SUMMARY
Objectives
The objective of this study was to compare the antimicrobial activity of commercially available antiseptic mouthrinses against saliva-derived plaque biofilms in static and flow-through biofilm systems in vitro.
Methods
Nine mouthrinses were tested in a recirculating flow-through biofilm model (RFTB) with viability assessment by ATP bioluminescence. In addition, five mouthrinses were evaluated in a batch chamber slide biofilm (BCSB) model, using live- dead staining and confocal laser scanning microscopy.
Results
In the RFTB model, essential oil (EO) and chlorhexidine (CHX)-containing rinses showed equivalent antimicrobial activity and were more effective than a range of cetyl pyridinium chloride (CPC1) formulations. In the BCSB model, twice-daily mouthrinse exposure demonstrated that the EO rinse was significantly more effective than rinses containing amine and stannous fluorides, a combination of CPC/CHX and CPC2. EO showed biofilm kill comparable to the CHX rinse.
Conclusions
The present studies have shown that mouthrinses vary significantly in their capability to kill plaque biofilm bacteria in BCSB and RFTB models. The EO mouthrinse demonstrated superior antiplaque biofilm activity to AFSF, CPC/CHX, and CPC rinses and comparable activity to CHX. The methods tested may be of value for the in-vitro screening of antiseptic rinses with different modes of antimicrobial action.
Keywords: biofilm, antiplaque, mouthrinse, antimicrobial, essential oils, chlorhexidine, cetylpyridinium chloride, amine fluoride, antiseptic, biocidal
1. Introduction
Mouthrinses are used as adjuncts to mechanical oral hygiene. Mechanical control alone for reducing recalcitrant biofilms in the oral cavity has been challenged because it is considered to be rather time-consuming and most importantly insufficient for effective oral hygiene1,2. An increasing number of innovative formulation technologies have led to a need for more predictive laboratory models to assess preclinical biocidal efficacy of mouthrinses. Recognizing that biofilm bacteria may be 10–1000 times more resistant to antimicrobial agents than planktonic cells3,4, a more predictive assessment of mouthrinse efficacy may be better achieved with biofilm tests. In the development of laboratory biofilm models5–7 several criteria must be considered: salivary pellicle; “wild type” microbiota inoculum; species specific nutrients; pH, temperature, redox potential; substantivity property of active agents; hydrodynamic shear simulation and end-point efficacy measures for visual and quantitative efficacy discrimination. Based on critical factors described herein, two biofilm models have been developed and validated8–11.
The objective of this study was to evaluate the efficacy of mouthrinses containing essential oils, cetylpyridinium chloride, chlorhexidine and amine fluoride/stannous fluoride by measuring ATP cell metabolism and showing confocal laser scanning microscopy images of vital stained biofilms in recirculating flow-through biofilm (RFTB) and batch chamber slide biofilm (BCSB) models.
2. Materials and Methods
2.1. Test mouthrinses
The test treatments were the following commercially available mouthrinses: an essential oil (0.064% thymol, 0.092% eucalyptol, 0.060% methyl salicylate and 0.042% menthol) rinse (EO, CoolMint Listerine, Johnson and Johnson); a 0.12% chlorhexidine rinse (CHX, Peridex, 3M Pharmaceuticals), seven 0.05% cetylpyridinium chloride rinses (CPC1, PLAX global mouthrinses, countries of origin shown in Figure 1, Colgate Palmolive); a 0.05% cetylpyridinium chloride/0.05% chlorhexidine rinse (CPC/CHX, PerioAid, Dentaid); an amine fluoride/stannous fluoride rinse (AFSF, Meridol, GABA); a 0.07% cetylpyridinium chloride rinse (CPC2, Crest Pro Health, Clean Mint, Proctor & Gamble); Sterile water or phosphate buffered saline (PBS) and 70% ethanol (Etoh) served as the negative control and positive controls respectively.
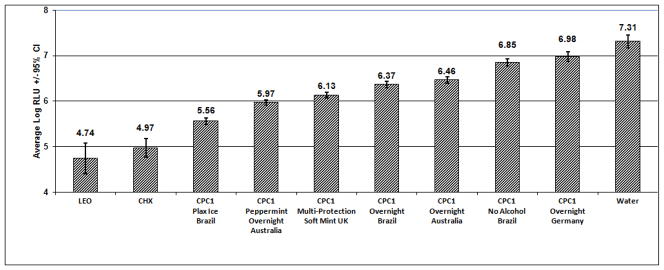
Figure 1.
Effect of EO, CHX and CPC1 Mouthrinses on saliva-derived biofilm (ATP determination, flow through biofilm model)
2.2 Recirculating flow-through biofilm model (RFTB)
Biofilms were cultivated on polystyrene pegs molded into a 96-place lid in a microtiter plate format (Nunc-TSP® Transferable Solid Phase Screening System), which was placed on a custom-made base containing 12 independent recessed channels with stainless steel inlet and outlet ports. Parafilm®-stimulated saliva was collected from 10–15 healthy adult volunteers, who had refrained from oral hygiene for 9–12 hours. The pegs were pre-incubated in pooled saliva at 30.5° C for 30 minutes in ambient atmosphere, then placed on the channel base, and BM/PBS/saliva medium was recirculated at a flow rate of 0.8mL/min for 24 hours. Five treatments were applied to the biofilms over a course of 60 hours, with medium changes after each treatment. After the last treatment, biofilms were dispersed by sonication for 30 seconds (Sonicator Ultrasonic Processor XL, MISONIX Inc.). Aliquots of biofilm were analyzed for ATP (Tropix TR717 microplate luminometer and Celsis RapidScreen ATP bioluminescence reagents), and reported as log values of relative light units (RLU) per well.
2.3 Batch chamber slide biofilm with confocal visualization model (BCSB)
Biofilms were grown in 8-well chambered coverglasses (Nunc) at 37 °C as described by Gu et al12. The growth medium was exchanged after 16–18 hours for biofilm growth. For the 30 or 60 second exposures, 16–18 hour biofilms were used. For twice daily 30 second treatments, biofilms were grown for 65 hours, medium was exchanged every 12 hours and treatments were applied 6 hours apart. Biofilms were stained with LIVE/DEAD Bacterial Viability staining kit (Invitrogen) and monitored through a 40x oil-immersion lens with a PASCAL LSM5 confocal laser scanning microscope (Zeiss). Image analysis software13,14 was used to calculate the proportion of damaged/dead cells within different biofilms. Statistical significance of results was performed using t-test and two-tailed determination of p-values.
3. Results
3.1 Flow-through biofilm model (RFTB)
EO and CHX rinses demonstrated equivalent antimicrobial activity in the assay (RLU 4.74 and 4.97 respectively, Figure 1). In contrast, all CPC1 rinses demonstrated significantly less activity compared to the EO and CHX rinses.
3.2 Batch chamber slide biofilm model (BCSB)
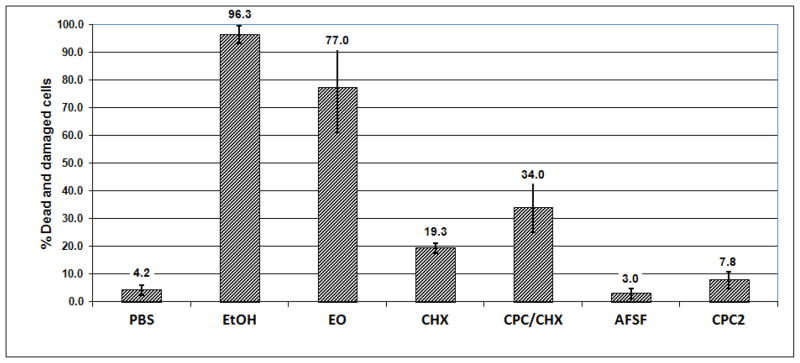
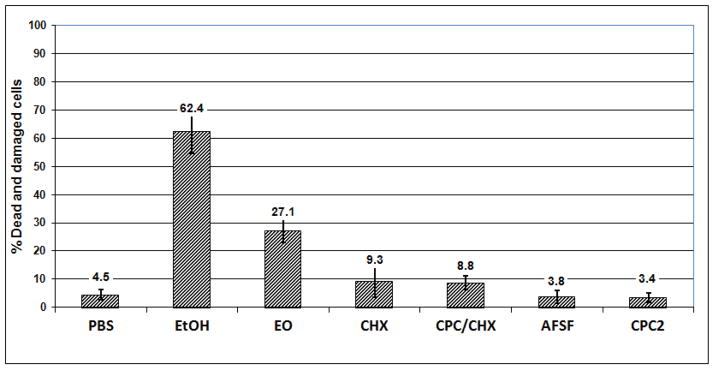
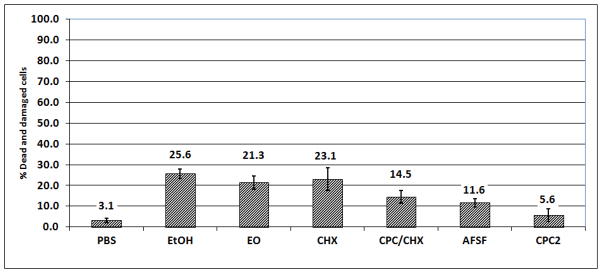
After a single 30-second treatment, EO was the only mouthrinse which caused significant kill or injury of biofilm cells 27.1 ± 4.1 % (p<0.001). CHX or CPC/CHX each elicited only a small extent of damage (9.3 ± 5.7 % and 8.8 ± 2.5%, p<0.05). The 30 second treatment with AFSF or CPC2 did not result in any significant killing of 16–18 hour - old biofilm cells (3.8 ± 2.3 % and 3.4 ± 1.8 % respectively). After a single 60-second treatment, EO mouthrinse showed greatest activity compared to the other mouth rinses tested, killing or severely damaging 77.0 ± 15.8 % of the total biofilm cells (p < 0.001) (Figure 3). CHX and CPC/CHX both caused considerable damage of 19.4 ± 1.8 % and 34.0 ± 9.0 % dead cells, respectively. In contrast, CPC2 treatment showed limited (7.8 ± 3.0 %) but still significant killing and cell damaging effects. AFSF rinse did not significantly kill the biofilm cells even after 60 seconds of treatment (3.0 ± 1.8 %). Following two 30 second treatments, EO and CHX showed antibacterial effects of 21.3 ± 3.1 % (p<0.001) and 23.1 ± 5.5%, (p<0.001). CPC/CHX exhibited about half of the biofilm cell damaging effect (14.5 ± 3.0 %) compared to EO or CHX, whereas AFSF rinse performed similarly to CPC/CHX by causing 14.5 ± 3.0 % cell death in 65 hour biofilms. Treatment with CPC2 showed little cidal effect even on 65 hour biofilms (5.6 ± 2.0 %).
Figure 3.
Effect of EO, CHX, CPC/CHX, AFSF and CPC2 mouthrinses on saliva-derived biofilms (single 60 second treatment, vital staining, batch biofilm model)
4. Discussion
The results of this study showed that the RFTB model is capable of discriminating differences among treatments with moderate-to-high antiseptic activity, with respect to ability to inhibit biofilm growth. The advantages of the present flow-through model are several relative to other models, including use of salivary microbiota as inoculum, multi-exposures to mouthrinse 6,15,16 and greater testing capacity.
The results obtained using the BCSB model showed that EO was consistently the most effective formulation against mixed species saliva-derived biofilms, independent of type of treatment or age of biofilm. In contrast, CPC/CHX exhibited less antimicrobial potency, showing biocidal activity in the intermediate range, whereas CPC2 did not cause significant killing under any of the conditions tested. Interestingly, both CHX and AFSF rinses were more effective when the 65 hour biofilm was exposed twice for 30 seconds and allowed to re-grow for 6 hours. These results may reflect the activity of mouthrinses which contain actives with substantive properties. Results obtained in the saliva-inoculated BCSB model demonstrated that exposing a relatively mature biofilm to two 30-second treatments with test mouthrinses over a 6 hour interval can provide biocidal data consistent with published long term clinical antiplaque studies17–20.
5. Conclusions
The models used in this study have provided data consistent with previous reports demonstrating EO and CHX mouthrinses to have greater anti-plaque activity than CPC1, AFSF, CPC2 or CPC/CHX. These results suggest that biofilm models may be useful additions to the screening pipeline for novel agents and formulations.
Figure 2.
Effect of EO, CHX, CPC/CHX, AFSF and CPC2 mouthrinses on saliva-derived biofilm - (single 30 second treatment, vital staining, batch biofilm model)
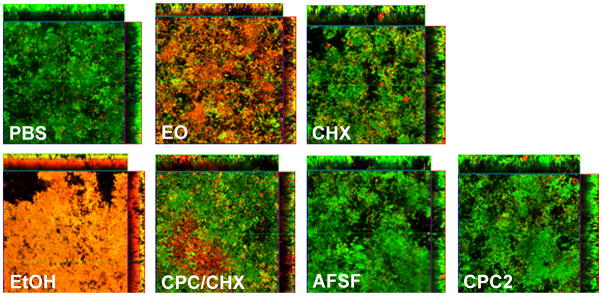
Figure 4.
Vital-stained salivary-derived biofilms following single 60 second exposure with different mouthrinses
Figure 5.
Effect of EO, CHX, CPC/CHX, AFSF and CPC2 mouthrinses on saliva-derived biofilms (two 30 second treatments, vital staining, batch biofilm model)
Footnotes
Conflict of interest statement
This study was funded by Johnson and Johnson Consumer Healthcare. Danette Ricci-Nittel and Pauline Pan are employees of Johnson and Johnson, and Scott Harper is a former employee of Johnson and Johnson. Wenyuan Shi has acted as a consultant for other oral healthcare companies.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infection. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Barnett M. Role of therapeutic antimicrobial mouthrinses in clinical practice. Journal of American Dental Research. 2003;134:699–702. doi: 10.14219/jada.archive.2003.0255. [DOI] [PubMed] [Google Scholar]
- 3.Fine DH, Furgang D, Barnett M. Comparative antimicrobial activities of antiseptic mouthrinses against isogenic planktonic and biofilm forms of Actinobacillus actinomycetemcomitans. Journal of Clinical Periodontology. 2001;28:697–700. doi: 10.1034/j.1600-051x.2001.028007697.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies D. Understanding biofilm resistance to antibacterial agents. Nature Reviews Drug Discovery. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 5.Foster JS, Pan PC, Kolenbrander PE. Effects of antimicrobial agents on oral biofilms in a saliva-conditioned flowcell. Biofilms. 2004;1:3–10. [Google Scholar]
- 6.Guggenheim BB, Giertsen W, Schupbach P, Shapiro S. Validation of an in-vitro biofilm model of supragingival plaque. Journal of Dental Research. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- 7.Filoche S, Wong S, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. Journal of Dental Research. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 8.Goodson JM, Yaskell T, Brogdon C, Harper DS, Pan PC. Characterizing a saliva-derived biofilm model by DNA checkerboard analysis. Journal of Dental Research. 2003;82(Supplement A) abstract #707. [Google Scholar]
- 9.Harper DS, Brogdon CL. Activity of chlorhexidine mouthrinses in a dynamic microplate-based biofilm model. Journal of Dental Research. 2005;82(Supplement A) abstract # 3470. [Google Scholar]
- 10.Ricci-Nittel D, Fourre T. Evaluation of antimicrobial mouthrinses using a microplate-based flow-through biofilm model. Journal of Dental Research. 2008;87(Supplement B) abstract #0287. [Google Scholar]
- 11.Lux R, Pan P, Casteneda SD, Sim JH, Harper DS, Shi W. Biocidal activity of antiseptic mouthrinses in saliva-derived biofilm model. Journal of Dental Research. 2008;87(Supplement B) abstract #1339. [Google Scholar]
- 12.Gu F, Lux R, Du-Thumm L, Stokes I, Kreth J, Anderson MH, et al. In-situ and non-invasive detection of specific bacterial species in oral biofilms using fluorescently labeled monoclonal antibodies. Journal of Microbiological Methods. 2005;62:145–60. doi: 10.1016/j.mimet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;46:2395–407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 14.Kreth J, Hagerman E, Tam K, Merritt J, Wu BM, Myung NB, et al. Quantitative analyses of Streptococcus mutans biofilms with quartz crystal microbalance, microjet impingement, and confocal microscopy. Biofilms. 2004;1:277–284. doi: 10.1017/S1479050504001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MI. Preclinical evaluation of plaque control agents: value of in-vitro models. In: Busscher HJ, Evans LV, editors. Oral Biofilms and Plaque Control. 1. Harwood Academic Publishers; Amsterdam: 1999. pp. 261–276. [Google Scholar]
- 16.Roberts SK, Wei GX, Wu CD. Evaluating biofilm growth of two oral pathogens. Letters in Applied Microbiology. 2002;35:552–556. doi: 10.1046/j.1472-765x.2002.01228.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma NC, Araujo MWB, Wu MM, Qaqish J, Charles C. Superiority of an essential oil mouthrinse when compared with a 0.05% cetylpyridinium chloride containing mouthrinse: a six month study. [accepted 12/20/2009];International Dental Journal. [PubMed] [Google Scholar]
- 18.Santos S, Sharma N, Araujo MWB, Coelho JF, Lynch M, McGuire JT. Antiplaque/antigingivitis efficacy of essential-oil fluoride mouthrinse and amine fluoride/stannous fluoride mouthrinse. Journal of Dental Research. 2006;83(Supplement B) abstract Seq #35. [Google Scholar]
- 19.Mankodi SM, Mostler KM, Charles CH, Bartels LL. Comparative antiplaque and antigingivitis effectiveness of a chorhexidine and an essential oil mouthrinse: 6 month clinical trial. Journal of Clinical Periodontology. 2004;31:878–884. doi: 10.1111/j.1600-051X.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 20.Gunsolley JC. A meta-analysis of six month studies of antiplaque and antigingivitis agents. Journal of the American Dental Association. 2006;137(12):1649–1657. doi: 10.14219/jada.archive.2006.0110. [DOI] [PubMed] [Google Scholar]