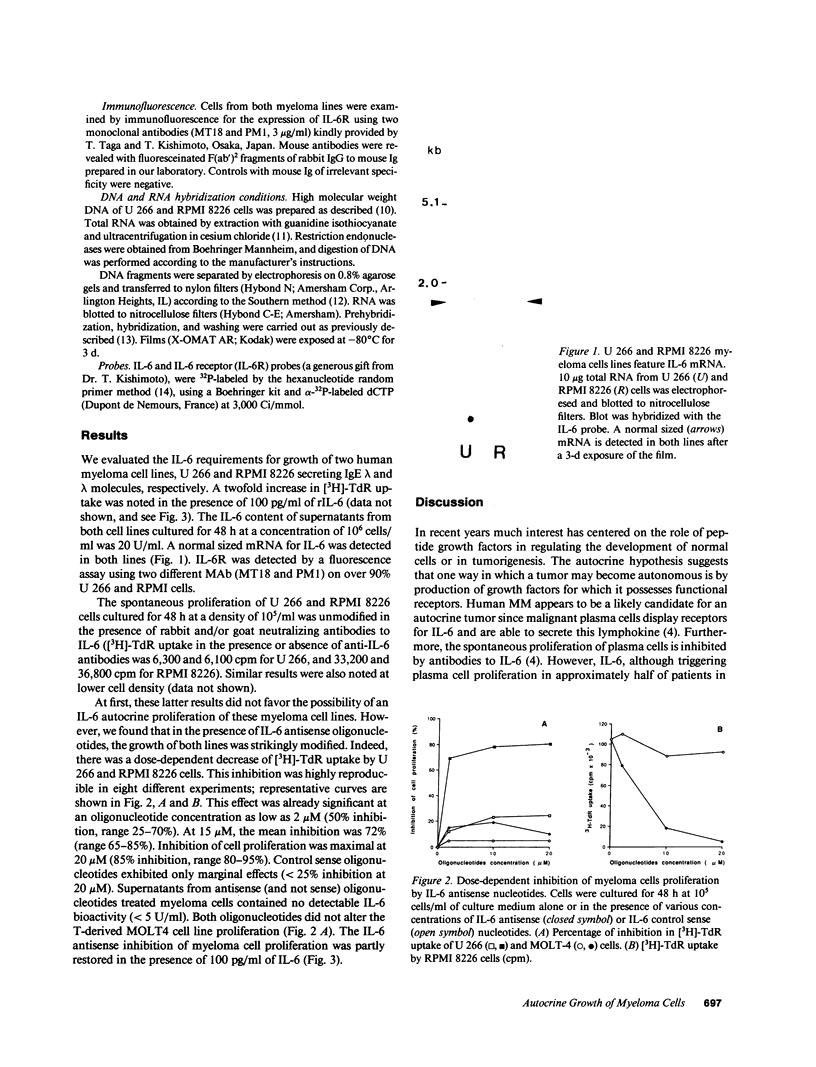
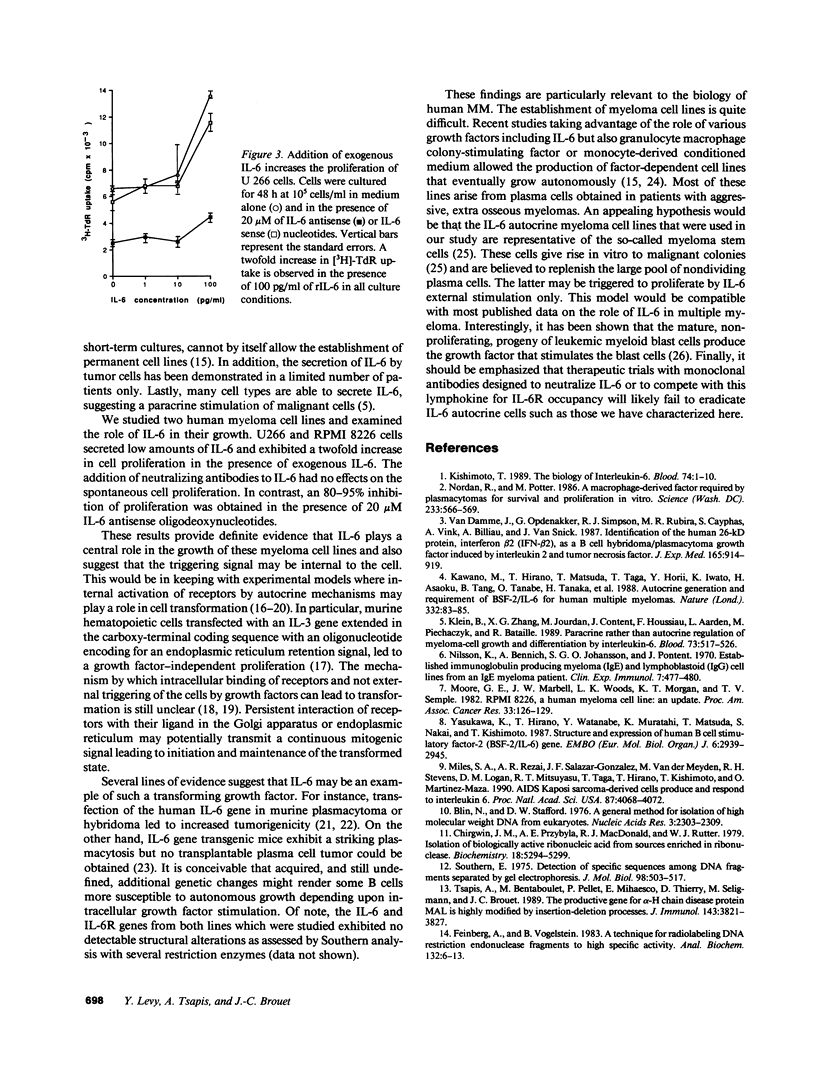
IL-6 has been shown to be a plasmacytoma growth factor in mice and is believed to play a key role in the development of human multiple myeloma. We investigated the IL-6 requirements for the growth of two human myeloma cell lines, U 266 and RPMI 8226. These cell lines secreted minute amounts of IL-6 (20 U/ml) and featured IL-6 mRNA. IL-6 receptors were detectable at the surface of malignant cells by immunofluorescence. Antibodies to IL-6 did not alter the proliferation of these myeloma cells. There was a dose-dependent decrease, however, in [3H]-thymidine uptake in the presence of IL-6 antisense (and not sense) oligodeoxynucleotides; in the presence of 20 microM IL-6 antisense, an 80 and 95% inhibition of the proliferation of U 266 and RPMI 8226 cells was observed, respectively. These results provide strong evidence for an IL-6 autocrine proliferation of myeloma cells which may occur via internal interaction between IL-6 and the IL-6 receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bejcek B. E., Li D. Y., Deuel T. F. Transformation by v-sis occurs by an internal autoactivation mechanism. Science. 1989 Sep 29;245(4925):1496–1499. doi: 10.1126/science.2551043. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder T. M., Abrams J. S., Wong P. M., Nienhuis A. W. Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol. 1989 Jan;9(1):204–213. doi: 10.1128/mcb.9.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dunbar C. E., Browder T. M., Abrams J. S., Nienhuis A. W. COOH-terminal-modified interleukin-3 is retained intracellularly and stimulates autocrine growth. Science. 1989 Sep 29;245(4925):1493–1496. doi: 10.1126/science.2789432. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hamburger A., Salmon S. E. Primary bioassay of human myeloma stem cells. J Clin Invest. 1977 Oct;60(4):846–854. doi: 10.1172/JCI108839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin. Implications for the mechanism of autocrine transformation. J Biol Chem. 1988 Sep 5;263(25):12608–12618. [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Boiron J. M., Portier M., Lu Z. Y., Wijdenes J., Brochier J., Bataille R. Interleukin-6 is the central tumor growth factor in vitro and in vivo in multiple myeloma. Eur Cytokine Netw. 1990 Oct-Nov;1(4):193–201. [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Content J., Houssiau F., Aarden L., Piechaczyk M., Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989 Feb;73(2):517–526. [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S. A., Rezai A. R., Salazar-González J. F., Vander Meyden M., Stevens R. H., Logan D. M., Mitsuyasu R. T., Taga T., Hirano T., Kishimoto T. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Nordan R. P., Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986 Aug 1;233(4763):566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- Scala G., Quinto I., Ruocco M. R., Arcucci A., Mallardo M., Caretto P., Forni G., Venuta S. Expression of an exogenous interleukin 6 gene in human Epstein Barr virus B cells confers growth advantage and in vivo tumorigenicity. J Exp Med. 1990 Jul 1;172(1):61–68. doi: 10.1084/jem.172.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Yoshioka R., Hirose Y., Sugai S., Tachibana J., Konda S. Establishment of two interleukin 6 (B cell stimulatory factor 2/interferon beta 2)-dependent human bone marrow-derived myeloma cell lines. J Exp Med. 1989 Jan 1;169(1):339–344. doi: 10.1084/jem.169.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapis A., Bentaboulet M., Pellet P., Mihaesco E., Thierry D., Seligmann M., Brouet J. C. The productive gene for alpha-H chain disease protein MAL is highly modified by insertion-deletion processes. J Immunol. 1989 Dec 1;143(11):3821–3827. [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink A., Coulie P., Warnier G., Renauld J. C., Stevens M., Donckers D., Van Snick J. Mouse plasmacytoma growth in vivo: enhancement by interleukin 6 (IL-6) and inhibition by antibodies directed against IL-6 or its receptor. J Exp Med. 1990 Sep 1;172(3):997–1000. doi: 10.1084/jem.172.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]