Abstract
Background.
We examined the association between extremely low-frequency magnetic fields (EMF) and the risk of dementia and Alzheimer’s disease using all 9,508 individuals from the Study of Dementia in Swedish Twins (HARMONY) with valid occupational and diagnostic data.
Methods.
Dementia diagnoses were based on telephone screening followed by in-person clinical workup. Main lifetime occupation was coded according to an established EMF exposure matrix. Covariates were age, gender, education, vascular risk factors, and complexity of work. Based on previous research, data were also analyzed separately for cases with disease onset by age 75 years versus later, men versus women, and those with manual versus nonmanual main occupation. We used generalized estimating equations with the entire sample (to adjust for the inclusion of complete twin pairs) and conditional logistic regression with complete twin pairs only.
Results.
Level of EMF exposure was not significantly associated with dementia or Alzheimer’s disease. However, in stratified analyses, medium and high levels of EMF exposure were associated with increased dementia risk compared with low level in cases with onset by age 75 years (odds ratio: 1.94, 95% confidence interval: 1.07–3.65 for medium, odds ratio: 2.01, 95% confidence interval: 1.10–3.65 for high) and in participants with manual occupations (odds ratio: 1.81, 95% confidence interval: 1.06–3.09 for medium, odds ratio: 1.75, 95% confidence interval: 1.00–3.05 for high). Results with 42 twin pairs discordant for dementia did not reach statistical significance.
Conclusions.
Occupational EMF exposure appears relevant primarily to dementia with an earlier onset and among former manual workers.
Keywords: Dementia, Magnetic fields, Occupation, Alzheimer’s disease
THE development of dementia appears to be the outcome of interactions between a large number of known and unknown genetic and environmental factors (1). Environmental factors, particularly those that are modifiable, have been of great interest to researchers, clinicians, and the public because they may facilitate development of potentially effective population-wide strategies to reduce the risk of dementia. Work-related environmental factors may play an important role in the search for strategies to postpone the onset of dementia and its most common type, Alzheimer’s disease (AD), because of the large amount of time most adults spend at work and the variety of influences that are at play.
Work-related exposure to extremely low-frequency magnetic fields (EMF), which tends to be relatively high in occupations such as electrical utility workers, railway engineers, metal workers and welders, may increase the risk of cancer (eg, 2,3). Some studies also point to a potential association between EMF exposure and dementia (4,5) or AD (5–8).
Nonetheless, whether work-related EMF exposure influences dementia onset remains unclear. Although findings of a recent meta-analysis support the role of EMF exposure in AD (9), the association was found to be rather weak and lacking a dose–response effect. Others have pointed to the possibility that the link between EMF exposure and dementia may exist primarily in specific subgroups such as cases with a relatively early disease onset (4,6), in men but not in women (5,10), and among manual workers in specific industries such as welders (7), electrical utility workers (3), and electrical/electronics, and metal workers (11).
There are biologically plausible reasons that EMF exposure might increase risk of dementia. With respect to AD, Sobel and colleagues (8) suggested that EMF exposure may play a role in the cleaving of amyloid precursor protein to form soluble beta-amyloid. Soluble beta-amyloid facilitates formation of insoluble toxic beta-amyloid in the brain, a known sign of AD pathology. This hypothesis remains empirically unconfirmed, and findings of an association between EMF exposure and all types of dementia (5,6) suggest that another mechanism nonspecific to AD may be at play. As suggested by Feychting and colleagues (6), EMF exposure may accelerate the general neuropathological process, leading to dementia diagnosis; that is, contributing to the depletion of brain reserve (12).
Our goal was to test the association between occupational EMF exposure and dementia in the population-based Swedish Twin Registry. We tested three hypotheses. First, we explored whether work-related exposure to EMF was associated with dementia or AD specifically. Second, we tested the hypotheses that EMF exposure may be associated with dementia primarily in (a) those with onset at or before 75 years of age versus later onset, (b) men versus women, and (c) those who had manual as opposed to nonmanual main lifetime occupations. Finally, we assessed the potential influence of genetic and early-life environmental factors shared by family members by exploring the association between work-related exposure to EMF and dementia in complete twin pairs where one twin was diagnosed with dementia, but the co-twin was not.
METHODS
Participants
Participants were members of the Swedish Twin Registry (13)—a population-based registry of all twins residing in Sweden—who were aged 65 years or older in 1998. In 1998, the HARMONY (taken from the Swedish words for “health” [Hälsa],“genes” [Rv], “environment” [Miljö], “and” [Och], and “new” [NY]) study began a follow-up of all twins from both same- and opposite-sex pairs who were at least 65 years of age. Cognitive screening and in-person evaluation for dementia were conducted as part of HARMONY. A random sample of twins was selected for contact by telephone each month. The cognitive screening used the previously validated TELE instrument (14) (TELE is the short name for the telephone screening instrument designed for the Study of Dementia in Swedish Twins). Individuals who screened positive for cognitive dysfunction and their co-twins were contacted for an in-person clinical diagnostic evaluation for dementia. Clinical diagnoses of dementia followed the diagnostic criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; (15)). Differential diagnoses were made according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria for AD, National Institute of Neurological Disorders and Stroke-Association Internationale pour la et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia (17), Lund and Manchester criteria for frontal temporal dementia (18), and consensus criteria for dementia with Lewy bodies (19). A complete description of the study design can be found elsewhere (20).
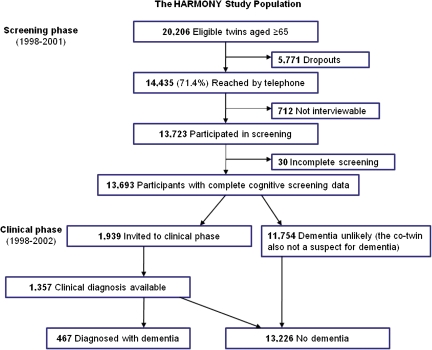
In the HARMONY study, 20,206 participants were eligible for screening (see Figure 1). The response rate for telephone screening was 71.4%; the response rate for those eligible for the clinical phase was 70.0%. The same telephone screening also included questions about demographic factors, health and behavioral information, and main lifetime occupation (13). For those who responded to the telephone screening, information about gainful lifetime occupation was available for 85.4%. Knowledgeable informants were used for participants who could not provide this information (7%).
Figure 1.
Flowchart of the HARMONY study.
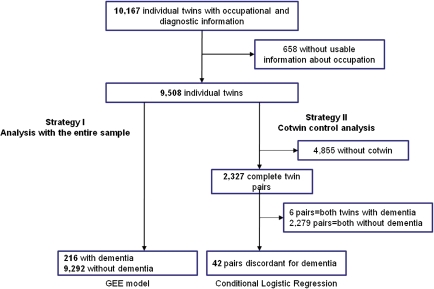
The sample for this study is described in Figure 2. Overall, 10,082 individuals aged 65 years and older had both complete data for cognitive status and main lifetime occupation. Of these, 573 could not be included because they had occupations for which EMF exposure data were not available. Therefore, 9,508 participants (4,654 who were members of complete twin pairs and 4,855 individual twins) were used in the analyses. Of these, 216 were classified as dementia cases (141 with AD) and 9,292 as controls. The average age at disease onset was 76.4 years (SD = 8.4 years) among dementia cases and 77.6 years (SD = 7.6 years) among the subset including only the cases with AD. There was no overlap in participants included in this analysis and a prior Swedish Twin study where an association between work-related EMF exposure and dementia was observed (4).
Figure 2.
Flowchart of the two analytical samples used in this study.
Of the 216 dementia cases, 42 had a co-twin in the study who was alive when the case developed dementia and who could be confirmed to be without dementia. Of these dementia discordant twin pairs, 13 were monozygotic and 29 were dizygotic (16 were opposite sex). In addition, 22 of the 42 pairs were discordant for AD, of whom 7 were monozygotic and 15 dizygotic (8 opposite sex). On average, the time between the estimated age of onset in a case and assessment in the co-twin was 6.9 years (SD = 5.6 years).
Measures
Occupation.—
The main independent variable in the study was exposure to extremely low-frequency magnetic fields in the participant’s main lifetime occupation. Information about occupation was collected in the 1998 telephone screening. Each participant, or a knowledgeable informant, was asked, “What kind of occupation did you have during the major part of your working life?”
Occupational information from these telephone interviews was sent to Statistics Sweden for coding according to categories from the 1980 Swedish Population and Housing Census, which used codes from the Nordic version of the International Standard Classification of Occupation manual (21).
EMF exposure.—
Information about work-related exposure to extremely low-frequency EMF was obtained from a job exposure matrix elaborated in a previous study (22), where workday measurements of EMF exposure were conducted on a sample from the general male Swedish population between 1989 and 1991. For each occupation, a summary estimate in microtesla was calculated as the geometric mean (due to skewness of the data) of all workday measurements in that particular occupation. This male job exposure matrix was later supplemented by separate measurements specifically for women (23). In our sample, 6% (573) of the 10,167 participants with known occupations had to be excluded due to missing values for EMF exposure for that occupational category.
In our sample, occupations with elevated exposure to extremely low-frequency EMF included, for example, railway workers (4.03 μT, n = 24), welders (1.12 μT, n = 79), forest workers (0.76 μT, n = 167), cashiers (0.45 μT, n = 48), retail traders (0.34 μT, n = 88), post office workers (0.31 μT, n = 99), cooks (0.31 μT, n = 136), electrical workers (0.31 μT, n = 133), dental nurses (0.30 μT, n = 46), chemical engineers (0.28 μT, n = 33), train dispatchers (0.25 μT, n = 85), and dentists (0.24 μT, n = 44).
Covariates.—
The covariates were age at screening (in years); gender; level of education (basic [<7 years; corresponding to mandatory education for this cohort] vs. more than basic [≥7 years]); complexity of work with data, people, and things; coronary artery disease; and stroke.
To measure complexity of work, we used scores developed for occupations from the 1970 U.S. Census data (24) that we applied to the parallel Swedish occupational coding scheme as described elsewhere (25). Then, 1970 U.S. Census scores for work characteristics were applied to the relevant occupations from the 1980 Swedish Census. Coronary artery disease and stroke were measured using records available in the national inpatient discharge registry. Each record contains up to eight discharge diagnoses coded according the International Classification of Diseases. Because Sweden uses a universal health care system, all hospitalizations are included in this registry.
Age at dementia onset—determined retrospectively via informants (26)—gender, and occupational status based on the main lifetime occupation were used for data stratification.
Data Analysis
In the analyses with the entire sample, we used categorization based on the previous study with Swedish Twin Registry data (4) with cutoffs at the 25th and 75th percentile of exposure distribution, thus stratifying participants into groups with low (bottom 25%), medium (middle 50%), and high (top 25%) exposure to EMF. The cutoffs were at 0.12 and 0.20 μT. We used generalized estimating equations models to adjust for clustering of participants, in this case, pairwise clustering. Results were adjusted for all covariates except for complexity of work with data, which was not related to dementia (p = .898) or AD (p = .695) in a bivariate regression model. Then, we conducted analyses with the sample stratified by age of onset (≤75 vs. >75), gender (men vs. women), and occupational status (manual vs. nonmanual work).
In co-twin control analyses, we used conditional logistic regression, which estimates odds ratios (ORs) based on comparisons across matched pairs. Due to the sample size limitations and the finding that cases were largely similar to their nondemented co-twins on covariates, we estimated crude ORs. Although the cases and controls did differ with respect to complexity of work, including these variables only did not appreciably alter the results when dementia was the outcome, and the models did not converge due to a small sample size when AD was the outcome. Also, stratified models could only be run for all types of dementia and not for AD alone, and meaningful results could not be obtained for the 13 monozygotic pairs alone. We used SAS software version 9 and a two-tailed .05 level of significance.
RESULTS
Analyses With the Entire Sample
Characteristics of participants across the levels of exposure are presented in Table 1. Women and those with nonmanual occupations were somewhat overrepresented among those with low EMF exposure. Occupations with medium exposure were also characterized by slightly higher levels of complexity of work compared with either low or high exposure.
Table 1.
Participant Characteristics by Level of Occupational Exposure to magnetic Fields (EMF)
| EMF Exposure in μT |
||||
| All | <0.12 | ≥0.12 to <0.20 | ≥0.20 | |
| n | 9,508 | 2,770 | 4,086 | 2,652 |
| Age at screening, mean (SD) | 72.6 (6.2) | 72.4 (6.1) | 72.6 (6.1) | 72.0 (6.3) |
| Women, % | 52 | 78 | 43 | 40 |
| Education, % more than basic | 47 | 51 | 49 | 40 |
| % With nonmanual occupation | 50 | 67 | 44 | 43 |
| Work complexity, mean (SD) | ||||
| With data | 3.0 (1.6) | 2.6 (1.3) | 3.3 (1.7) | 2.8 (1.6) |
| With people | 1.7 (1.5) | 1.4 (1.1) | 2.1 (1.9) | 1.3 (1.1) |
| With things | 2.9 (2.2) | 3.4 (1.4) | 2.6 (2.4) | 2.9 (2.4) |
| Coronary artery disease, % | 23 | 20 | 24 | 26 |
| Stroke, % | 9 | 8 | 9 | 10 |
Results for the association between level of exposure to EMF at work and risk of dementia and AD are summarized in Table 2. Although the ORs were above 1.00 for dementia among those with occupations reflecting medium or high level of EMF exposure, these results did not reach statistical significance. To assess whether controlling for complexity of work or cardiovascular factors attenuated the results appreciably, we subsequently removed these covariates but found that the results were actually weakened, suggesting some, although insubstantial, suppression effect.
Table 2.
Level of Work-Related Exposure to Magnetic Fields and Dementia or Alzheimer’s Disease
| n (%) Cases | n (%) Controls | AOR | 95% CI | p Value | |
| All types of dementia | 216 (100) | 9,292 (100) | |||
| EMF exposure in μT | |||||
| <0.12 (reference) | 50 (23) | 2,720 (29) | 1.00 | ||
| ≥0.12 to <0.20 | 93 (43) | 3,994 (43) | 1.41 | 0.96–2.06 | .079 |
| ≥0.20 | 73 (34) | 2,579 (28) | 1.38 | 0.93–2.03 | .108 |
| Alzheimer’s disease | 141 (100) | 9,292 (100) | |||
| EMF exposure in μT | |||||
| <0.12 (reference) | 35 (25) | 2,720 (29) | 1.00 | ||
| ≥0.12 to <0.20 | 58 (41) | 3,994 (43) | 1.35 | 0.85–2.14 | .211 |
| ≥0.20 | 48 (34) | 2,579 (28) | 1.38 | 0.88–2.26 | .153 |
Note: AOR = adjusted odds ratio; 95% CI = 95% confidence interval. Results adjusted for age, gender, education, complexity of work with people and things, coronary artery disease, and stroke.
Next, we tested whether EMF exposure with data stratified by age of onset, gender, and manual/nonmanual occupation (see Table 3). We found divergent results for analyses stratified by age of onset and by type of work. Specifically, medium or high EMF work-related exposure was related to approximately double the risk of dementia compared with low exposure when only cases with onset by age 75 years were considered. The parallel estimates were nonsignificant for AD only, and no significant results emerged from analyses with cases with onset after age 75 years.
Table 3.
Work-Related EMF Exposure and Dementia or Alzheimer’s Disease Shown Separately for Age of Onset in Cases, Gender, and Type of Occupation
| EMF Exposure in μT | n (%) Cases | n (%) Controls | AOR | 95% CI | p Value | n (%) Cases | n (%) Controls | AOR | 95% CI | p Value |
| Age of onset ≤75 | Age of onset >75 | |||||||||
| All types of dementia | 87 (100) | 9,292 (100) | 129 (100) | 9,292 (100) | ||||||
| <0.12 (reference) | 16 (18) | 2,720 (29) | 1.00 | 34 (26) | 2,720 (29) | 1.00 | ||||
| ≥0.12 to <0.20 | 40 (46) | 3,993 (43) | 1.94 | 1.07–3.54 | .030 | 53 (41) | 3,993 (43) | 1.12 | 0.68–1.87 | .652 |
| ≥0.20 | 31 (36) | 2,579 (28) | 2.01 | 1.10–3.65 | .022 | 42 (33) | 2,579 (28) | 1.02 | 0.59–1.75 | .954 |
| Alzheimer’s disease | 47 (100) | 9,292 (100) | 94 (100) | 9,292 (100) | ||||||
| <0.12 (reference) | 10 (21) | 2,720 (29) | 1.00 | 25 (27) | 2,720 (29) | 1.00 | ||||
| ≥0.12 to <0.20 | 20 (43) | 3,993 (43) | 1.69 | 0.74–3.87 | .215 | 38 (40) | 3,993 (43) | 1.17 | 0.66–2.08 | .585 |
| ≥0.20 | 17 (36) | 2,579 (28) | 1.94 | 0.90–4.18 | .090 | 31 (33) | 2,579 (28) | 1.20 | 0.62–2.11 | .656 |
| Men | Women | |||||||||
| All types of dementia | 95 (100) | 4,462 (100) | 121 (100) | 4,830 (100) | ||||||
| <0.12 (reference) | 9 (9) | 598 (13) | 1.00 | 41 (34) | 2,122 (44) | 1.00 | ||||
| ≥0.12 to <0.20 | 50 (53) | 2,426 (54) | 1.62 | 0.77–3.42 | .202 | 43 (36) | 1,567 (32) | 1.39 | 0.83–2.31 | .210 |
| ≥0.20 | 36 (38) | 1,438 (32) | 1.81 | 0.84–3.88 | .127 | 37 (31) | 1,141 (24) | 1.14 | 0.67–1.94 | .623 |
| Alzheimer’s disease | 54 (100) | 4,462 (100) | 94 (100) | 4,830 (100) | ||||||
| <0.12 (reference) | 5 (9) | 598 (13) | 1.00 | 30 (34) | 2,122 (44) | 1.00 | ||||
| ≥0.12 to <0.20 | 27 (50) | 2,426 (54) | 1.34 | 0.48–3.73 | .266 | 31 (36) | 1,567 (32) | 1.44 | 0.78–2.66 | .247 |
| ≥0.20 | 22 (41) | 1,438 (32) | 1.80 | 0.64–5.05 | .264 | 26 (29) | 1,141 (24) | 1.08 | 0.57–2.07 | .805 |
| Manual workers | Nonmanual workers | |||||||||
| All types of dementia | 140 (100) | 4,570 (100) | 76 (100) | 4,722 (100) | ||||||
| <0.12 (reference) | 19 (14) | 894 (20) | 1.00 | 31 (41) | 1,826 (39) | 1.00 | ||||
| ≥0.12 to <0.20 | 69 (49) | 2,050 (45) | 1.81 | 1.06–3.09 | .030 | 24 (32) | 1,943 (41) | 1.15 | 0.57–2.32 | .689 |
| ≥0.20 | 52 (37) | 1,626 (36) | 1.75 | 1.00–3.05 | .049 | 21 (28) | 953 (20) | 1.07 | 0.56–2.03 | .844 |
| Alzheimer’s disease | 90 (100) | 4,570 (100) | 51 (100) | 4,722 (100) | ||||||
| <0.12 (reference) | 11 (12) | 894 (20) | 1.00 | 24 (47) | 1,826 (39) | 1.00 | ||||
| ≥0.12 to <0.20 | 46 (51) | 2,050 (45) | 2.09 | 1.04–4.19 | .038 | 12 (24) | 1,943 (41) | 0.81 | 0.31–2.16 | .675 |
| ≥0.20 | 33 (37) | 1,626 (36) | 2.00 | 0.98–4.09 | .056 | 15 (29) | 953 (20) | 1.02 | 0.46–2.28 | .957 |
Note: AOR = adjusted odds ratio; 95% CI = 95% confidence interval. Results adjusted for age, gender, education, complexity of work with people and things, and coronary artery disease.
Among participants with manual occupations, those exposed to medium or high level of EMF exposure had about double the risk of dementia and AD compared with those with occupations in the low-exposure range, although the effect only approached significance for high EMF exposure and AD (p = .056). No substantive differences in risk based on level of EMF exposure were observed among those with nonmanual occupations. No statistically significant results emerged from analyses conducted separately for men and women.
A trend test, performed by entering the three levels of exposure as one ordinal variable, indicated a nonsignificant dose–response relationship increased exposure to EMF and dementia in all models but the model that considered dementia cases with age of onset by age 75 years (OR = 1.35, 95% confidence interval [CI]: 1.03–1.74, p = .024).
In post hoc analyses conducted with the entire sample, we estimated whether any specific type of manual occupation known for elevated exposure to EMF such as electrical or electronics work, welding, metal work, or tailoring would be associated with an increased risk of dementia. None of the results approached significance (p > .40), although we cannot preclude the possibility that the small sample size and the small number of cases was the culprit of these findings.
Analyses With Complete Dementia-Discordant Twin Pairs
Table 4 describes the results of the conditional logistic regression models. The risk did not appear to be elevated for medium level of EMF exposure (compared with low). Although the ORs for high level of EMF exposure were above 1.00 for both risk of dementia and AD, these results were not statistically significant. We also estimated models with data stratified in a fashion parallel to that used with the entire sample. The results were not significant when cases with onset by age 75 years (vs. later) were considered (OR = 6.80, 95% CI: 0.62–74.80, p = .117 vs. OR = 1.86, 95% CI: 0.34–10.02, p = .471) or among pairs where both had had manual versus nonmanual occupations (OR = 2.33, 95% CI: 0.47–11.65, p = .301 vs. OR = 1.07, 95% CI: 0.56–2.23, p = .844). It is possible that the small sample size played a role in these findings.
Table 4.
Exposure to Magnetic Fields in Twin Pairs Discordant for Dementia
| EMF Exposure in μT | n Cases | n Co-twins | OR | 95% CI | p Value |
| All types of dementia | 42 | 42 | |||
| <0.12 (reference) | 8 | 11 | 1.00 | ||
| ≥0.12 to <0.20 | 19 | 24 | 1.00 | 0.32–3.13 | .999 |
| ≥0.19 | 15 | 7 | 3.00 | 0.79–11.43 | .107 |
| Alzheimer’s disease | 22 | 22 | |||
| <0.12 (reference) | 3 | 5 | 1.00 | ||
| ≥0.12 to <0.20 | 9 | 14 | 0.84 | 0.11–6.27 | .864 |
| ≥0.20 | 10 | 3 | 7.14 | 0.62–81.98 | .115 |
Note: OR = odds ratio; 95% CI = 95% confidence interval.
DISCUSSION
We examined the association between work-related exposure to extremely low-frequency magnetic fields (EMF) and risk of dementia in the population-based Swedish Twin Registry as well as the possibility that EMF exposure plays a particular role in age of onset by 75 years, men, and those with manual occupations. The overall models yielded positive associations between EMF exposure and dementia that did not reach the threshold for statistical significance. However, we found further evidence that even a medium level of EMF exposure at work may increase (double in our study) the risk of dementia when the onset of disease is by age 75 years. In addition, we found novel evidence that among former manual workers, the risk of dementia may increase substantially with even medium exposure to extremely low-frequency EMF.
Our overall finding that increased EMF exposure may by itself pose only a limited risk with respect to dementia goes along with the null results found previously by some studies based on clinical evaluation (27) and mortality data (28) but contrary to the conclusion of a recent meta-analysis (9). Covariates did not seem to influence this association. This is particularly notable with respect to controlling for complexity of work with people and things, which tap into the intellectual challenge at work. Previously (11), it was reported that the risk of dementia and AD observed in occupations with high EMF exposure, namely electrical and metal work, was attenuated when challenge at work was controlled.
Stronger findings after restricting cases to those with age of onset by age 75 years was observed previously in a study using national mortality data (6) and in a study based on earlier data from the Swedish Twin Registry (4), when the last occupation prior to disease onset was considered. It may be that cumulative EMF exposure, or exposure at an age when preclinical neurodegenerative changes may occur, accelerates the neuropathology seen at clinical dementia onset. Alternatively, other health risks associated with EMF exposure may, in some individuals, cause death before dementia develops. Although an association between occupational EMF exposure and dementia in men at least 75 years of age was also found in one study (5), the possibility that EMF exposure may influence the development of dementia primarily early after retirement may deserve further investigation.
In analyses stratified by gender, the associations appeared to be stronger for men than women, although no results emerged as statistically significant. This gender pattern appears to correspond to the pattern found in several previous studies with participants clinically evaluated by the Kungsholmen project (5) and by U.S.-based (10) and Sweden-based (6) studies using mortality data, which yielded a significant association between work-related EMF exposure and risk of dementia in men but not in women.
We found an association between work-related EMF exposure and dementia when data were restricted to only manual workers. Previously, an increased risk of dementia and/or AD relative to the rest of the sample was found in specific manual occupations with relatively high EMF exposure, including welders (7) and electrical, electronics, or metal workers (6,11). Although it is not clear why manual workers may be particularly susceptible to dementia after greater exposure to EMF, it is possible that EMF acts as another contributing factor to the existing group of risk factors associated with manual work such as other adverse exposures in the workplace, poor lifestyle habits such as excessive alcohol drinking, or elevated stress levels. Together, these factors could accelerate the depletion of brain reserve by facilitating the general disease-related neurodegenerative process.
In addition, restricting the analyses to manual workers created a more homogeneous sample, which likely led to control over other unmeasured and potentially important factors. The assumption of overall greater EMF exposure among manual workers seems like another viable alternative explanation. However, the median values for EMF exposure among manual (0.16 μT) and nonmanual (0.14 μT) workers were quite similar. Finally, we did not find significant results for individual occupations, possibly due to small sample size.
Several limitations should be noted. First, this study involves analysis of prevalent dementia cases, which may expose the results to confounding by differential survival. Second, the sample of twins discordant for dementia was rather small, making the observed estimates difficult to interpret. In addition, 29 of the 42 pairs were dizygotic; hence, control over genetic factors was only partial for co-twin analyses. Third, EMF measurements were performed between 1989 and 1991 when many of the participants were already retired. Fourth, informants were used for participants who could not provide information about main lifetime occupation. However, the bias of using informants has been found to be minimal in previous studies (29), and this may be particularly true for relatively objective variables, such as main lifetime occupation. Fifth, we were not able to take into account the duration of occupational exposure to EMF, which was likely to vary across participants. For example, average exposure was probably longer for men than women as women from this older cohort were more likely to exit the labor force to tend to family matters. However, we did not find substantially stronger results for men, and it is unlikely that exposure varied in any other systematic way that could strengthen the association between EMF and dementia. If anything, considering time of exposure to be uniform across participants may have led to underestimation of the effects. Also important to note is that exit rates and job mobility were very low in Sweden until the late 1980s (30), suggesting that the issue of time of exposure may be more pertinent with younger cohorts.
Sixth, some members of the HARMONY study could not be interviewed as a result of illness and absence of an informed proxy, leading to some data missing not at random. Finally, because only major cardiovascular events are recorded in the national health registries, vascular factors requiring only ambulatory care were not controlled in the analyses. However, the current scientific evidence does not support an association between extremely low frequency magnetic field exposure and cardiovascular disease (31).
In conclusion, using a population-based sample, we found nuanced support for the possibility that work-related exposure to EMF may be associated with dementia. We found that EMF exposure may increase the risk of dementia when the clinical onset occurs before age 75 years or among those with manual main lifetime occupations, even when results are adjusted for previously identified risk factors for dementia, such as age, gender, level of education, complexity of work, and cardiovascular risk factors.
FUNDING
Supported by National Institute on Aging grant No. R01 AG08724 and P30 AG17265 and by an Alzheimer’s Association/Zenith Fellows Award.
References
- 1.Kuller LH. Dementia epidemiology research: it is time to modify the focus of research. J Gerontol A Biol Sci Med Sci. 2006;61(12):1314–1318. doi: 10.1093/gerona/61.12.1314. [DOI] [PubMed] [Google Scholar]
- 2.Kheifets L, Monroe J, Vergara X, Mezei G, Afifi AA. Occupational electromagnetic fields and leukemia and brain cancer: an update to two meta-analyses. J Occup Environ Med. 2008;50:677–688. doi: 10.1097/JOM.0b013e3181757a27. [DOI] [PubMed] [Google Scholar]
- 3.McElroy JA, Egan KM, Titus-Ernstoff L, et al. Occupational exposure to electromagnetic field and breast cancer risk in a large, population-based, case-control study in the United States. J Occup Environ Med. 2007;49:266–274. doi: 10.1097/JOM.0b013e318032259b. [DOI] [PubMed] [Google Scholar]
- 4.Feychting M, Pedersen NL, Svedberg P, Floderus B, Gatz M. Dementia and occupational exposure to magnetic fields. Scand J Work Environ Health. 1998;24:46–53. doi: 10.5271/sjweh.277. [DOI] [PubMed] [Google Scholar]
- 5.Qui C, Fratiglioni L, Karp A, Winblad B, Bellander T. Occupational exposure to electromagnetic fields and risk of Alzheimer’s disease. Epidemiology. 2004;15:687–694. doi: 10.1097/01.ede.0000142147.49297.9d. [DOI] [PubMed] [Google Scholar]
- 6.Feychting M, Jonsson F, Pedersen NL, Ahlbom A. Occupational magnetic field exposure and neurodegenerative disease. Epidemiology. 2003;14:413–419. doi: 10.1097/01.EDE.0000071409.23291.7b. [DOI] [PubMed] [Google Scholar]
- 7.Hakansson N, Gustavsson P, Johansen C, Floderus B. Neurodegenerative diseases in welders and other workers exposed to high levels of magnetic fields. Epidemiology. 2003;14:420–426. doi: 10.1097/01.EDE.0000078446.76859.c9. [DOI] [PubMed] [Google Scholar]
- 8.Sobel E, Dunn M, Davanipour Z, Qian Z, Chui HC. Elevated risk of Alzheimer’s disease among workers with likely electromagnetic field exposure. Neurology. 1996;47:1477–1481. doi: 10.1212/wnl.47.6.1477. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AM, Sisternas A, Perez Hoyos S. Occupational exposure to extremely low frequency electric and magnetic fields and Alzheimer disease: a meta-analysis. Int J Epidemiol. 2008;37:329–340. doi: 10.1093/ije/dym295. [DOI] [PubMed] [Google Scholar]
- 10.Schulte PA, Burnett CA. EMFs and Alzheimer’s disease. Neurology. 1997;49:312–313. doi: 10.1212/wnl.49.1.312. [DOI] [PubMed] [Google Scholar]
- 11.Seidler A, Geller P, Nienhaus A, et al. Occupational exposure to low frequency magnetic fields and dementia: a case-control study. Occup Environ Med. 2007;64:108–114. doi: 10.1136/oem.2005.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2018. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique source for clinical, epidemiological, and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 14.Gatz M, Reynolds C, John R, Johansson B, Mortimer J, Pedersen NL. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int Psychogeriatr. 2002;14:273–289. doi: 10.1017/s1041610202008475. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. 4th ed. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:256–560. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Neary D, Snowdon JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 20.Gatz M, Fratiglioni L, Johansson B, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Sweden [Statistiska centralbyrån] Population and Housing Census 1980 [Folk och Bostadsråkningen 1980] Stockholm, Sweden: Statistics Sweden; 1980. [Google Scholar]
- 22.Floderus B, Persson T, Stenlund C. Magnetic-field exposures in the workplace: reference distribution and exposures in occupational groups. Int J Occup Environ Health. 1996;2:226–238. doi: 10.1179/oeh.1996.2.3.226. [DOI] [PubMed] [Google Scholar]
- 23.Forssen UM, Mezei G, Nise G, Feychting M. Occupational magnetic field exposure among women in Stockholm County, Sweden. Occup Environ Med. 2004;61:594–602. doi: 10.1136/oem.2003.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roos PA, Treiman DJ. DOT scales for the 1970 Census classification. In: Miller AR, Treiman DJ, Cain PS, Roos PA, editors. Work, Jobs, and Occupations: A Critical Review of Occupational Titles. Washington, DC: National Academy Press; 1980. pp. 336–389. [Google Scholar]
- 25.Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60:P251–P258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 26.Fiske A, Gatz M, Aadnoy B, et al. Assessing age of dementia onset: validity of informant reports. Alzheimer Dis Assoc Disord. 2005;19:128–134. doi: 10.1097/01.wad.0000174947.76968.74. [DOI] [PubMed] [Google Scholar]
- 27.Li CY, Sung FC, Wu SC. Risk of cognitive impairment in relation to elevated exposure to electromagnetic fields. J Occup Environ Med. 2002;44:66–72. doi: 10.1097/00043764-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Noonan CW, Reif JS, Yost M, et al. Occupational exposure to magnetic fields in case-referent studies of neurodegenerative diseases. Scand J Work Environ Health. 2002;28:42–48. doi: 10.5271/sjweh.645. [DOI] [PubMed] [Google Scholar]
- 29.Demissie S, Green RC, Mucci L, et al. Reliability of information collected by proxy in family studies of Alzheimer’s disease. Neuroepidemiology. 2001;20:105–111. doi: 10.1159/000054768. [DOI] [PubMed] [Google Scholar]
- 30.Oyer P. Wage structure and labor mobility in Sweden, 1970–1990. In: Lazear EP, Shaw KL, editors. The Structure of Wage, an International Comparison. Chicago, IL: University of Chicago Press; 2008. pp. 419–448. [Google Scholar]
- 31.World Health Organization. Environmental Health Criteria (EHC) Document on ELF Fields, Doc No. 238. WHO EMF Project Web site; 2007. www.who.int/emf. Accessed February 15, 2010. [Google Scholar]