Abstract
In the songbird brain, dehydroepiandrosterone (DHEA) is metabolized to the active and aromatizable androgen androstenedione (AE) by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD). Thus, brain 3β-HSD plays a key role in regulating the steroidal milieu of the nervous system. Previous studies have shown that stress rapidly regulates brain 3β-HSD activity in a sex-specific manner. To elucidate endocrine regulation of brain 3β-HSD, we asked whether 17β-estradiol (E2) regulates DHEA metabolism in adult zebra finch (Taeniopygia guttata) and whether there are sex-specific effects. Brain tissue was homogenized and centrifuged to obtain supernatant lacking whole cells and cell nuclei. Supernatant was incubated with [3H]DHEA and radioinert E2 in vitro. Within only 10 min, E2 significantly reduced 3β-HSD activity in both male and female brain. Interestingly, the rapid effects of E2 were more pronounced in females than males. These are the first data to show a rapid effect of estrogens on the songbird brain and suggest that rapid estrogen effects differ between male and female brains.
Keywords: 3beta-HSD, 5beta-reductase, androstenedione, aromatase, bird, corticosterone, dehydroepiandrosterone, estradiol, HPLC, neurosteroid, non-genomic, song, stress, testosterone, zebra finch
Dehydroepiandrosterone (DHEA) – a gonadal, adrenal and neural steroid – is a sex steroid precursor with no known classical intracellular steroid receptor. Nonetheless, DHEA has a variety of effects on the central nervous system in humans and animals (Baulieu 1998; Kimonides et al. 1998; Wolf and Kirschbaum 1999). In songbirds, studies have shown that male song sparrows (Melospiza melodia) have high levels of plasma DHEA during the non-breeding season, when plasma testosterone (T) levels are low (Soma and Wingfield 2001; see also Hau et al. 2004). In addition, DHEA treatment during the non-breeding season increases song behavior and the size of a brain region controlling song (HVC; Reiner et al. 2004), and also modulates neural responses to an aggressive challenge (Soma et al. 2002; Goodson et al. 2005a). These behavioral and neural effects of DHEA are similar to the effects of T and 17β-estradiol (E2) (Soma et al. 2004b), suggesting that the effects of DHEA depend upon its conversion to active sex steroids within the brain (Soma 2006; Demas et al. 2007; Schlinger et al. 2007).
Dehydroepiandrosterone can be converted into potent androgens and estrogens locally within target tissues, such as the brain, that have the appropriate steroidogenic enzymes (Labrie et al. 2005). DHEA can be metabolized to androstenedione (AE), an active and aromatizable androgen, by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) (Fig. 1). AE can be subsequently converted to estrone (E1), T, 5α-androstanedione (5α-A), or 5β-A by various enzymes (Fig. 1). 3β-HSD has been detected in peripheral tissues (Mason 1993) and also in the central nervous system (Mensah-Nyagan et al. 1994; Guennoun et al. 1995). In a songbird, the zebra finch (Taeniopygia guttata), high levels of 3β-HSD have been detected in the brain of developing (Vanson et al. 1996; Tam and Schlinger 2007) and adult animals (Soma et al. 2004a; London et al. 2006).
Fig. 1.
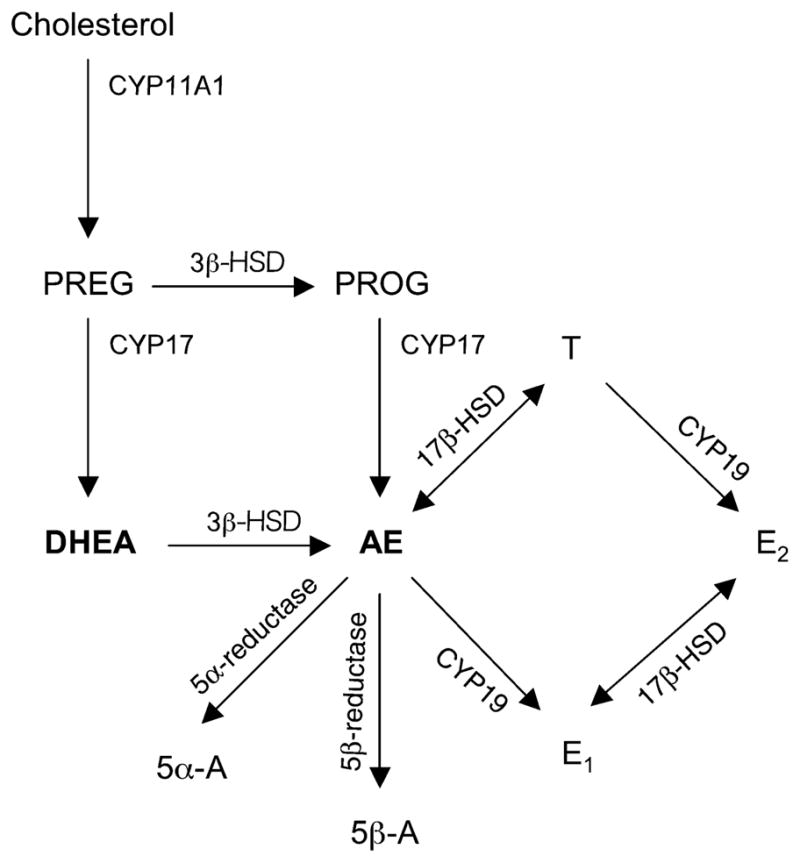
Simplified diagram of sex steroid synthesis. Steroids: PREG, pregnenolone; PROG, progesterone. Enzymes: CYP11A1, cytochrome P450 side chain cleavage; CYP17, cytochrome P450 17α-hydroxylase/C17, 20 lyase. The enzyme 3β-HSD metabolizes DHEA into AE. AE can then be converted to other steroids: 5β-A, 5β-androstanedione; 5α-A, 5α-androstanedione; E1, estrone; T, testosterone.
Interestingly, 3β-HSD activity in the adult zebra finch brain is rapidly regulated by restraint stress in a sex-specific manner (Soma et al. 2004a). Restraint stress rapidly (<10 min) decreases brain 3β-HSD activity in females but not in males (Soma et al. 2004a). This is the first demonstration that 3β-HSD is rapidly modulated. The rapid effects of stress suggest that the physiological mechanisms controlling 3β-HSD activity are also rapid. Although corticosterone is a possible regulator of 3β-HSD, plasma corticosterone levels are similar in male and female zebra finches following stress (Soma et al. 2004a), and in vitro corticosterone treatment does not rapidly regulate brain 3β-HSD activity (D. Pradhan, L. Lau, K. Soma, unpublished results).
Another possible regulator of brain 3β-HSD is E2. Plasma E2 levels are rapidly increased by stress in female rats (Shors et al. 1999), and brain aromatase activity is rapidly increased by stress in Japanese quail (Baillien et al. 2004). Songbirds have very high levels of brain aromatase, and E2 is a potent regulator of behavior, neuroanatomy, and steroidogenic enzymes in songbirds (Freking et al. 1998; Soma et al. 2000, 2004b). Moreover, previous studies have shown that estrogens can regulate brain 3β-HSD. For example, in female rats, in vivo 17β-estradiol benzoate (EB) treatment increases 3β-HSD mRNA and activity in the hypothalamus (Soma et al. 2005). In contrast, in male rats, in vitro E2 treatment decreases 3β-HSD activity in the sciatic nerve, and E2 was the most potent steroidal regulator of 3β-HSD (Coirini et al. 2003a,b). Lastly, E2 has numerous rapid effects on the brain (Cornil et al. 2006; Woolley 2007) and might also rapidly regulate brain 3β-HSD.
Songbirds, such as zebra finches are an excellent model species for studying the regulation of neural steroidogenic enzymes. The expression and activity of steroidogenic enzymes, such as aromatase and 3β-HSD, are extremely high in the zebra finch brain (Goodson et al. 2005b). In addition, zebra finch song behavior and neuroanatomy are highly sexually dimorphic and sex steroid-sensitive (Nottebohm and Arnold 1976; Gurney 1981). Therefore, this species is excellent for examining (i) rapid regulation and (ii) sex differences in regulation of brain 3β-HSD. Here, we test the hypothesis that E2 rapidly regulates DHEA metabolism by brain 3β-HSD in a sex-specific manner.
Experimental procedures
Materials
Tritiated steroids, [1,2,6,7-3H]AE (specific activity = 91 Ci/mmol) and [1,2,6,7-3H]DHEA (specific activity = 60 or 63 Ci/mmol), were purchased from Perkin-Elmer. Radioinert steroids were purchased from Steraloids (Newport, RI, USA). Ultima-Flo M scintillation cocktail was purchased from Perkin-Elmer (Waltham, MA, USA). RediSafe liquid scintillation cocktail was purchased from BeckmanCoulter (Mississauga, ON, Canada). Bovine serum albumin, nicotinamide adenine dinucleotide (NAD+), and primulin were purchased from Sigma (St Louis, MO, USA). Trilostane (3β-HSD inhibitor) was a gift from Micron Technologies (Kent, UK). Silica gel thin layer chromatography plates were purchased from Analtech (Newark, DE, USA). All other chemicals were of HPLC grade.
Subjects
Experimental procedures were carried out under University of British Columbia Animal Care permit (A04–277), following the guidelines of the Canadian Council on Animal Care. Subjects were adult male and female zebra finches (90 + days old), kept in single-sex group housing, but in visual and auditory contact with each other. Animals were maintained on a 14-h light, 10-h dark cycle, and food and water were available ad libitum.
Tissue collection and preparation
Within 2 min of entering the animal colony, subjects were killed by rapid decapitation. Care was taken to minimize stress, in order to avoid the effects of stress on 3β-HSD activity (Soma et al. 2004a).
The whole brain was immediately removed, placed in ice-cold sucrose-phosphate buffer (3 mL), and homogenized on ice with a glass-teflon homogenizer (15 strokes). The resulting homogenate was immediately centrifuged for 30 min at 1000 g at 4°C (Coirini et al. 2003b; Soma et al. 2005). The supernatant (containing mitochondria, microsomes, synaptosomes, and cytosol; Schlinger and Callard 1989; Rohmann et al. 2007) was frozen on dry ice and then stored at −80°C. The pellet (containing unbroken cells, cell debris, and cell nuclei; Rohmann et al. 2007) was discarded.
Measurement of DHEA metabolism by 3β-HSD
We measured 3β-HSD activity using an in vitro assay that quantifies the conversion of [3H]DHEA to [3H]AE. The assay methods were based on previous studies (Soma et al. 2004a) with slight modifications. In particular, we did not use a ‘cold trap’ of radioinert AE, which was found to inhibit 3β-HSD activity in pilot experiments. Brain homogenate or supernatant (180 μL) was incubated with 200 nmol/L [3H]DHEA (Soma et al. 2004a). [3H]DHEA was repurified by thin layer chromatography (TLC) before use. NAD+ (20 μL; 1 mmol/L final concentration), a cofactor of 3β-HSD and an electron receiver, was added. Exogenous NAD+ facilitates 3β-HSD activity and inhibits aromatase activity, which requires NADPH as an electron donor (K. Soma, unpublished results). Control tubes contained everything but tissue.
Incubations were carried out at 41°C with shaking, and reactions were terminated by snap freezing in methanol/dry ice. To determine procedural losses, tubes containing a known amount of [3H]AE were processed in parallel. Steroids were extracted with diethyl ether (two times), and extracts were dried with nitrogen. Steroids were then separated using either HPLC or TLC. HPLC and TLC provided separate but complementary methods of measuring enzyme activity (e.g., Matsunaga et al. 2001; Mensah-Nyagan et al. 2007). While HPLC has greater ability to separate steroids, TLC provides higher sensitivity.
High performance liquid chromatography
The dried residues obtained after ether extraction were resuspended in 500 μL HPLC-grade methanol. To remove neutral lipids, samples were stored at −20°C overnight, centrifuged at 3000 g for 5 min at 4°C, and the supernatants were decanted into new tubes.
Tritiated steroids were separated using reversed-phase HPLC (Gilson 322) and quantified with a radioflow detector (as in Mensah-Nyagan et al. 2007). A Waters SymmetryShield C18 column (4.6 × 250 mm, 5-μm silica particles) was used for steroid separation. The solvents used were 40% acetonitrile in water (solvent A) and 68% methanol in water (solvent B). Before sample injection, the column was equilibrated with 55% solvent B for 20 min. 10 μL of sample was injected onto the column with an autoinjector (in triplicate). Samples were eluted at a flow rate of 0.5 mL/min over 60 min. The mobile phase was increased to 95% solvent B over 55 min, in three isocratic steps of 75% (20–25 min), 85% (35–40 min), and 95% (45–47 min) of solvent B. The mobile phase was then brought back to 55% solvent B for 3 min. The column was equilibrated for 10 min prior to the next injection.
Eluates were mixed with an equal amount of flow scintillant, and radioactivity was measured with a radioflow detector (Berthold LB 509; Guelph, ON, Canada). We quantified the area of the [3H]AE peak (Unipoint software; Winnipeg, MB, Canada). The [3H]AE peak area was corrected for background values, recovery (average = 94%), and protein content of samples, and the data are presented as area per mg protein. Protein content was measured by the Bradford method (Bradford 1976), using bovine serum albumin as a standard.
Thin layer chromatography
All samples were processed in duplicate for TLC analysis. The dried residues obtained after ether extraction were resuspended in dichloromethane: methanol (1: 1), and radioinert DHEA, AE, 5β-A, and 5α-A were added as markers. Samples were spotted onto silica gel plates, and run in chloroform: ethyl acetate (4: 1) for 18 min (two times) (Soma et al. 2004a). Steroids were visualized under UV light after spraying with primulin. The appropriate bands were scraped from the plates, tritiated steroids were eluted from the silica with 900 μL methanol: water (8: 1), and 200 μL aliquots were counted in a scintillation counter (BeckmanCoulter LS 6500). The dpm were corrected for background values and recovery (average = 90%), and all data are presented as fmole per mg protein.
Initial validations
First, using TLC, timecourse studies with adult male brain tissue determined an appropriate incubation time. One timecourse study compared 3β-HSD activity in brain homogenates and supernatants, using incubation times from 15 to 180 min. A second timecourse study with supernatants only used incubation times from 2.5 to 15 min.
Second, we examined the effects of trilostane, a specific inhibitor of 3β-HSD (Potts et al. 1978). We tested the effect of trilostane (4000 nmol/L, as in Soma et al. 2004a) when HPLC was used to measure 3β-HSD in males. In addition, we tested the effect of trilostane when TLC was used to measure 3β-HSD in males and females.
Third, using TLC, with pooled brain supernatant (n = 4 males), we tested the effects of various E2 doses (2, 4, 20, 40, 200, 1000, and 2000 nmol/L) on 3β-HSD. E2 doses were based on plasma E2 levels in zebra finches (Hutchison et al. 1984) and previous studies examining E2 regulation of aromatase and 3β-HSD in vitro (Freking et al. 1998; Coirini et al. 2003b; Micevych et al. 2007).
Rapid regulation of 3β-HSD activity by E2: sex difference
We then measured the effects of in vitro E2 on brain 3β-HSD activity in males and females. We prepared supernatants from the brains of adult males (n = 6) and females (n = 6). Based on initial validations, we used 0, 4 and 1000 nmol/L E2. The incubation duration was 10 min. Importantly, we used a within-subject design, in which samples from each subject were treated with all three doses of E2. Furthermore, we measured 3β-HSD activity in each subject using both HPLC and TLC. With TLC, we were able to detect the two main metabolites downstream of 3β-HSD, [3H]AE and [3H]5β-A, but note that the amount of [3H]5β-A produced was minor (<8% of total 3β-HSD metabolites). With HPLC, we were able to detect [3H]AE but not [3H]5β-A (i.e., the [3H]5β-A peak was too small).
Statistics
Data are shown as mean ± SEM. If necessary, data were log-transformed prior to analysis. Data were analyzed using SPSS 11 (Chicago, IL, USA). To analyze the effects of E2 in males and females, we used a mixed design ANOVA with E2 dose as a within-subjects factor and sex as a between-subjects factor, and we used Fisher’s protected least significant difference (LSD) tests for post hoc analyses. To further examine the E2 effects, we expressed the data as a percent of control, and compared males and females using Mann–Whitney U-tests. All tests were two-tailed, and α was set at 0.05.
Results
3β-HSD assay validations
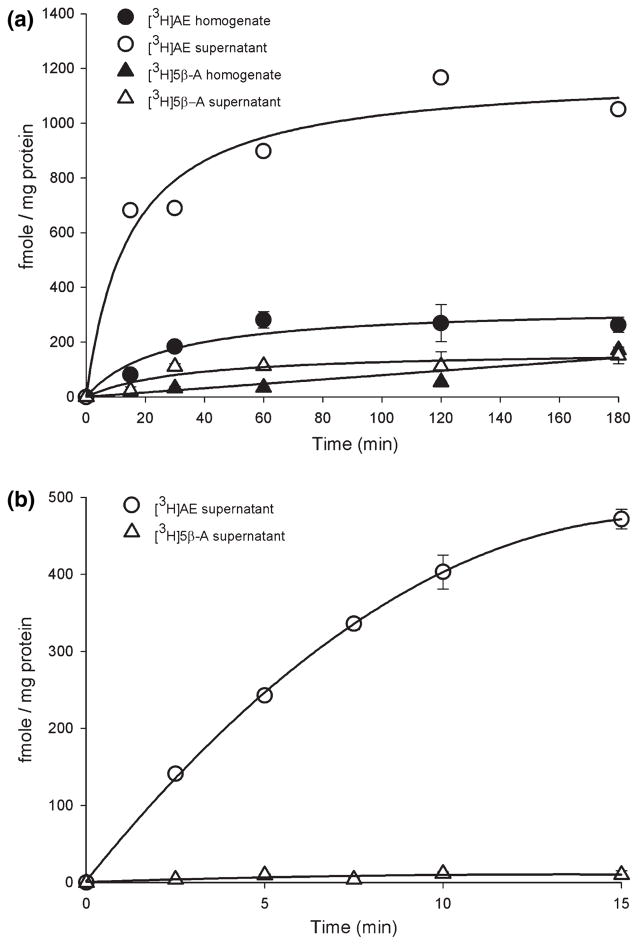
Timecourse studies using TLC determined the appropriate incubation time and compared 3β-HSD activity in brain homogenates and supernatants. In the first timecourse study (Fig. 2a), the amount of formed [3H]AE was much higher in supernatants than homogenates. Therefore, in all subsequent studies, we used supernatants. [3H]AE formation in supernatants was already non-linear by the earliest timepoint (15 min), so we examined earlier timepoints. In the second timecourse study, [3H]AE formation was linear from 2.5 to 10 min (Fig. 2b). Based on these data, we chose a 10 min incubation time. Thus, in vitro E2 treatment lasted 10 min, which matches the 10 min restraint stress used in previous studies examining rapid regulation of 3β-HSD (Soma et al. 2004a). Furthermore, in these timecourse studies using TLC, [3H]AE was the main product formed (>92% of total 3β-HSD metabolites). [3H]5β-A levels were much lower (<8% of total 3β-HSD metabolites). [3H]5α-A was non-detectable and not measured in subsequent experiments. Therefore, for TLC analyses, we used the sum of [3H]AE and [3H]5β-A to represent 3β-HSD activity.
Fig. 2.
Timecourse of DHEA metabolism by 3β-HSD in adult male zebra finch brain. Incubations were carried out at 41°C with 200 nmol/L [3H]DHEA and 1 mmol/L NAD+, and products were separated using TLC. (a) DHEA metabolism in brain homogenate and supernatant from 15 to 180 min. Open symbols represent product formation in supernatants, and closed symbols represent product formation in homogenates. n = 3 replicates per timepoint. (b) DHEA metabolism in brain supernatants from 2.5 to 15 min. n = 2 replicates per timepoint.
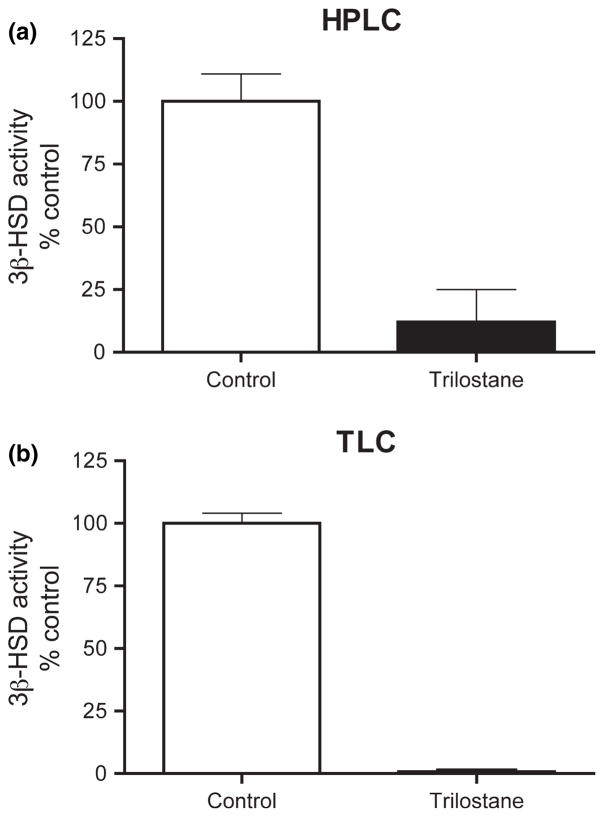
Next, we tested the effects of in vitro trilostane, a specific 3β-HSD inhibitor. The trilostane concentration was 20× substrate concentration (Soma et al. 2004a). As measured by HPLC or TLC, 3β-HSD activity in males was greatly reduced by trilostane (Fig. 3a and b). Using TLC, we obtained similar results with females.
Fig. 3.
Effect of trilostane, a specific 3β-HSD inhibitor, on 3β-HSD activity in adult male zebra finch brain. [3H]DHEA metabolism to [3H]AE was determined by (a) HPLC and (b) TLC analyses. n = 4 replicates per group.
We then tested the effects of various E2 concentrations in vitro. Using pooled brain supernatant (n = 4 males), we examined E2 doses from 2 to 2000 nmol/L. Qualitatively, E2 concentrations above 40 nmol/L appeared to reduce 3β-HSD activity (Fig. 4).
Fig. 4.
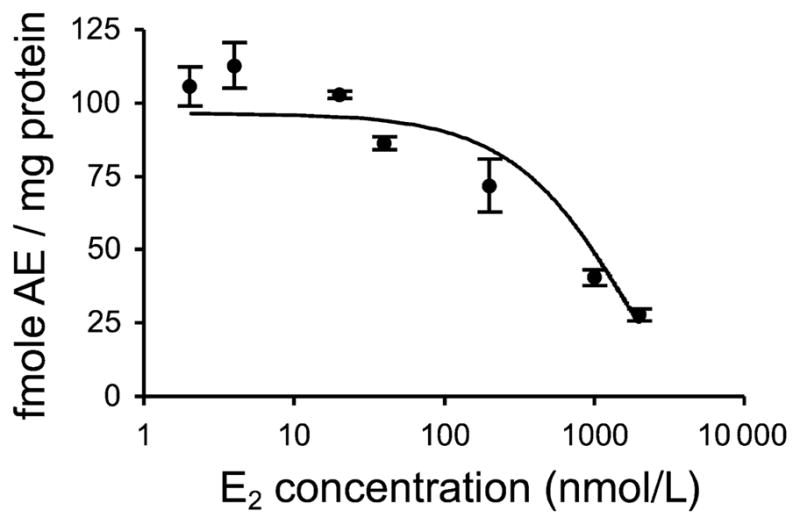
Effect of in vitro E2 on DHEA metabolism by 3β-HSD in adult male zebra finch brain, as measured by TLC. E2 concentrations ranged from 0 to 2000 nmol/L. n = 3 replicates per group.
Using HPLC (Fig. 5), we identified peaks for [3H]AE, [3H]DHEA, and an unknown metabolite ([3H]UNK). Formed [3H]5β-A was too low to detect via HPLC. With regard to the [3H]UNK, trilostane did not affect the peak area (p = 0.81), indicating that [3H]UNK is not a product of 3β-HSD. Therefore, for HPLC analyses, we used [3H]AE peak area to represent 3β-HSD activity.
Fig. 5.
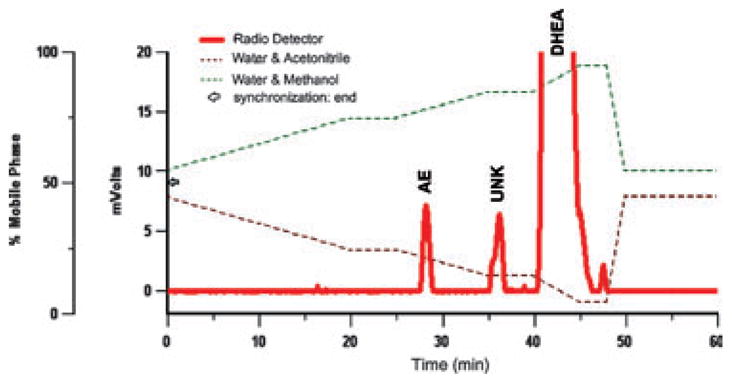
Representative HPLC chromatograph illustrating the peaks and retention times of [3H]AE, [3H]UNK (an unknown steroid), and [3H]DHEA. [3H]UNK peak area was not affected by trilostane or estradiol.
E2 rapidly reduced 3β-HSD activity: sex difference
Based on initial validations, we measured the rapid effects of 0, 4, and 1000 nmol/L E2in vitro on 3β-HSD activity in male (n = 6) and female (n = 6) zebra finch brain. Tissue was incubated with E2 for 10 min. We used a within-subjects design, in which samples from each subject were treated with all three doses of E2. Furthermore, we measured 3β-HSD activity using both HPLC and TLC.
HPLC
Using HPLC, E2 treatment significantly affected 3β-HSD activity within 10 min (Fig. 6; F2, 20 = 10.878, p = 0.008). There was no effect of sex (F1,10 = 1.238, p = 0.292). There was no significant interaction between E2 treatment and sex (F2, 20 = 3.145, p = 0.107). Post hoc Fisher’s LSD tests revealed a significant inhibition of 3β-HSD by 1000 nmol/L E2 (p = 0.010) but not by 4 nmol/L E2 (p = 0.256). There was a trend for a sex difference in baseline activity of 3β-HSD (t = 1.994, p = 0.074).
Fig. 6.
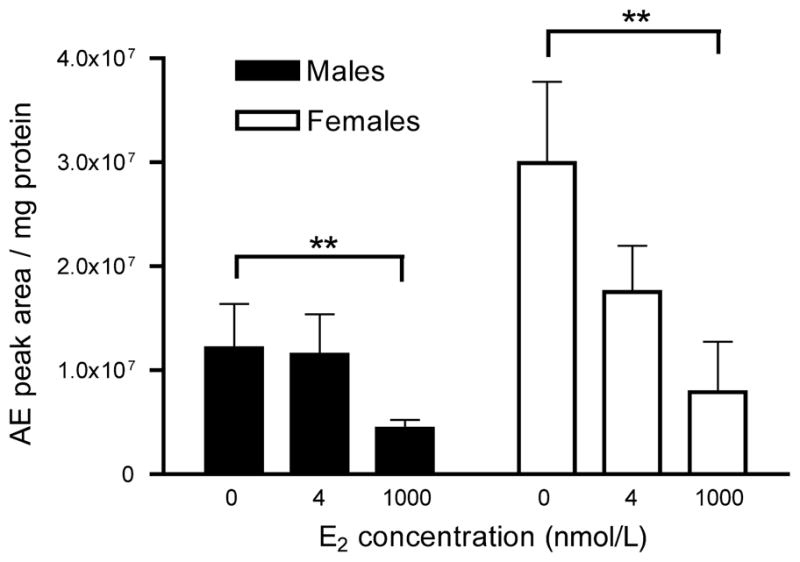
HPLC analysis of the rapid effect of E2 on 3β-HSD activity in adult male and female zebra finch brain. 3β-HSD activity in the presence of 0, 4, or 1000 nmol/L E2 for 10 min was determined using HPLC for steroid separation and product quantification. n = 6 males and 6 females. **p < 0.01.
TLC
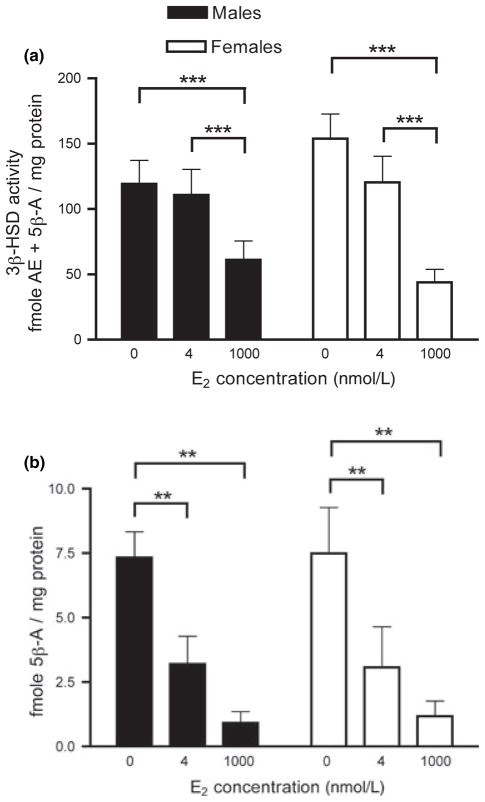
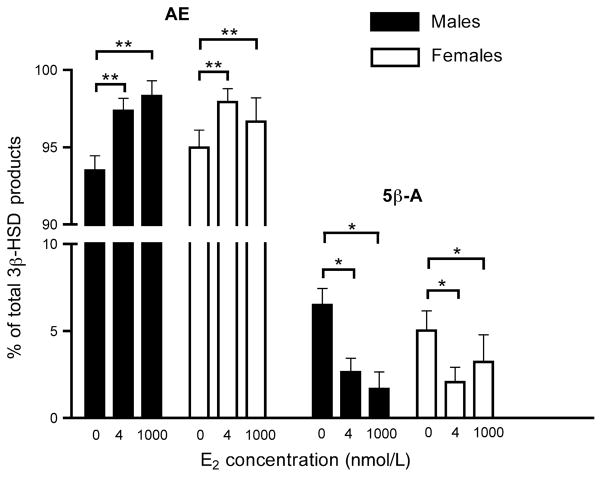
Using TLC, we obtained a similar pattern of results for 3β-HSD activity (Fig. 7a). There was a significant within-subjects effect of E2 treatment (F2, 20 = 36.499, p < 0.0001) and no effect of sex (F2, 10 = 0.001, p = 0.981). There was a trend for an interaction between E2 treatment and sex (F2, 20 = 3.134, p = 0.065). Post hoc Fisher’s LSD tests revealed that 1000 nmol/L E2 significantly decreased 3β-HSD activity (p < 0.0001) but 4 nmol/L E2 did not have a significant effect (p = 0.230). There was also a significant difference between 4 and 1000 nmol/L E2 (p = 0.001).
Fig. 7.
TLC analysis of the rapid effect of E2 on 3β-HSD activity in adult male and female zebra finch brain. 3β-HSD activity in the presence of 0, 4, or 1000 nmol/L E2 for 10 min was determined using TLC for steroid separation. n = 6 males and 6 females. (a) Effect of E2 on total 3β-HSD metabolites ([3H]AE and [3H]5β-A). (b) Effect of E2 on only [3H]5β-A levels. **p < 0.01, ***p < 0.001.
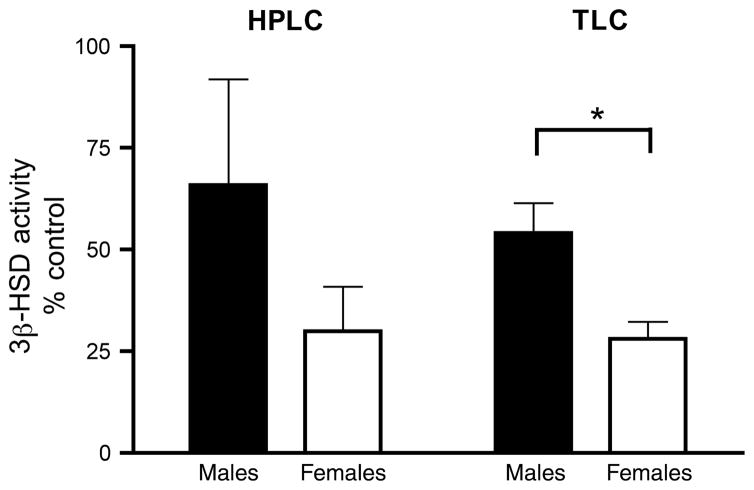
Because there was a trend for an interaction between E2 treatment and sex and because the power for this analysis was relatively low (observed power = 0.684), we examined this potential interaction further. We focused on the 1000 nmol/L E2 dose, which was significantly different from controls. When 3β-HSD activity is expressed as a percentage of control (0 nmol/L E2), there is a greater effect of 1000 nmol/L E2 in females than in males (Fig. 8b; Mann–Whitney U-test, p = 0.025). The HPLC data yielded a similar pattern, but the sex difference was not significant (Fig. 8a; Mann–Whitney U-test, p = 0.132).
Fig. 8.
Effect of 1000 nmol/L E2 on 3β-HSD activity as determined by HPLC and TLC analyses. All data are expressed relative to within-subject baseline (0 nmol/L E2). TLC analysis revealed a significant sex difference in inhibition of 3β-HSD activity by E2. n = 6 males and 6 females. *p < 0.05.
Furthermore, we specifically examined the effects of E2 on [3H]5β-A, an inactive 5β-reduced metabolite of [3H]AE (Fig. 7b). There was a significant effect of E2 treatment on [3H]5β-A levels (F2, 20 = 31.274, p < 0.0001). There was no effect of sex (F2, 10 = 0.005, p = 0.945) and no interaction between E2 treatment and sex (F2, 20 = 0.031, p = 0.970). Post hoc Fisher’s LSD tests revealed that both 4 and 1000 nmol/L E2 significantly decreased [3H]5β-A, relative to controls (p < 0.001 in both cases). There was a trend for a difference between 4 and 1000 nmol/L E2 (p = 0.073). Changes in [3H]5β-A levels could reflect changes in either 5β-reductase and/or 3β-HSD. To assess whether 5β-reductase activity was reduced by E2, we expressed [3H]5β-A as a percentage of total 3β-HSD metabolites ([3H]AE + [3H]5β-A) (as in Soma et al. 2004a). There was a significant effect of E2 on percentage of [3H]5β-A (F2, 20 = 9.055, p = 0.002; Fig. 9). Relative to controls, 4 and 1000 nmol/L E2 significantly decreased the percentage of [3H]5β-A (4 nmol/L E2: p = 0.034; 1000 nmol/L, p = 0.031). These data indicate that E2 can also rapidly inhibit brain 5β-reductase activity.
Fig. 9.
[3H]AE and [3H]5β-A expressed as a percentage of total 3β-HSD metabolites, as determined by TLC. n = 6 males and 6 females. *p < 0.05, **p < 0.01.
Discussion
The main conclusions of this study are (i) E2 decreases brain 3β-HSD and 5β-reductase activities within 10 min; (ii) these E2 effects occur in supernatants that have few, if any, intact cells or cell nuclei; and (iii) the rapid effect of E2 on 3β-HSD activity is greater in females than males. To our knowledge, this is the first demonstration of a rapid effect of E2 on the songbird brain. Given the pronounced effects of physiological doses of DHEA on songbird behavior and adult neuroplasticity (Soma et al. 2002), it is important to understand the neural metabolism of DHEA to active sex steroids, a process in which brain 3β-HSD plays a critical role (Soma et al. 2004a; Demas et al. 2007; Schlinger et al. 2007). The regulation of brain 3β-HSD remains largely unknown and may differ dramatically from the regulation of adrenal and gonadal 3β-HSD (Soma et al. 2005). Also, it is noteworthy that 3β-HSD activity is much higher in the nervous system of zebra finches and song sparrows (Soma et al. 2004a; unpublished results) than that of rats (e.g., Coirini et al. 2003a,b; Soma et al. 2005), reinforcing the idea that songbirds are an excellent model system for studying steroid synthesis in the brain (Goodson et al. 2005b).
Physiological relevance of E2 doses
In several recent experiments, E2 doses used to elicit rapid, non-genomic actions in vitro have been in the high nanomolar to low micromolar range (Freking et al. 1998; Sinchak et al. 2003; Cornil et al. 2006; Micevych et al. 2007; Woolley 2007). In the present study, inhibition of 3β-HSD by E2 occurred with doses greater than 40 nmol/L (Fig. 4), which are higher than plasma levels of E2 in adult zebra finches (Hutchison et al. 1984; Cornil et al. 2006). We chose 4 nmol/L E2 to represent high circulating levels of E2 (Hutchison et al. 1984) and 1000 nmol/L E2 to potentially represent levels of E2 at specific brain regions or specific synapses. Aromatase has been identified in pre-synaptic terminals in the adult zebra finch brain, suggesting that local estrogen levels at the level of the synapse may be high (Peterson et al. 2005). However, currently it is not technically possible to directly measure E2 concentrations at the synapse (see Taziaux et al. 2007). Nonetheless, Palkovits punch or microdialysis can be used to estimate E2 concentrations in small brain regions. Using Palkovits punch on adult male zebra finch brain tissue, we have collected preliminary data suggesting that E2 levels in specific brain regions (nucleus taeniae of the amygdala, caudomedial nidopallium) are up to 30 times higher than E2 levels in plasma (Newman et al. 2007; E. Chin, K. Po, K. Soma, unpublished results). Concentrations of E2 at aromatase-positive synapses might be even higher.
E2 inhibition of brain 3β-HSD and 5β-reductase occurs in supernatants
We found that brain 3β-HSD activity is markedly higher in 1000 g supernatants than homogenates (as in rodents: Coirini et al. 2003a,b; Soma et al. 2005). By using supernatants, we were able to employ an incubation time as short as 10 min. In contrast, in homogenates, little [3H]AE is formed within 10 min (Fig. 2a). Also, previous studies using homogenates of zebra finch brain report lower levels of 3β-HSD activity than the present results with supernatants (Soma et al. 2004a). The supernatants contain mitochondria, synaptosomes, microsomes and cytosol, but are unlikely to contain intact cells and cell nuclei (Schlinger and Callard 1989; Rohmann et al. 2007). 3β-HSD is present in mitochondria and endoplasmic reticulum membranes (Pelletier et al. 2001), and these organelles are enriched in supernatants, which may explain the high 3β-HSD activity seen with supernatants.
E2 inhibited DHEA metabolism in supernatants. In HPLC and TLC experiments, 1000 nmol/L E2 significantly reduced 3β-HSD activity in both males and females. Similarly, in studies of supernatants prepared from male rat sciatic nerve (Coirini et al. 2003a), E2 inhibits 3β-HSD activity (as indicated by the metabolism of pregnenolone to progesterone). In these studies, E2 was the most potent steroidal regulator of 3β-HSD activity, even more potent than progesterone, the direct product of 3β-HSD (Coirini et al. 2003a). Taken together, these results with supernatants suggest a non-genomic effect of E2 on 3β-HSD.
In addition, E2 affected 5β-reductase activity in supernatants. Both 4 and 1000 nmol/L E2 significantly decreased [3H]5β-A levels. To specifically assess 5β-reductase activity, we expressed [3H]5β-A as a percentage of total 3β-HSD metabolites ([3H]AE + [3H]5β-A). The percentage of [3H]5β-A was significantly reduced by E2 treatments (see Fig. 9), suggesting a non-genomic effect of E2 on 5β-reductase also.
E2 inhibition of brain 3β-HSD and 5β-reductase is rapid
Traditionally, the effects of E2 were thought to be the result of binding to intracellular receptors that regulated gene transcription, a process that typically takes hours to days. More recent evidence indicates a variety of rapid, non-genomic mechanisms of E2 action (Cornil et al. 2006; Woolley 2007). E2 can have profound effects on neurophysiology and behavior within a short time span (seconds to minutes). For example, rapid electrophysiological effects of E2 have been documented in the rodent preoptic area (Kelly et al. 1976), and rapid behavioral effects of E2 are seen in Japanese quail (Cornil et al. 2006). In the present experiments, E2 inhibited 3β-HSD and 5β-reductase activities within 10 min, which argues for a non-genomic effect. In previous studies of female rats, EB treatment in vivo increased 3β-HSD mRNA and activity in the hypothalamus, an effect that required more than 12 h to occur (Soma et al. 2005). Thus, estrogens may regulate brain 3β-HSD via multiple pathways involving both non-genomic and genomic mechanisms. Future studies of zebra finches will administer E2in vivo to elucidate rapid and long-term effects of E2 on brain 3β-HSD.
Sex difference in rapid E2 effect
The present data suggest a sex difference in rapid E2 regulation of brain 3β-HSD. When compared to baseline activity, the inhibition by 1000 nmol/L E2 is greater in females than males. However, this sex difference was only significant for TLC data and thus should be interpreted cautiously. In the present studies using whole brain, there is a trend for a sex difference in baseline 3β-HSD activity (F > M), but previous studies using specific telencephalic regions found significant sex differences in baseline activity (F > M) (Soma et al. 2004a). Moreover, the sex difference in 3β-HSD activity was reversed following a 10 min restraint stress: females, but not males, showed a dramatic decline in 3β-HSD activity (Soma et al. 2004a). In rats, acute stress increases plasma E2 in females (Shors et al. 1999), and in quail, acute stress increases brain aromatase activity (Baillien et al. 2004). Taken together, these data raise the hypothesis that acute stress inhibits brain 3β-HSD in female zebra finches by rapidly increasing circulating and/or brain estrogens.
Few studies have documented sex differences in rapid E2 effects. For example, in gonadectomized rats, EB rapidly (within 30 min) affects striatal D2 dopamine receptor binding in females but not males (Bazzett and Becker 1994). More recently, in gonadectomized mice, E2 rapidly (within 60 min) increased pCREB-immunoreactive cell numbers in the medial preoptic area and ventromedial nucleus of females but not males (Abraham and Herbison 2005). In addition, E2 rapidly increased the number of GnRH neurons expressing pCREB in female but not male mice (Abraham and Herbison 2005). Similar to the present results, these studies indicate that rapid E2 effects are more prominent in the female brain. In contrast, in vocalizing fish, rapid E2 effects on neuronal firing are similar in males and females (Remage-Healey and Bass 2007).
Possible mechanisms of E2 action
There are several possible mechanisms by which E2 might rapidly decrease brain 3β-HSD activity in supernatants. One hypothesis is that E2 directly binds to 3β-HSD and acts as a competitive inhibitor. However, this possibility appears unlikely because there is a sex difference in the E2 effect. Currently, there is no evidence that the structure of 3β-HSD differs between the sexes.
Second, E2 may act via estrogen receptors (ER)α or ERβ, which are located at nuclear and extranuclear sites in the brain, including mitochondria, endoplasmic reticulum, and synapses (e.g., Blaustein et al. 1992; Milner et al. 2001, 2005; Romeo et al. 2005; Hart et al. 2007). In rodents, brain estrogen receptors have been reported in mitochondria (Yang et al. 2004; Milner et al. 2005) and endoplasmic reticulum (Blaustein et al. 1992), both organelles that contain membrane-bound 3β-HSD in peripheral organs (Pelletier et al. 2001). Estrogen receptors have also been detected at pre-synaptic terminals (e.g., Horvat et al. 2001; Milner et al. 2001; Hart et al. 2007). In zebra finch brain, pre-synaptic terminals contain high levels of aromatase (Peterson et al. 2005; Rohmann et al. 2007), suggesting that local E2 concentrations are high at specific synaptic boutons, and we are currently examining 3β-HSD in synaptosomes.
Third, E2 may act via other estrogen receptors. E2 can act via G protein coupled receptors (Kelly et al. 2003), such as GPR30 (Thomas et al. 2005). GPR30 has been reported in endoplasmic reticulum (Revankar et al. 2005). In addition, a membrane-associated ER, ER-X, might mediate some effects of E2 (Toran-Allerand et al. 2002).
Regardless of which estrogen receptor may be involved, E2 can induce rapid changes in calcium concentrations (Lobaton et al. 2005; Micevych et al. 2007), leading to changes in protein kinases (Driggers and Segars 2002). These mechanisms are important in the rapid modulation of brain aromatase activity (Cornil et al. 2006). Studies in quail indicate that brain aromatase is modulated, in part, by phosphorylation of the aromatase protein by protein kinases (Balthazart et al. 2006). The songbird 3β-HSD sequence also contains potential phosphorylation sites (T. Charlier and K. Soma, unpublished results), suggesting that similar mechanisms might rapidly modulate the activity of brain 3β-HSD.
Conclusions
These results show that in vitro E2 rapidly inhibits the metabolism of DHEA by 3β-HSD in the brain. The rapid effect of E2 is greater in females than males. Zebra finches have high levels of brain aromatase (Saldanha et al. 2000), including aromatase in pre-synaptic boutons (Peterson et al. 2005). Taken together, these data raise the hypothesis that brain 3β-HSD is rapidly regulated by neurally-synthesized E2. Because E2 is a downstream product of DHEA, E2 could thus limit its own production via a local negative feedback loop (Gower and Cooke 1983). A local negative feedback loop might be important for creating short pulses of E2 signals at the synapse, if one considers brain E2 as a neurotransmitter (Balthazart and Ball 2006).
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Michael Smith Foundation for Health Research, and the Canada Foundation for Innovation to KKS. We thank Loretta Lau for help with assays and Drs. Jeff Richards and Thierry Charlier for comments on the manuscript.
Abbreviations used
- 3β-HSD
3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase
- 5α-A
5α-androstanedione
- 5β-A
5β-androstanedione
- AE
androstenedione
- DHEA
dehydroepiandrosterone
- E2
17β-estradiol
- EB
17β-estradiol benzoate
- NAD
nicotinamide adenine dinucleotide
- T
testosterone
- E1
estrone
References
- Abraham IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. J Neurosci. 2005;131:945–951. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Baillien M, Cornil CA, Ball GF, Balthazart J. Soc Neurosci Abstr. San Diego: 2004. Sexual performance and stress rapidly alter the preoptic aromatase activity in quail. Program No. 88.4. [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? TINS. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziauz M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–791. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinol. 1998;23:8–963. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coirini H, Gouézou M, Delespierre B, Liere P, Pianos A, Eychenne B, Schumacher M, Guennoun R. Characterization and regulation of the 3β-HSD enzyme in the rat sciatic nerve. J Neurochem. 2003a;84:119–126. doi: 10.1046/j.1471-4159.2003.01512.x. [DOI] [PubMed] [Google Scholar]
- Coirini H, Gouézou M, Delespierre B, Schumacher M, Guennoun R. 3β-hydroxysteroid dehydrogenase isomerase (3β-HSD) activity in the rat sciatic nerve: kinetic analysis and regulation by steroids. J Steroid Biochem Mol Biol. 2003b;85:89–94. doi: 10.1016/s0960-0760(03)00133-x. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Cooper MA, Albers HE, Soma KK. Novel mechanisms underlying neuroendocrine regulation of aggression: a synthesis of rodent, avian and primate studies. In: Blaustein JD, Lajtha A, editors. Behavioral Neurochemistry and Neuroendocrinology. Handbook of Neurochemistry and Molecular Neurobiology. Vol. 21. Springer; Berlin, Germany: 2007. pp. 337–372. [Google Scholar]
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freking F, Ramachandran B, Schlinger BA. Regulation of aromatase, 5α- and 5β-reductase in primary cell cultures of developing zebra finch telencephalon. J Neurobiol. 1998;36:30–40. doi: 10.1002/(sici)1097-4695(199807)36:1<30::aid-neu3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. NeuroReport. 2005a;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Saldanha CJ, Hahn TP, Soma KK. Recent advances in behavioral neuroendocrinology: insights from studies on birds. Horm Behav. 2005b;48:461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower DB, Cooke GM. Regulation of steroid-transforming enzymes by endogenous steroids. J Steroid Biochem. 1983;19:1527–1556. doi: 10.1016/0022-4731(83)91130-5. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouézou M, Lombés M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD), is expressed in rat brain. Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Gurney ME. Hormonal control of cell form and number in the zebra finch song system. J Neurosci. 1981;1:658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-α-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territoral aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Horvat A, Nikezic G, Petrovic S, Kanazir DT. Binding of estradiol to synaptosomal mitochondria: physiological significance. Cell Mol Life Sci. 2001;58:636–644. doi: 10.1007/PL00000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103:363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qui J, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Lobaton CD, Vay L, Hernandez-SanMiguel E, SantoDomingo J, Moreno A, Montero M, Alvarez J. Modulation of mitochondrial Ca2+ uptake by estrogen receptor agonists and antagonists. Br J Pharmacol. 2005;145:862–871. doi: 10.1038/sj.bjp.0706265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JI. The 3β-hydroxysteroid dehygdrogenase gene family of enzymes. Trends Endocrinol Metab. 1993;4:199–203. doi: 10.1016/1043-2760(93)90117-w. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Ukena K, Tsutsui K. Expression and localization of cytochrome P450 17α-hydroxylase/c17,20-lyase in the avian brain. Brain Res. 2001;899:112–122. doi: 10.1016/s0006-8993(01)02217-x. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Feuilloley M, Dupont E, Do-Rego JC, Leboulenger F, Pelletier G, Vaudry H. Immunocytochemical localization and biological activity of 3beta-hydroxys-teroid dehydrogenase in the central nervous system of the frog. J Neurosci. 1994;14:7306–7318. doi: 10.1523/JNEUROSCI.14-12-07306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Saredi S, Schaeffer V, Kibaly C, Meyer L, Melcangi RC, Patte-Mensah C. Assessment of neuroactive steroid formation in diabetic rat spinal cord using high-performance liquid chromatography and continuous flow scintillation detection. Neurochem Int. 2007 doi: 10.1016/j.neuint.2007.06.010. in press. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Dewing P, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Newman AEM, Chin EH, Schmidt KL, Bond L, Whynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2007 doi: 10.1016/j.ygcen.2007.08.007. in press. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Tremblay Y, Belanger A, Labrie F. Immunoelectron microscopic localization of three key steroidogenic enzymes (cytochrome P450(scc), 3beta-hydroxysteroid dehydrogenase and cytochrome P450(c17)) in rat adrenal cortex and gonads. J Endocrinol. 2001;171:373–383. doi: 10.1677/joe.0.1710373. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GO, Creange JE, Harding HR, Schane HP. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids. 1978;32:257–267. doi: 10.1016/0039-128x(78)90010-7. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce L, et al. Songbirds and the revised avian brain nomenclature. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-M-D-Aspartic acid binding and ultrastructural localization of estrogen receptors. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telen-cephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix co-turnix japonica) brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Pradhan DS, Soma KK. 3β-HSD activates DHEA in the songbird brain. Neurochem Int. 2007 doi: 10.1016/j.neuint.2007.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Pickett J, Wood GE, Paczynski M. Acute stress enhances estrogen levels in the female rat. Stress. 1999;3:163–171. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone (DHEA) in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001;123:144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc R Soc Lond B. 2000;267:1089–1096. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. doi: 10.1006/hbeh.2001.1750. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydroxysteroid dehydroge-nase/Δ5-Δ4 isomerase in adult zebra finch brain: sex difference and rapid effect of stress. Endocrinology. 2004a;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004b;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam H, Schlinger BA. Activities of 3β-HSD and aromatase in slices of developing and adult zebra finch brain. Gen Comp Endocrinol. 2007;150:26–33. doi: 10.1016/j.ygcen.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan C, MacLusky NJ, et al. ER-X: a novel, plasma-membrane associated, putative estrogen receptor that is regulated during development and following ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanson A, Arnold AP, Schlinger BA. 3β-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yang S-H, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]