Abstract
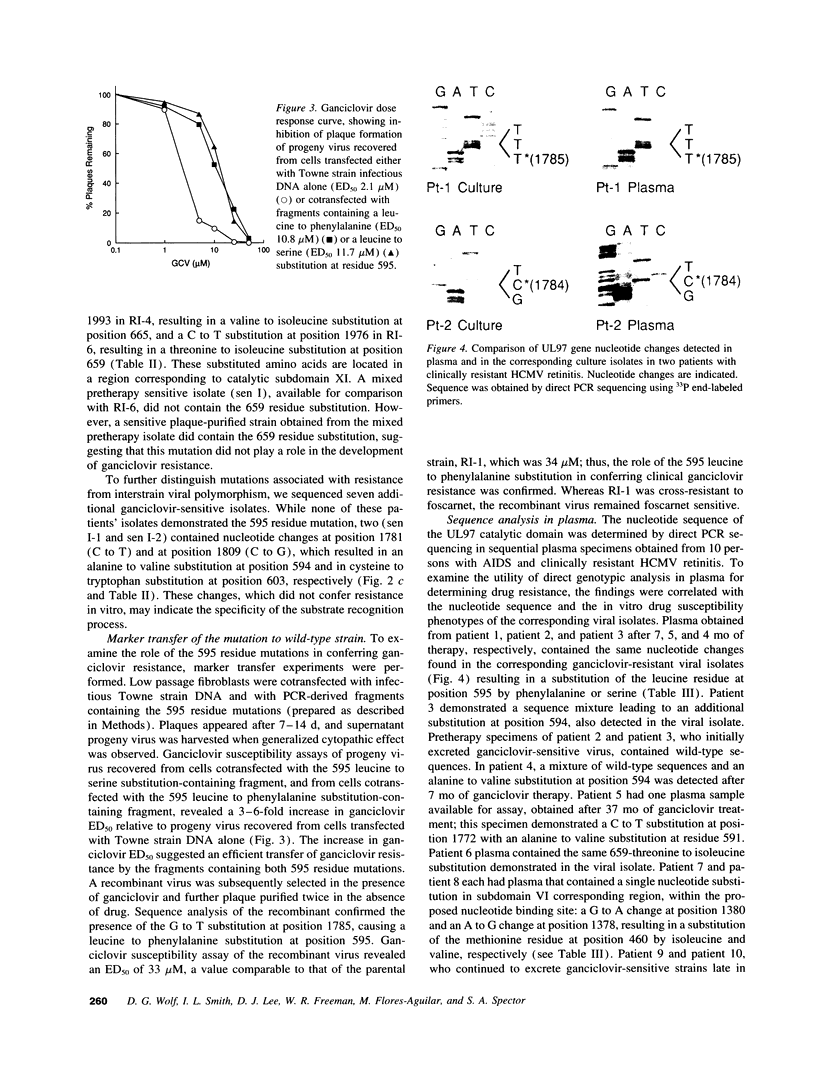
Specific mutations in the UL97 region of human cytomegalovirus (HCMV) have been found to confer resistance to laboratory-adapted strains subjected to ganciclovir selection. In this study, mutations in the UL97 region of HCMV isolates obtained from patients receiving ganciclovir therapy were examined to determine whether they would confer ganciclovir resistance, and if these mutations could be detected directly in the plasma of AIDS patients with progressive HCMV disease despite ganciclovir treatment. A single nucleotide change within a conserved region of UL97 was found in five resistant isolates, resulting in an amino acid substitution in residue 595: from leucine to phenylalanine in one, and from leucine to serine in four resistant isolates. A sixth resistant isolate demonstrated a single nucleotide change, leading to a threonine to isoleucine substitution in residue 659. The role of the 595 amino acid substitution in conferring ganciclovir resistance was confirmed by marker transfer experiments. In further studies, direct sequencing of HCMV DNA present in plasma obtained from persons with resistant viruses revealed the identical amino acid substitutions in plasma as those present in the cultured viruses. These findings indicate that clinical resistance to ganciclovir can result from specific point mutations in the UL97 gene, and that the emergence of the resistant genotype can be detected directly in patient plasma.
Full text
PDF


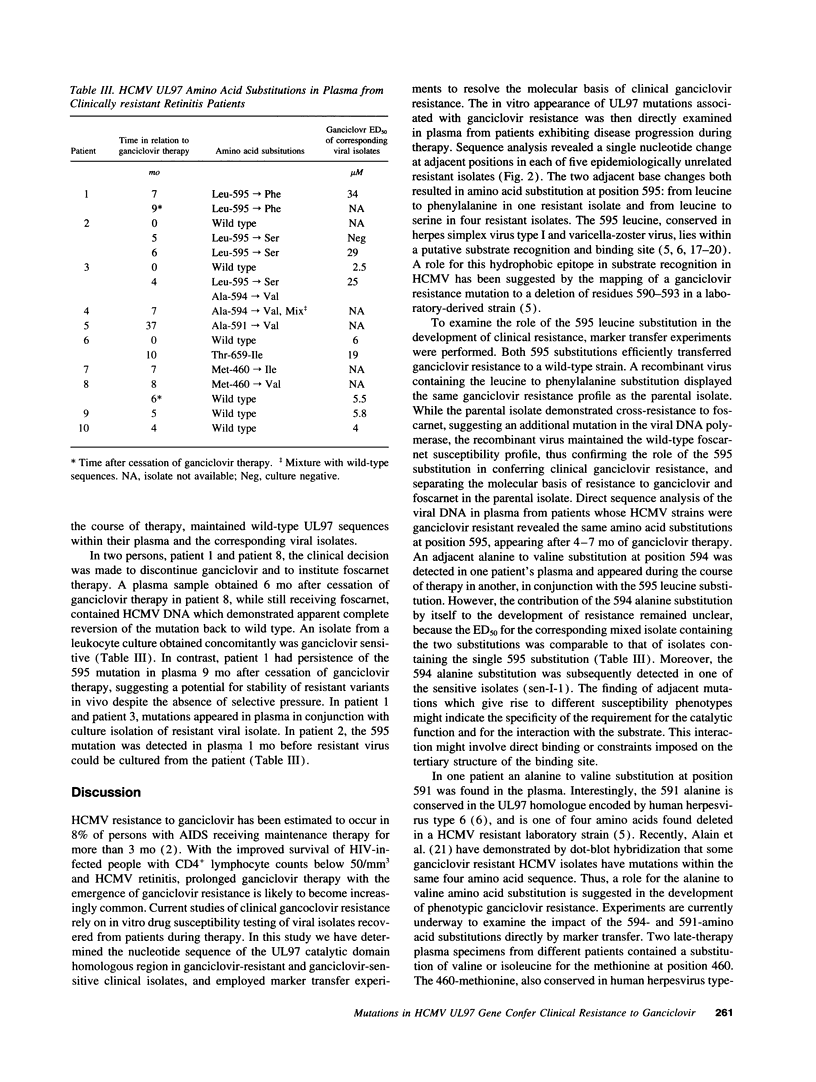


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alain S., Mazeron M. C., Pépin J. M., Morinet F., Raskine L., Sanson-Le Pors M. J. Rapid detection of cytomegalovirus strains resistant to ganciclovir through mutations within the gene UL97. Mol Cell Probes. 1993 Dec;7(6):487–495. doi: 10.1006/mcpr.1993.1072. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Lawrence G. L., Barrell B. G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989 May;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- Chou S. W., Dennison K. M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991 Jun;163(6):1229–1234. doi: 10.1093/infdis/163.6.1229. [DOI] [PubMed] [Google Scholar]
- Chou S. Effect of interstrain variation on diagnostic DNA amplification of the cytomegalovirus major immediate-early gene region. J Clin Microbiol. 1992 Sep;30(9):2307–2310. doi: 10.1128/jcm.30.9.2307-2310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner W. M., Scholl D., Stanat S. C., Martin M., Sonke R. L., Spector S. A. Rapid antiviral DNA-DNA hybridization assay for human cytomegalovirus. J Virol Methods. 1990 Jun;28(3):293–298. doi: 10.1016/0166-0934(90)90122-v. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Miner R. C., Busch D. F., Follansbee S. E., Gullett J., Mehalko S. G., Gordon S. M., Owen W. F., Jr, Matthews T. R., Buhles W. C. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991 Apr;163(4):716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K. K., Stanat S. C., Balfour H. H., Jr, Jordan M. C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989 Feb 2;320(5):289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Flores-Aguilar M., Kuppermann B. D., Quiceno J. I., Dankner W. M., Wolf D. G., Capparelli E. V., Connor J. D., Sherwood C. H., Fullerton S., Gambertoglio J. G. Pathophysiology and treatment of clinically resistant cytomegalovirus retinitis. Ophthalmology. 1993 Jul;100(7):1022–1031. doi: 10.1016/s0161-6420(93)31523-x. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Littler E., Stuart A. D., Chee M. S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992 Jul 9;358(6382):160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Lurain N. S., Thompson K. D., Holmes E. W., Read G. S. Point mutations in the DNA polymerase gene of human cytomegalovirus that result in resistance to antiviral agents. J Virol. 1992 Dec;66(12):7146–7152. doi: 10.1128/jvi.66.12.7146-7152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Ng T. I., Grose C. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology. 1992 Nov;191(1):9–18. doi: 10.1016/0042-6822(92)90161-h. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989 Jan;63(1):450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A. Diagnosis of cytomegalovirus infection. Semin Hematol. 1990 Apr;27(2 Suppl 1):11–29. [PubMed] [Google Scholar]
- Spector S. A., Merrill R., Wolf D., Dankner W. M. Detection of human cytomegalovirus in plasma of AIDS patients during acute visceral disease by DNA amplification. J Clin Microbiol. 1992 Sep;30(9):2359–2365. doi: 10.1128/jcm.30.9.2359-2365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Schmidt K., Ticknor W., Grossman M. Cytomegaloviruria in older infants in intensive care nurseries. J Pediatr. 1979 Sep;95(3):444–446. doi: 10.1016/s0022-3476(79)80532-6. [DOI] [PubMed] [Google Scholar]
- Stanat S. C., Reardon J. E., Erice A., Jordan M. C., Drew W. L., Biron K. K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991 Nov;35(11):2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Biron K. K., Talarico C., Stanat S. C., Davis M., Pozzi L. M., Coen D. M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother. 1993 Jan;37(1):19–25. doi: 10.1128/aac.37.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Talarico C. L., Stanat S. C., Davis M., Coen D. M., Biron K. K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992 Jul 9;358(6382):162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Wolf D. G., Spector S. A. Early diagnosis of human cytomegalovirus disease in transplant recipients by DNA amplification in plasma. Transplantation. 1993 Aug;56(2):330–334. doi: 10.1097/00007890-199308000-00014. [DOI] [PubMed] [Google Scholar]