Abstract
Background.
Because cognitive impairment and frailty share common risk factors (eg, high proinflammatory cytokines), we examined whether poor cognition predicts subsequent risk of frailty in initially nonfrail Mexican Americans aged 67 years and older.
Methods.
Frailty was defined as meeting one or more of the following components: (a) unintentional weight loss of >10 pounds, (b) weakness, (c) self-reported exhaustion, and (d) slow walking speed. Sociodemographic factors, Mini-Mental State Examination, medical conditions (stroke, heart attack, diabetes, arthritis, cancer, and hypertension), and depressive symptoms were obtained. Main outcome measure was risk of becoming frail over 10 years.
Results.
Out of 942 participants who were nonfrail at baseline (1995–1996), 57.8% were women and the mean age was 73.7 years (SD = 5.3). In general estimation equation models testing the relationship between Mini-Mental State Examination (<21 vs. ≥21) and the risk of becoming frail over a 10-year period, there was a significant association (odds ratio = 1.09, 95% confidence interval = 1.00–1.19; p = .0310)] between the cognition-by-time interaction and odds of becoming prefrail or frail over time. This association was independent of age, sex, marital status, education, time, and medical conditions, indicating that nonfrail participants with poor cognition had a 9% odds per year of becoming frail over time compared with those with good cognition.
Conclusion.
Low Mini-Mental State Examination score was independently associated with increased risk of frailty over a 10-year period in older Mexican Americans. Low Mini-Mental State Examination score may be an early marker for future risk of frailty.
Keywords: Cognition, Frailty, Aging
FRAILTY is a syndrome of progressive decline in body weight, muscle strength, self-reported energy level, walking speed, and physical activity level (1–4). The incidence of both frailty syndrome and cognitive impairment increases with age (2,5–8). Preliminary evidence suggests that cognitive decline may be an early manifestation of older adults in transition to frailty. The evidence derives mainly from studies showing independent associations between cognitive impairments and increased risk of acquiring individual components of frailty syndrome (eg, cognition and slow walking speed, cognition and weight loss, cognition and subsequent muscle weakness) (9–13).
Other evidence derives from data supporting the existence of shared risk factors. For example, brain infarcts and high proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-6, and C-reactive protein are associated with both impaired cognition and frailty syndrome (3,4,14–18). In a longitudinal study of 104 cognitively normal participants, Silbert and colleagues found an association between progression of white matter hyperintensity on brain magnetic resonance imaging and a decline in gait and cognitive function over 13 years of follow-up (17). These data suggest that inflammation, brain infarcts, and other pathological factors might be contributing to both the development of frailty and the onset of cognitive decline in older adults.
Most studies on predictors of frailty have been conducted in the non-Hispanic White population. Little is known about risk factors of frailty in minority populations, particularly the fastest growing minority group—the Hispanic older adults. Also, little research exists regarding how cognitive function affects transitions to frailty because studies in cognitive aging tend to use individual components of frailty versus the full frailty measure (2).
The present study examined the longitudinal association between cognition and subsequent risk of becoming frail (using the full components of the frailty measure) over 10 years in a large sample of initially nonfrail Mexican-American older adults living in the community. We hypothesized that participants with low cognitive scores would be at higher risk for becoming frail over time than those with high cognitive scores. A better understanding of the relationship between cognition and frailty may allow early identification of (and potential interventions for) older adults at the highest risk of becoming frail.
METHODS
Sample and Procedures
We used data from the Hispanic Established Populations for Epidemiologic Study of the Elderly (EPESE), a longitudinal study of Mexican Americans aged 65 years and older at initial interview and residing in Texas, New Mexico, Colorado, Arizona, and California. The sample and its characteristics have been described elsewhere (19,20). The sampling procedure assured a sample that is generalizable to approximately 500,000 older Mexicans Americans living in the southwest. Five waves of data have been collected (1993–1994, 1995–1996, 1998–1999, 2000–2001, and 2004–2005). The current study used data obtained at second, third, fourth, and fifth waves (1995–2005). Data from the second wave were used because it included all the measures necessary to compute the frailty measure (2). We did not allow proxy interviews due to the physical nature of some of the frailty measurements. The final sample included 1994 participants in 1995–1996 with complete data. The inclusion criteria for the present study were the ability to perform the items necessary to complete an operationally defined measure of frailty (2) and a standardized assessment of global cognition with the Mini-Mental State Examination (MMSE) (21). Because we were interested in examining how cognition influenced transitions from nonfrail state to being prefrail or frail, we excluded all participants with one or more scores on the frailty measure (see description later) at Wave 2 (1995–1996). Thus, the final sample consisted of 942 nonfrail participants with complete data on frailty and all covariates at Wave 2 (hereafter referred as baseline) and follow-up. The average follow-up was 4.8 years (SD = 3.3). Seven hundred and ninety-one participants were reinterviewed at second follow-up (1998–1999), 717 participants at third follow-up (2000–2001), and 540 participant at the end of follow-up (2004–2005). In total, 47 participants refused to be reinterviewed, 67 participants were lost to follow-up, and 288 were confirmed dead through the National Death Index and by relatives at the end of follow-up. Those participants who were not present at the end of the 10-year follow-up in 2004–2005 (n = 402) were more likely to be older, to be female, nonmarried, to have lower MMSE score, and to report more hypertension and diabetes at baseline.
Participants excluded from the analysis (N = 1,496) were significantly more likely to be older, to have lower level of education, lower MMSE score, and to have a history of heart attack, stroke, arthritis, diabetes, hip fracture, and cancer when compared with included participants. Of the excluded participants, 34% were frail, 63.6% were prefrail, and 2.2% were nonfrail. The University of Texas Medical Branch Institutional Review Board on human protection and research ethics approved the study.
Study Variables
Measurements.—
Frailty was assessed using a modified version of the Fried and Walston frailty measure (1). The modified scale has a range of 0–4 and includes weight loss, exhaustion, walking speed, and grip strength (22,23) (Table 1). We used the modified scale because the physical activity data were not available in all the four waves needed for our longitudinal analysis. Weight loss was calculated as the difference between weight at the previous interview and current weight. Participants with unintentional weight loss of >10 lbs were categorized as positive for the weight loss criterion (score = 1). Exhaustion was assessed using two items from the Center for Epidemiological Studies-Depression scale: (24) “I felt that everything I did was an effort” and “I could not get going.” The items asked, “How often in the last week did you feel this way?” 0 = rarely or none of the time (<1 day), 1 = some or a little of the time (1–2 days), 2 = a moderate amount of the time (3–4 days), or 3 = most of the time (5–7 days). Participants answering “2” or “3” to either of these two items were categorized as positive for the exhaustion criterion (score = 1).
Table 1.
Definition of Frailty* Criteria
| Characteristic | Definition |
| Shrinking | Unintentional Weight Loss of >10 Pounds |
| Weakness | Lowest 20% in grip strength (adjusted by gender and BMI [kg/m2]) |
| Men | Strength ≤21 for BMI ≤24.2 |
| Strength ≤24.5 for BMI 24.3–26.8 | |
| Strength ≤25.4 for BMI 26.9–29.5 | |
| Strength ≤25.5 for BMI >29.5 | |
| Women | Strength ≤13.5 kg for BMI ≤24.7 |
| Strength ≤14.2 kg for BMI 24.8–28.3 | |
| Strength ≤15.0 kg for BMI 28.4–32.1 | |
| Strength ≤15.0 kg for BMI >32.1 | |
| Exhaustion | Self-report positive answer from either of two question on CES-D scale: I felt that everything I did was an effort, I could not get going |
| Slowness | Slowest 20% of walking time from 4.87-m walk test adjusted by gender and height (cm) |
| Men | Time ≥11.2 s for height ≤167.6 cm |
| Time ≥9.7 s for height >167.6 cm | |
| Women | Time ≥12.0 s for height ≤153.7 cm |
| Time ≥11.2 s for height >153.7 cm |
Notes: BMI = body mass index; CES-D = Center for Epidemiological Studies-Depression scale.
Frailty was defined as meeting one or more of the following components: (a) unintentional weight loss of >10 pounds, (b) weakness, (c) self-reported exhaustion, and (d) slow walking speed.
Walking speed was assessed over an 8-foot timed walk. Participants were asked to walk at their normal pace timed to the nearest second. Height- and gender-adjusted time points were used based on the slowest 20% and scored as positive for this criterion. Those unable to perform the test were also categorized as positive (score = 1). Grip strength was assessed by different criteria for men and women using a handheld dynamometer (Jaymar Hydraulic Dynamo-meter model #5030J1; J.A. Preston, Corp., Jackson, MI). Participants unable to perform the grip strength test and those in the lowest 20% adjusted for body mass index and stratified by gender were categorized as positive for the weakness criterion (score = 1). Cut-points for handgrip strength and walking speed were calculated at baseline and used to create the frailty index for each follow-up period. The summary frailty score measured at each wave ranged from 0 to 4 with a higher score indicating increased frailty. Participants were categorized as “not frail,” “prefrail,” or “frail.” Participants who scored 0 on the summary frailty measure were categorized as notfrail. Frailty in this study was defined has having 1 or more components. The original frailty scale has shown good predictive validity among older (≥65 years of age) White and Black men and women (1). The original scale was predictive of reduced mobility or activity of daily living function, hospitalization, and death and has been widely used in the aging research literature (2,25).
Cognitive function was assessed with the 30-item MMSE, the most frequently used cognitive screening measure in cognitive aging research (21,26,27). The English and Spanish versions of the MMSE were adopted from the Diagnostic Interview Scale and have been used in prior community surveys (21,26,27). Similar to previous studies on cognitive aging, especially in populations with low educational attainment, the MMSE score was dichotomized as less than 21 (impaired or poor cognition [PC]) and 21 or greater (normal or good cognition) (13,28).
Covariates.—
Baseline sociodemographic variables included age, sex, marital status, and years of education. The presence of medical conditions was assessed by asking whether a doctor had ever told respondents that they had diabetes mellitus, heart attack, stroke, hypertension, arthritis, hip fracture, or cancer.
Outcome.—
The primary outcome was becoming frail, defined as acquisition of 1 or more components of the frailty measure over the 10-year follow-up interview period. Participants included in the analyses were not frail at baseline (n = 942).
Statistical Analyses
Sociodemographic and health characteristics were examined at baseline for nonfrail participants stratified by MMSE score (<21 vs ≥21) using descriptive and univariate statistics for continuous variables and contingency tables (chi-square) for categorical variables. To test whether cognitive function was related to becoming frail over 10 years of follow-up, a general estimation equation model was fitted using the GENMOD procedure in SAS (SAS Institute, Inc., Cary, NC), adjusting for age, sex, marital status, education, and medical conditions (diabetes mellitus, heart attack, stroke, hypertension, arthritis, hip fracture, or cancer). All the variables (female, marital status, MMSE, and medical conditions) were analyzed as time-dependent variables except variables of age and education. Those participants who died, refused, and were lost to follow-up were included in the study until their last follow-up (last interview date for the 10-year follow-up). Two models were constructed to test the independent relationship between MMSE scores and new onset of becoming frail over the 10-year follow-up period. Model 1 included time, cognitive function, age, sex, marital status, education, and cognition-by-time interaction. In Model 2, medical conditions were added to the variables included in Model 1. We also analyzed cognitive function using MMSE as a continuous measure to investigate if there was a gradient of risk on becoming frail over the 10-year follow-up. For all models, testing was two-sided using an α of .05. We conducted standard tests of regression diagnostics for outliers, goodness of fit, and assumptions for the cumulative logit models. All assumptions were met (data not shown). All analyses were performed using the SAS for Windows, version 9.2 (SAS Institute, Inc.).
RESULTS
Table 1 presents the criteria used for frailty in this study. We used the modified frailty scale because the physical activity data were not available in all the four waves needed for our longitudinal analysis. The grip strength test was adjusted for body mass index and stratified by gender. Walking speed (slowness) was adjusted for height and gender.
Table 2 presents baseline characteristics of participants who were not frail at baseline as a function of baseline MMSE score less than 21 versus 21 or greater. Participants with MMSE scores less than 21 were significantly more likely to be older and less educated.
Table 2.
Descriptive Characteristics of the Sample by Cognitive Status Among Nonfrail Participants at Baseline (1995–1996; N = 942)
| MMSE < 21, N = 159 |
MMSE = ≥ 21, N = 783 |
||
| Predictor Variables | n (%) | n (%) | p Value |
| MMSE (units), mean ± SD | 18.6 ± 2.4 | 26.1 ± 3.2 | <.0001 |
| Age (years), mean ± SD | 74.9 ± 6.3 | 73.5 ± 5.1 | <.0001 |
| Gender (female) | 80 (50.3) | 464 (59.3) | .0374 |
| Marital status (married) | 80 (50.3) | 440 (56.2) | .1741 |
| Education (years), mean ± SD | 2.9 ± 3.0 | 5.7 ± 4.1 | <.0001 |
| Diabetes | 34 (21.4) | 202 (25.8) | .2415 |
| Heart attack | 10 (6.3) | 51 (6.5) | .9166 |
| Stroke | 11 (6.9) | 37 (4.7) | .2516 |
| Hypertension | 70 (44.0) | 349 (44.6) | .8993 |
| Arthritis | 53 (33.3) | 325 (41.5) | .0552 |
| Hip fracture | 2 (1.3) | 3 (0.4) | .2000 |
| Cancer | 8 (5.0) | 39 (4.9) | .9787 |
Note: MMSE = Mini-Mental State Examination.
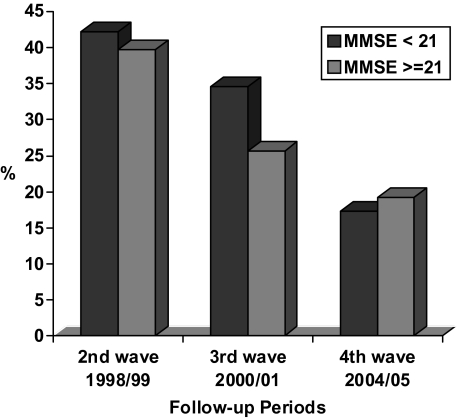
Figure 1 shows incidence of frailty (acquisition of one or more frailty components) by baseline cognitive status at each wave of follow-up: 1998–1999 (second wave), 2000–2001 (third wave), and 2004–2005 (fourth wave). Incidence of frailty was, in the second wave, 42.1% in the PC group and 39.7% in the normal cognition (NC) group; in the third wave, 34.6% in PC and 25.6% in NC group; and in the fourth wave, 17.3% in PC and 19.2% in NC group.
Figure 1.
Incidence of frailty over time by cognitive status at baseline (1995–1996, N = 942).
Table 3 shows number and percents of frailty transitions and number of deaths by baseline cognitive status for each follow-up interval period from 1995–1996 to 1998–1999 (second wave), from 1998–1999 to 2000–2001 (third wave), and from 2000–2001 to 2004–2005 (fourth wave). Participants at beginning of each period were not frail. As shown in Table 3, at every follow-up wave, the PC group had higher mortality rates than those in the NC group. Transition to frailty from nonfrail state was higher among the PC group in the second and third waves (13.8% vs 11.6% and 11.0% vs 9.8% respectively), but the trend was reversed in the fourth wave (6.8% vs 7.3%).
Table 3.
Number and Percent of Frailty Transitions and Deaths by Baseline Cognitive Status for Each Follow-up Interval Among NonFrail Older Mexican Americans. (N = 942 participants who were nonfrail at baseline in 1995/1996)
| 1995/1996–1998/1999 |
1998/1999–2000/2001 |
2000/2001–2004/2005 |
||||
| Second Wave |
Third Wave |
Fourth Wave |
||||
| MMSE < 21 | MMSE ≥ 21 | MMSE < 21 | MMSE ≥ 21 | MMSE < 21 | MMSE ≥ 21 | |
| Nonfrail* | N = 159 | N = 783 | N = 82 | N = 387 | N = 73 | N = 329 |
| Nonfrail, n (%) | 85 (53.5) | 422 (53.9) | 56 (68.3) | 238 (61.5) | 51 (60.0) | 247 (75.1) |
| Prefrail, n (%) | 34 (21.4) | 216 (27.6) | 12 (14.6) | 96 (24.8) | 9 (12.3) | 33 (1 0.0) |
| Frail, n (%) | 22 (13.8) | 91 (11.6) | 9 (11.0) | 38 (9.8) | 5 (6.8) | 24 (7.3) |
| Deaths, n (%) | 18 (11.3) | 54 (6.9) | 5 (6.1) | 15 (3.9) | 8 (10.9) | 25 (7.6) |
Note: *Participants at beginning of each period were not frail.
We also examined percentage of participants who developed only one of each of the frailty components at each wave over the 10-year follow-up period (table not shown). By 1998–1999, among participants with no frailty at baseline in 1995–1996, 30.9% of the PC group and 26.3% of the NC group met frailty criteria solely on the basis of weight loss component alone. By 2000–2001 and 2004–2005, the percentage of participants that met the criteria solely on weight loss component diminished. For example, by 2004–2005, only 3.7% of the PC group and 5.4% of the good cognition group became frail from nonfrail by weight loss criteria alone. On the other hand, the percentage of participants who became frail from nonfrailty status by slowness (walk speed) criterion alone increased from 17.6% in PC group and 15.8% in NC group in 1998–1999 to 25% (PC) and 18.1% (NC) in 2004–2005, respectively.
Table 4 presents general estimation equation models testing the relationship between cognitive function (MMSE <21 vs ≥21) and odds of becoming frail (one or more components) over a 10-year period. In Model 1, there was a significant association between the cognition-by-time interaction and risk of becoming prefrail or frail; participants with PC (MMSE <21) had a higher odds of become prefrail or frail over time compared with those with high cognition (MMSE ≥21), independent of age, sex, marital status, education, and time (odds ratio [OR] = 1.09, 95% confidence interval [CI] = 1.01–1.17; p = .0262). In Model 2, we added medical conditions (diabetes mellitus, heart attack, stroke, hypertension, arthritis, hip fracture, or cancer) as time-dependent covariates. The association remained significant (OR = 1.09, 95% CI = 1.00–1.19; p = .0310), indicating that nonfrail participants with PC had a 9% odds per year of becoming frail at the 10-year follow-up compared with those with good cognition after adjusting for all covariates. With MMSE as a continuous time-dependent variable, the OR for frailty after controlling for all covariates was 1.00 (95% CI = 0.99–1.01; p = .0954). Additional analyses were performed using incident frailty as 2 or more components; the odds ratio of becoming frail over time for subjects with MMSE <21 was 1.02 (95% CI = 0.95–1.09; p = .6137).
Table 4.
General Estimation Equations for Becoming Frail (one or more components) Over 10-year of Follow-up as a Function of Cognitive Impairment (MMSE < 21) Among Nonfrail Participants at Baseline (n = 942)
| Model 1 |
Model 2 |
|
| Predictor Variables | OR (95% CI) | OR (95% CI) |
| Time | 1.15 (1.09–1.22) | 1.20 (1.11–1.29) |
| Cognitive impairment (MMSE < 21) | 1.05 (0.76–1.44) | 1.04 (0.75–1.44) |
| Age (years) | 1.05 (1.04–1.07) | 1.05 (1.04–1.07) |
| Gender (female) | 1.21 (0.96–1.53) | 1.11 (0.86–1.43) |
| Marital status (married) | 0.87 (0.69–1.10) | 0.88 (0.69–1.13) |
| Education | 0.99 (0.97–1.02) | 0.99 (0.97–1.02) |
| Cognitive impairment (MMSE < 21) × time | 1.09 (1.01–1.17) | 1.09 (1.00–1.19) |
| Gender (female) × Time | 1.02 (0.93–1.08) | 1.04 (0.98–1.11) |
| Marital status (married) × Time | 1.07 (1.01–1.13) | 1.06 (1.00–1.13) |
| Diabetes | 1.20 (0.94–1.54) | |
| Heart attack | 0.89 (0.55–1.46) | |
| Stroke | 1.06 (0.63–1.78) | |
| Hypertension | 1.25 (0.97–1.61) | |
| Arthritis | 1.23 (0.96–1.56) | |
| Hip fracture | 1.07 (0.34–3.33) | |
| Cancer | 1.10 (0.65–1.86) | |
| Diabetes × Time | 1.05 (0.98–1.12) | |
| Heart attack × Time | 1.05 (0.94–1.18) | |
| Stroke × Time | 1.00 (0.89–1.13) | |
| Hypertension × Time | 0.91 (0.85–0.96) | |
| Arthritis × Time | 0.99 (0.93–1.04) | |
| Hip fracture × Time | 1.28 (1.00–1.65) | |
| Cancer × Time | 1.00 (0.88–1.13) |
Note: MMSE = Mini-Mental State Examination; OR = odds ratio.
DISCUSSION
The findings can be summarized as follows: nonfrail older Mexican Americans with low cognitive scores were significantly more likely to acquire one or more components of frailty over 10 years than those with higher cognitive scores, independent of age, sex, education, diabetes mellitus, heart attack, stroke, hypertension, arthritis, hip fracture, or cancer. Our findings support and extend prior studies examining the association of impaired cognition with individual components of frailty (eg, cognition and weight loss, cognition and slow gait) (11,29) by showing the relationship between low cognitive scores and higher odds of acquiring one or more components of frailty syndrome over time, with simultaneous adjustments for time-dependent changes in demographic and health covariates, in a large community-based sample of initially nonfrail elderly Mexican Americans.
Using data from the Women’s Health Initiative Memory Study (n = 1,793 women; mean age = 70.3 years, 89% White), Atkinson and colleagues showed that women with low or declining scores on the Modified Mini-Mental State (3MS) Examination were significantly more likely to have slower gait speed and weaker muscle strength over a 6-year follow-up period, independent of demographics, comorbid conditions, and other relevant confounders (29). These studies (11,29) suggest that PC might contribute to acquiring individual components of frailty (eg, weight loss, slow gait, or reduced muscle strength). The current study advances the literature by showing that PC predicts increased risk of acquiring one or more components of the frailty measure in the same study sample.
Our findings also support results of past studies arguing for inclusion of cognitive function in the assessment of frailty (30,31). Such inclusion might enhance the ability of the frailty measure to better predict clinically relevant outcomes, such as disability, health care use, and death. In a 4-year longitudinal study of more than 6,000 French participants aged 65 years and older, cognitively impaired frail participants were significantly more likely to become disabled, demented, or hospitalized compared with cognitively intact frail participants, independent of other health indicators (30). Future studies are needed to test whether adding cognition as a component of frailty might increase ability of frailty measure to predict key clinical outcomes.
Several mechanisms might explain the association between PC and increased risk of frailty. First, low cognition in nonfrail persons may be associated with underrecognition of risk factors for frailty (eg, suboptimal nutrition, poor exercise engagement) and clustering of unrecognized and undertreated comorbidities (eg, diabetes and atrial fibrillation) known to affect components of frailty. In that scenario, the persistence of the risk factors and comorbidities eventually leads to manifestations of the frailty syndrome. Supporting this explanation are previous studies showing an association between declining cognition—as in Alzheimer’s disease—and decreasing physical activity and food intake (32,33), a decrease that could promote further muscle loss, easy exhaustion, weight loss, and other components of frailty. We do not have evidence for this explanation in our study. This scenario, however, suggests the potential to screen for and develop interventions for these risk factors as a means of reducing the odds of frailty, especially in seniors with low cognitive function.
Second, the association between PC and frailty could reflect the existence of shared factors (such as low sex steroids, strokes, and high inflammation markers) that may be causing cognitive decline and the onset of frailty. Findings from previous studies support this explanation (3,4,14–17,34). In a scenario of shared risk factors, cognitive deficits might manifest earlier, but over time and with persistence of the pathogenic factors, other components of frailty (eg, muscle loss, slow gait, or easy exhaustion) as captured by the frailty measures become apparent. We were not able to examine this hypothesis due to the lack of brain imaging or blood markers in our sample, but this is an important area for future experimental research.
This study has several limitations. First, we were limited to self-reports of medical conditions. However, previous studies have reported good agreement between self-reported medical conditions and actual medical diagnoses (35,36). Second, by including participants in the sample who were interviewed at each follow-up, we are examining the cohort of healthier participants. This might underestimate the relationship between cognitive function and becoming frail. To test whether the healthier cohort effect might alter our findings, we repeated the analyses by including the data of the participants who died, refused to be reinterviewed, or were lost to follow-up and with the assumption that they remained in the same frailty status as in their previous interview; the relationship between PC and frailty risk still remained significant (OR = 1.09; 95% CI = 1.01–1.19).
The use of different measures for frailty by different investigators in frailty research limits data comparison between studies. The noninclusion of physical activity measure as a part of the frailty index in this study could bias our findings toward underestimation of incidence rate of frailty on older Mexican Americans. Also, we used slower gait speed cut-points (<0.21–0.25 m/s adjusted for gender and height) than cut-point (<0.65–0.76 m/s) used by Fried and colleagues; this also likely may bias our frailty incidence toward underestimation.
The higher death rate in the PC group increases the possibility that a cognitively impaired participant could transition from nonfrail to frail to death without having an interview between the nonfrail state and death either because they transitioned between waves or because they missed a follow-up wave due to illness prior to death. Not capturing such participants in our frailty incidence measure likely bias our findings on association between impaired cognition and frailty incidence toward an underestimation. Another limitation is the lack of tissue and blood samples in our study sample, precluding testing for apolipoprotein E genotype and other previously published biologic markers of frailty (5,18). Investigating the potential role of these markers on frailty transitions in older Mexican Americans is an important area for future study.
This study had several strengths, including its large community-based sample, its prospective design, and its exploration of the potential role of cognition in odds of becoming frail in older Mexican Americans, in an understudied but rapidly growing segment of the older population in the United States.
In conclusion, our results showed that initially nonfrail older Mexican Americans with PC had higher odds of becoming frail over a period of 10 years than those with NC, independent of other demographic and health factors. The findings raise a number of important questions for future research. Can level of cognitive performance identify older adults at highest risk for frailty? Does cognitive functioning moderate the trajectory and health impact of frailty? Examining these questions may provide evidence of new risk factors for frailty associated with cognitive function. An important step toward reducing the adverse impacts of frailty is early identification of older adults at the highest risk of becoming frail and the subsequent development of prevention programs and intervention strategies.
FUNDING
This study was supported by the National Institutes of Health, National Institute on Aging (R01 AG10939, R01 AG17638, P30 AG024832, R01 AG031178); National Cancer Institute (P50 CA105631); and the National Institute for Child Health and Human Development (K12 HD052023). The sponsors had no role in the design, methods, participant recruitment, data collections, analysis, or preparation of the manuscript.
Acknowledgments
The authors thank the anonymous reviewers for their helpful comments. Preliminary results of the study were presented as a Poster Presentation at the 2010 American Geriatrics Society Meeting, Orlando, FL.
References
- 1.Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard W, Blass J, Ettinger WH, et al., editors. Principles of Geriatric Medicine and Gerontology. New York: McGraw-Hill Professional; 1999. pp. 1387–1402. [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 4.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 5.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 6.Ottenbacher KJ, Graham JE, Al SS, et al. Mexican Americans and frailty: findings from the Hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009;99(4):673–679. doi: 10.2105/AJPH.2008.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottenbacher KJ, Ostir GV, Peek MK, Snih SA, Raji MA, Markides KS. Frailty in older Mexican Americans. J Am Geriatr Soc. 2005;53(9):1524–1531. doi: 10.1111/j.1532-5415.2005.53511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deary IJ, Corley J, Gow AJ, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 11.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res. 2004;12(9):1519–1526. doi: 10.1038/oby.2004.189. [DOI] [PubMed] [Google Scholar]
- 12.Raji MA, Kuo YF, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53(9):1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 13.Raji MA, Ostir GV, Markides KS, Goodwin JS. The interaction of cognitive and emotional status on subsequent physical functioning in older Mexican Americans: findings from the Hispanic established population for the epidemiologic study of the elderly. J Gerontol A Med Sci. 2002;57(10):M678–M682. doi: 10.1093/gerona/57.10.m678. [DOI] [PubMed] [Google Scholar]
- 14.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 15.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmelli D, DeCarli C, Swan GE, et al. The joint effect of apolipoprotein E epsilon4 and MRI findings on lower-extremity function and decline in cognitive function. J Gerontol A Med Sci. 2000;55(2):M103–M109. doi: 10.1093/gerona/55.2.m103. [DOI] [PubMed] [Google Scholar]
- 19.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano) 1993;5(1):27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 20.Markides KS, Stroup-Benham CA, Black SA, Satish S, Perkowski LC, Ostir G. The health of Mexican American elderly: selected findings from the Hispanic EPESE. In: Wykle ML, Ford AB, editors. Serving Minority Elderly in the 21st Century. New York: Springer Pub; 1999. pp. 72–90. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Samper-Ternent R, Al SS, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008;56(10):1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Snih S, Graham JE, Ray LA, Samper-Ternent R, Markides KS, Ottenbacher KJ. Frailty and incidence of activities of daily living disability among older Mexican Americans. J Rehabil Med. 2009;41(11):892–897. doi: 10.2340/16501977-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CED-S Scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 suppl):1–29. [PubMed] [Google Scholar]
- 26.Bird HR, Canino G, Stipec MR, Shrout P. Use of the Mini-mental State Examination in a probability sample of a Hispanic population. J Nerv Ment Dis. 1987;175(12):731–737. doi: 10.1097/00005053-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Uhlmann RF, Larson EB. Effect of education on the Mini-Mental State Examination as a screening test for dementia. J Am Geriatr Soc. 1991;39(9):876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 28.Leveille SG, Guralnik JM, Ferrucci L, Corti MC, Kasper J, Fried LP. Black/white differences in the relationship between MMSE scores and disability: the Women’s Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 1998;53(3):201–208. doi: 10.1093/geronb/53b.3.p201. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2010;65(3):300–306. doi: 10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59(12):1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 32.Wang PN, Yang CL, Lin KN, Chen WT, Chwang LC, Liu HC. Weight loss, nutritional status and physical activity in patients with Alzheimer’s disease. A controlled study. J Neurol. 2004;251(3):314–320. doi: 10.1007/s00415-004-0316-4. [DOI] [PubMed] [Google Scholar]
- 33.White HK, McConnell ES, Bales CW, Kuchibhatla M. A 6-month observational study of the relationship between weight loss and behavioral symptoms in institutionalized Alzheimer’s disease subjects. J Am Med Dir Assoc. 2004;5(2):89–97. doi: 10.1097/01.JAM.0000110646.48753.EF. [DOI] [PubMed] [Google Scholar]
- 34.Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biol Psychol. 2002;61(1–2):139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger M, Samsa GP, Schmader K, Greenberg SM, Carr DB, Wildman DS. Comparing proxy and patients’ perceptions of patients’ functional status: results from an outpatient geriatric clinic. J Am Geriatr Soc. 1992;40(6):585–588. doi: 10.1111/j.1532-5415.1992.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 36.Magaziner J, Simonsick EM, Kashner TM, Hebel JR. Patient-proxy response comparability on measures of patient health and functional status. J Clin Epidemiol. 1988;41(11):1065–1074. doi: 10.1016/0895-4356(88)90076-5. [DOI] [PubMed] [Google Scholar]