Abstract
Tumor necrosis factor–like cytokine 1A (TL1A) is expressed in endothelial cells and contributes to T-cell activation, via an extracellular fragment TL1AL72-L251, generated by ectodomain shedding. Fragments of TL1A, referred to as vascular endothelial growth inhibitor, were found to induce growth arrest and apoptosis in endothelial cells; however, the underlying mechanisms remained obscure. Here, we show that full-length TL1A is the major detectable gene product in both human umbilical vein endothelial cells and circulating endothelial progenitor cells. TL1A expression was significantly enhanced in senescent circulating endothelial progenitor cells, and knockdown of TL1A partially reverted senescence. TL1A overexpression induced premature senescence in both circulating endothelial progenitor cells and human umbilical vein endothelial cells. We also identified a novel extracellular fragment of TL1A, TL1AV84-L251, resulting from differential ectodomain shedding, which induced growth arrest and apoptosis in human umbilical vein endothelial cells. These findings suggest that TL1A is involved in the regulation of endothelial cell senescence, via a novel fragment produced by differential ectodomain shedding.
Keywords: TL1A, HUVEC, CEP, Senescence, Ectodomain shedding
WHEREAS cellular senescence is now well recognized as a potent tumor suppressor mechanism, its role in aging is still controversial (1). Nevertheless, cellular senescence has been widely used as a model system to study aging of various human tissues. Thus, senescent cells have been described in mitotic tissue of aging rodents (2), nonhuman primates (3), as well as in human aged tissues, such as the skin (4,5), the vascular system (6), and the kidney (7). The premature appearance of senescent cells in the vascular endothelium has been associated with the onset of age-associated cardiovascular diseases, such as arteriosclerosis (8). Furthermore, apoptosis and senescence are considered important factors contributing to vascular dysfunction and pathology (9).
Senescent cells can also have detrimental effects on their microenvironment and neighboring cells due to the dysregulation of their metabolism and changes in the secretome (for review, see [10]). The secretome of senescent cells, referred to as the senescence-associated secretory phenotype (11) or the senescence-messaging secretome (12), is complex and, besides cytokines and chemokines (13), includes other factors that are involved in senescence-associated proliferation arrest, such as insulin-like growth factors and insulin-like growth factor binding proteins (14,15), and extracellular matrix remodeling, such as matrix metalloproteinase-1 and -3 (16). Cytokines of the senescence-associated secretory phenotype, like interleukin (IL)-1 or members of the tumor necrosis factor (TNF) superfamily, are known regulators of nuclear factor kappa B (17), consistent with an emerging role for nuclear factor kappa B in the regulation of cellular senescence (18).
In 1997, a new member of the TNF-ligand superfamily was discovered by searching a cDNA database (19). After preliminary experiments with a partial cDNA, which blocked the proliferation of endothelial cells, the novel TNF ligand was named vascular endothelial growth inhibitor (VEGI) (20). The full-length gene product (251 amino acids) was referred to as TNF-like cytokine 1A (TL1A), VEGI-251 (21), or, most recently, as TNF (ligand) superfamily, member 15 (TNFSF15) (22). For convenience, the gene is referred to as TL1A throughout this article. Two isoforms of TL1A, referred to as VEGI-174 and VEGI-192, supposed to be produced by alternative splicing, were also described (23–25); however, the physiological relevance of these isoforms remained largely unclear (see subsequently).
TL1A is a unique TNF ligand because it is predominantly expressed in endothelial cells (20). To a minor extent, TL1A is also expressed in tissue macrophages, lamina propria T cells and plasma cells, FcγR-activated peripheral blood mononuclear cells (PBMC), and monocyte-derived dendritic cells (26–29). TL1A is the only known ligand for the death receptor DR3 (TNFRSF12) (21). DR3 was shown to be primarily expressed in activated lymphocytes (30,31) and to a minor extent in natural killer (NK) cells, macrophages, and endothelial cells, such as human umbilical vein endothelial cells (HUVEC) (27,32–34). TL1A is also a ligand for decoy receptor 3 (DcR3/ TNFRSF6B) (21). DcR3 messenger RNA (mRNA) is expressed in several human tissues like spleen, lung, and the gastrointestinal tract, as well as in HUVEC (35). TL1A was shown to be involved in several inflammatory diseases, such as renal inflammation (25), Crohn’s disease (36), inflammatory bowel disease (26), rheumatoid arthritis (37,38), and autoimmune diseases (39). Recent research on DR3- and TL1A-knockout mice confirmed an important role of TL1A/DR3 in autoimmune and inflammatory diseases (40,41). An involvement of TL1A and DR3 in atherosclerosis was also shown (27,42,43). This is reasonable because the development of atherosclerosis has an autoimmune component and is considered as an inflammatory disease (44).
Although research on TL1A is already in progress for more than 10 years, studies on the consequences of ectopic overexpression of TL1A cDNA in human cells are sparse and conflicting (21,23). In fact, the main focus of research until today was the exploration of the antiangiogenic, apoptosis stimulating, and immunomodulatory functions of recombinant fragments of TL1A, most of which have no defined counterpart in vivo. In a limited RNA profiling study comparing young and senescent HUVEC, we have found previously that TL1A expression was increased in senescent HUVEC (45); however, no in-depth analysis or functional studies were performed. This is the focus of the present study.
METHODS
Chemicals to Manipulate Ectodomain Shedding
The tumor necrosis factor-α protease inhibitor-1 (TAPI-1) is an ADAM17/10 (TACE) inhibitor (Cat. No. 579051; Calbiochem Merck Chemicals, Nottingham, UK). TAPI-1 was dissolved in DMSO to a final concentration of 10 mM. The end concentration of TAPI-1 in the culture medium was 10 or 20 μM. Stock solutions were stored at −20°C. Phorbol-12-myristat-13-acetate (PMA) (Sigma-Aldrich, Vienna, Austria) was dissolved in DMSO to a stock concentration of 10 μg/mL and stored at −20°C. To induce ectodomain shedding activity 10–50 ng/mL PMA in culture medium was used. H2O2 (Sigma H1009) was stored at 4°C as a 30% solution, and 1–5 mM H2O2 was used in experiments.
Cell Culture
The growth and maintenance of the 293FT cell line was performed like described in Invitrogen’s (Lofer, Austria) user manual (Cat. No. R700-07, WFGE08S). Circulating endothelial progenitor cells (CEP) were isolated from peripheral blood of healthy donors with written consent and maintained according to the methods described in (46). Cells were propagated in Endothelial Cell Growth Medium-2 (EGM-2, CC-3162; Lonza, Verviers, Belgium). HUVEC were isolated and maintained according to the methods described in (47). Cells were propagated in Endothelial Cell Growth Medium (EGM, CC-3124; Lonza). U-2OS cells were obtained from ATCC and propagated in Dulbecco’s modified Eagle’s medium (Sigma D5546) supplemented with 10% heat-inactivated fetal bovine serum, 4 mM L-glutamine (Gibco Invitrogen, Lofer, Austria), and 1% penicillin streptomycin (Gibco). For determining cell number and viability, the CASY Model DT was used from Innovatis AG (Reutlingen, Germany).
Cell Proliferation and Apoptosis
Cell proliferation was assayed using the 5-bromo-2′-deoxy-uridine labeling and detection kit I (Cat. No. 11296736001; Roche, Vienna, Austria) as described in the manufacturer’s manual. The cell nuclei were counterstained with 4′-6-diamidino-2-phenylindole (2 ng/mL) in DABCO mounting medium. Bound anti-bromodeoxyuridine monoclonal antibody was visualized by immunofluorescence microscopy, and the percentage of bromodeoxyuridine-stained nuclei was calculated.
For detection of apoptosis, the cells were detached, washed once with phosphate-buffered saline (PBS), and incubated with 100 μL Annexin V buffer containing 10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2 (pH 7.4) and 3 μL of Annexin V-fluoresceinisothiocyanate (Pharmingen BD Biosciences, Heidelberg, Germany) for 15 minutes at room temperature. After the incubation, 300 μL of Annexin V buffer was added and the cell suspension was measured by fluorescence activated cell sorting (FACS).
Tube Formation Assay
The tube formation assay for endothelial cells determines their ability to form a capillary-like tubular structure on a special extracellular matrix called Matrigel basement membrane (basement membrane matrix, growth factor reduced matrigel, Cat. No. 356231; BD Biosciences). This assay served an in vitro angiogenesis assay. Matrigel stored at −20°C was thawed at 4°C. Two hundred microliters of Matrigel was transferred in each well of a 24-well plate on ice using cold pipet tips. The plate was incubated at 37°C for 30 minutes, and 20,000 up to 40,000 cells were seeded with 300 μL medium. After 12 hours of incubation, branching points of the tubes were counted.
Lentiviral Overexpression and Knockdown of TL1A
As a transfer vector for overexpression of TL1A, the lentiviral pLenti6/UbC/V5-DEST Gateway vector (Invitrogen) was used. Cloning procedure involved the TOPO cloning of TL1A into pENTR/D-TOPO thereby the V5 Tag was removed by introduction of a stop codon after the coding sequence of TL1A (GenBank NM_005118). The resulting vector was used to introduce the TL1A coding sequence into pLenti6/UbC/V5-DEST by recombination to generate the transfer vector pLenti6/UbC/TL1A (see Invitrogen's ViraPower Lentiviral Expression System manual for further details). Control vector (Mock) was generated by removing the TL1A coding sequence from pLenti6/UbC/TL1A with EcoRV and religation of the lentiviral backbone. As a transfer vector for knockdown of TL1A, we used the lentiviral pLKO.1-TRC short-hairpin vector from Addgene/Open Biosystems (Epsom, UK). Following sequences within the TL1A, mRNAs were chosen as target: pLKO1 within the 3′ noncoding region of TL1A (5′-GAGGAGACTGAGTGATTAA-3′) and pLKO99 within the coding region (5′-GCCATGTTCTCCTTGCAAGAA-3′). The short hairpin RNA for pLKO1 was designed using the Dharmacon siDESIGN center (for cloning procedure, refer to the pLKO.1-TRC Cloning Vector manual from Addgene). Transfer vector pLKO99 was obtained from Open Biosystems. As a control the pLKO.1-TRC control vector (Addgene plasmid 10879) was used. For packaging of the lentivirus in 293FT cells (Invitrogen), the corresponding overexpression or knockdown transfer vector was used together with psPAX2 (Addgene plasmid 12260) and pMD2.G (Addgene plasmid 12259). 293FT cells were cultivated in T75 flasks to 90% confluence and transfected with a mixture of 3 μg of the transfer vector, 7.5 μg psPAX2 and 2.5 μg pMD2.G using Lipofectamine 2000 (Invitrogen). On the next day, the supernatant was exchanged with 10 mL of fresh 293FT growth medium. The supernatant was then harvested 48 hours later, centrifuged at 400g to get rid of cell debris, filtered through 0.45-μm filter (Millipore, Vienna, Austria), and stored at −80°C. The titer of lentiviral supernatant was determined by limiting dilution on 50,000 seeded U-2OS in six-well plates with 8 μg/mL hexadimethrine bromide (polybrene) as transduction enhancer. To produce 4 mg/mL stock solution, hexadimethrine bromide (Cat. No. 10,768-9; Sigma-Aldrich) was diluted in sterile water and filtered trough a 0.22-μm sterile filter. To avoid freeze/thaw cycles, only small aliquots were stored at −20°C. To select for transduced cells, 10 μg/mL blasticidin for overexpression and 200 ng/mL puromycin for knockdown virus was added 1 day after transduction. After 6 days of selection, the colonies were stained using crystal violet (1% in 10% ethanol). In general, titers for overexpression virus were about 1 × 106 transforming units per milliliter (TU/mL) and for knockdown virus 5 × 106 TU/mL. For transduction of HUVEC and CEP, a multiplicity of infection of 4–8 was used together with 8 μg/mL hexadimethrine bromide. Four multiplicity of infection was sufficient to transduce 100% of seeded HUVEC as assessed by fluorescence microscopy of green fluorescent protein–transduced cells.
Reverse Transcriptase–Polymerase Chain Reaction
For RT-PCR, the mRNA of cells was isolated using the RNeasy mini kit (Cat. No. 74104; Qiagen, Vienna, Austria) and reverse transcribed into cDNA using the transcriptor first-strand cDNA synthesis kit (Cat. No. 04896866001; Roche), following procedure A of the provided protocol with anchored oligo(dT)18 primers. The cDNA was then used as a template for a standard PCR followed by standard agarose gel analysis. Following isoform-specific primer pairs were used for the detection of TL1A (VEGI-251) and its isoforms: TL1A (VEGI-251) (fw 5′-TGCAGGACTCACCACATA-3′ rev 5′-CTTGGCTTATCTCCGTCT-3′); VEGI-174 (fw 5′-GCAAGTCTACAGTTTCCC-3′ rev 5′-TTCGGTTCTTGGTGAAGG-3′); VEGI-192 (fw 5′-TTCAGTCACCCTTTGTCTC-3′ rev 5′-AGGCCTAGTTCATGTTCC-3′).
Real-Time-quantitative-PCR
Isolation of mRNA and cDNA generation was performed like described previously. To quantify the amount of cDNA template, a real-time-quantitative-PCR (RTq-PCR) was established using isoform-specific primer pairs (see previously) and SYBR. For relative quantification, an efficiency-corrected calculation model of the Δ-Δ CT method was used (48). For normalization of the isoform-specific RTq-PCR, levels of β-2 microglobulin were quantified in parallel.
Microarray Hybridization and Evaluation
To prepare RNA for cDNA microarray hybridization, the RNeasy Mini Kit (Cat. No. 74104; Qiagen) was used. For hybridization, the human genome U133 plus 2.0 arrays from Affymetrix (High Wycombe, UK) were used. The microarrays were hybridized and analyzed by the microarray facility (Microarray Facility Tübingen, Tübingen, Germany, www.microarray-facility.com). A compact disc containing the image analysis of the microarray experiment with the raw data was provided by the Microarray Facility. These raw data sets were prepared for analysis using CARMAweb (Institute for Genomics and Bioinformatics, Graz, Austria, https://carmaweb.genome.tugraz.at/carma) (49). For normalization, the algorithm “gcrma” was used.
Deglycosylation of TL1A
In order to detect N- and O-linked oligosaccharides in TL1A, the glycoprotein deglycosylation kit from Calbiochem Merck Chemicals (Cat. No. 362280) was used. According to the manufacturer’s manual, cell lysate or supernatant of TL1A overexpressing U-2OS together with supplied reaction buffer and denaturation solution was heated at 100°C for 5 minutes. After cooling to room temperature, Triton X-100 detergent solution was added. N-glycosidase F was added to cleave N-glycans. To cleave N- and O-linked oligosaccharides, N-glycosidase F, α2-3,6,8,9-neuraminidase, endo-α-N-acetylgalactosaminidase, β-1,4-galactosidase, and β-N-acetylglucosaminidase were added sequentially. Reactions were incubated for 3 hours at 37°C. The shift in molecular mass by deglycosylation was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Western blotting.
Western Blot of Cell Lysates and Supernatants
TL1A-transfected U-2OS cells were grown in six-well plates to about 80%–100% confluency. To produce cell supernatants, the cells were washed three times with PBS and incubated with serum-free Dulbecco’s modified Eagle’s medium for 5 hours to get rid of the residual fetal bovine serum. Then, the medium was exchanged with 800 μL per well fresh serum-free Dulbecco’s modified Eagle’s medium containing 1% penicillin streptomycin and 4 mM L-glutamine. Depending on the experiment after 2–48 hours, the supernatants were harvested, centrifuged at 1000g for 5 minutes to get rid of cell debris and filtered through 0.45-μm filter (Millipore). If the concentration of TL1A in the supernatant was too low to detect by Western blot, it was concentrated about eightfold by speed-vac evaporation. The resulting supernatant was mixed with sodium dodecyl sulfate protein buffer containing 300 mM Tris–HCl pH 6.8, 500 mM dithiothreitol, 10% sodium dodecyl sulfate, 0.5% bromphenol blue, and 50% glycerin. Lysis buffer contained 50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate, 0.5% Na-deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 10 μg/mL aprotinin, and 10 μg/mL leupeptin (pH 7.5). Cell lysates and supernatants were assayed by standard Western blot protocol using primary goat anti-human TL1A/TNFSF15 antibody (Cat. No. AF744; R&D Systems, Biomedica, Vienna, Austria) and mouse β-Tubulin antibody (Sigma). Horseradish peroxidase–conjugated secondary antibodies were purchased from Dako, Vienna, Austria.
AMAXA Electroporation
To introduce expression plasmids into HUVEC, the HUVEC nucleofector kit (Cat. No. VPB-1002) from AMAXA, Lonza, Verviers, Belgium was used. The method was performed like described in the manufacturer’s protocol.
Staining for Senescence-Associated β-Galactosidase
The senescent status was verified by in situ staining for senescence-associated β-galactosidase (SA-β-gal) as described (4). Cells were grown on six-well cell culture dishes, washed three times with PBS, and fixed with 2% formaldehyde, 0.2% glutaraldehyde in PBS for 5 minutes. After another washing step with PBS, the cells were incubated with β-galactosidase staining solution (150 mM NaCl, 2 mM MgCl, 2.5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 40 mM citric acid, 12 mM sodium phosphate, pH 6.0, adding 1 mg/mL 5-bromo-4-chloro-3-indolyl-b-D-galactoside [X-gal] directly before staining of the cells) for 24 hours at 37°C. The reaction was stopped by washing the cells with PBS.
Expression and Purification of TL1A Fragments From Escherichia coli
For expression of recombinant TL1A in Escherichia coli, the pET-3a vector from Novagen (Merck; Cat. No. 69418-3) was used. For directional cloning, forward primers were designed that harbor an NdeI restriction site and reverse primers with a BamHI site. For TL1AL72-L251, the primer pair (fw 5′-TATACATATGCTGCACTGGGAACATGAACTA-3′) and (rev 5′-TATAGGATCCCTATAGTAAGAAGGCTCCAAAGAAGGT-3′) was used. For TL1AV84-L251, (fw 5′-TATACATATGGTTTATGCACCTCTTAGAGCAGA-3′) in combination with the above-mentioned reverse primer was used. After PCR using full-length TL1A vector as a template, the amplified fragments were cloned into the NdeI and BamHI site of the pET-3a vector. TL1A insert sequences were verified by DNA sequencing. Note that in the resulting recombinant protein, there will be a methionine at the beginning of the TL1A fragment but no T7 Tag. For recombinant protein expression, the BL21(DE3) (Novagen) E coli strain was transformed with the respective TL1A overexpression constructs, selected on LB plates containing 100 μg/mL ampicillin and 25 μg/mL chloramphenicol and incubated at 37°C. Twenty milliliters of LB medium containing antibiotics as described previously was inoculated with a single colony from the LB plate and incubated at 225 rpm/37°C overnight. Two milliliters of the overnight culture was centrifuged and resuspended in 200 mL of NZCYM medium (10 g NZ amine, 5 g NaCl, 5 g yeast extract, 1 g casamino acids, 2 g MgSO4×7H2O, H2O ad 950 mL, adjusted to pH 7.4 with NaOH and filled to 1000 mL) containing ampicillin (100 μg/mL) and chloramphenicol (25 μg/mL). The flask was incubated at 225 rpm/37°C until the optical density at 600 nm reached 0.5, then expression was induced by adding isopropyl-β-D-thiogalactopyranoside to a final concentration of 0.4 mM. After 3 hours of shaking at 225 rpm/37°C, bacteria were collected by centrifugation. TL1A inclusion bodies were isolated by lysis of the bacterial cells and washing thoroughly using inclusion body wash buffer containing 25 mL of 1 M Tris–HCl pH 8, 2 mL of 0.5 M EDTA pH 8, 0.35 μL/mL β-mercaptoethanol, 25 mL glycerol, 0.25 g sodium deoxycholic acid, 5 mL igepal CA-630 (NP40) filled to 500 mL with H2O. Inclusion bodies were solubilized in 5 mL of 8 M urea and loaded on a 5 mL Ni2+ HiTrap Chelating HP column (GE Healthcare, Vienna, Austria) that was equilibrated with 8 M urea. TL1A was slowly refolded on the column with a linear PBS gradient at a flow rate of 1.5 mL/min (40 bed volumes). Refolded TL1A was eluted with a linear imidazole gradient of 0–100 mM (10 bed volumes) at a flow rate of 1.5 mL/min. Typically, the fragments eluted at about 37 mM imidazole. TL1A containing fractions were pooled and dialyzed against PBS. Concentration of TL1A protein was assessed by measurement at optical density of 280 nm.
Treatment and Analysis of PBMC
Peripheral blood samples were taken from three healthy volunteers. Informed written consent was obtained from all participants, and the blood collection was approved by the Ethics Committee of the Innsbruck Medical University. PBMC were purified by Ficoll-Hypaque density gradient centrifugation (Amersham Biosciences, Vienna, Austria). About 0.5 Mio PBMC were incubated in 48-well plates for 3 days with IL-12 (10 ng/mL), IL-18 (50 ng/mL), IL-15 (50 ng/mL), and/or TL1A fragments (50 μg/mL). Brefeldin A (10 μg/mL) was added for the last 12 hours. PBMC were washed with PBS and stained for 30 minutes at 4–8°C using the conjugated antibodies anti-CD3 (APC-Cy7) and anti-CD8 (PerCP; all BD Biosciences). After washing with PBS, PBMC were resuspended in Cytofix/Cytoperm (BD Biosciences) for 20 minutes at 4–8°C. Cells were resuspended in Cytowash buffer and stained with anti-interferon (IFN)-γ (fluoresceinisothiocyanate; BD Biosciences) for 30 minutes at 4–8°C. After a washing step with Cytowash buffer, PBMC were resuspended in PBS and analyzed on a FACSCanto II flow cytometer and FACSDiva Software (BD Biosciences). NK cells were defined within the lymphocyte gate as CD3−CD8αdim.
RESULTS
TL1A Is the Main Isoform in HUVEC
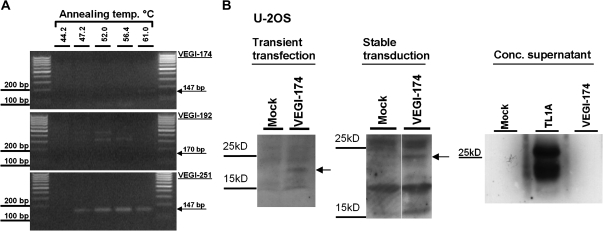
In addition to canonical TL1A (VEGI-251), two isoforms, referred to as VEGI-174 and VEGI-192, respectively, were described that are believed to be generated by differential splicing (23). To analyze the major isoforms in HUVEC, mRNA was isolated and reverse transcribed. About 250 ng cDNA was then used to perform a gradient RTq-PCR with isoform-specific primers and 35 amplification cycles (Figure 1A). A faint band was obtained at 61°C annealing temperature for the VEGI-174 isoform, whereas no specific band (170 base pairs) was detected for VEGI-192. With a distinct band of the expected size (147 base pairs), VEGI-251 was the main isoform in HUVEC. There was no unspecific amplification or contamination of the PCR reaction (data not shown).
Figure 1.
Abundance of tumor necrosis factor–like cytokine 1A (TL1A) and isoforms in human umbilical vein endothelial cells (HUVEC) and expression of vascular endothelial growth inhibitor (VEGI)-174 in U-2OS. (A) cDNA of senescent HUVEC was used to perform a gradient RT-PCR using isoform-specific primers for VEGI-174, VEGI-192, and TL1A (VEGI-251). The annealing temperature gradient reached from 44.2 to 61°C. After 35 amplification cycles, polymerase chain reaction (PCR) products were subjected to agarose gel analysis. The expected size of PCR product is indicated for each isoform. (B) U-2OS cells were transiently transfected (left) or stably transduced (middle) with a eukaryotic overexpression vector (pcDNA3) or a lentiviral vector encoding VEGI-174 cDNA, respectively. Empty vector (Mock) was used as a control. After 6 days, cell extracts were analyzed by Western blot using polyclonal antibodies specific for TL1A. This antibody was raised against the C-terminal part of TL1A and therefore should also be able to detect VEGI-174. The concentrated supernatant of VEGI-174 and TL1A (VEGI-251) transduced cells was also subjected to Western blotting (right).
The VEGI-174 mRNA was nearly undetectable in HUVEC and CEP, consistent with previous reports that VEGI-174 might be a cloning artifact (21). To address this point, total RNA from human placenta was reverse transcribed, whereby VEGI-174 cDNA was readily detected after 30 amplification cycles (data not shown). The identity of VEGI-174 was verified by sequencing, and a lentiviral vector for the overexpression of VEGI-174 was generated. To monitor protein products resulting from the expression of VEGI-174, U-2OS cells were transiently transfected with empty vector (Mock) or pcDNA3.1 expressing VEGI-174 and analyzed by Western blotting (Figure 1B, left). The same strategy was applied for lentiviral expression of VEGI-174 (Figure 1B, middle). With both expression methods, a relatively weak protein band of an apparent molecular weight (MW) of 22 kDa was observed, which probably corresponds to VEGI-174. When the supernatant of U-2OS cells overexpressing VEGI-174 was examined by Western blot using TL1A antibodies, no immunoreactive fragment was detected, whereas a fragment corresponding to TL1AL72-L251 was readily detected in cellular supernatants after overexpression of TL1A/VEGI-251 (Figure 1B, right). Based on these findings, VEGI-174 was not further investigated.
Transmembrane Location and Ectodomain Shedding of TL1A
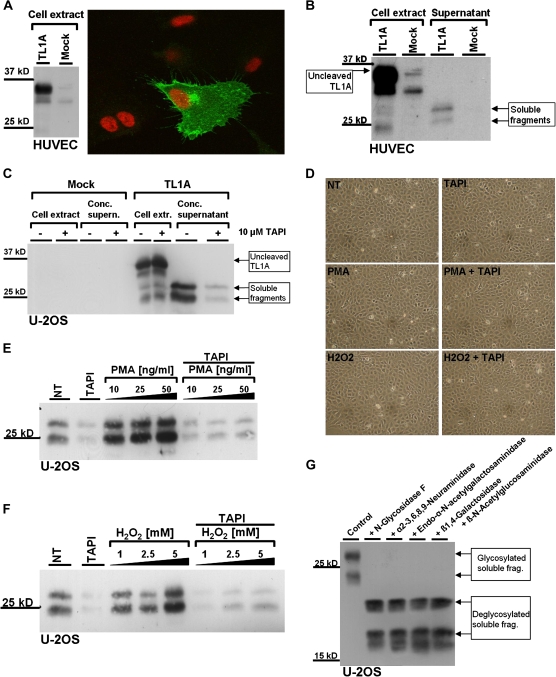
To determine the subcellular localization of TL1A in endothelial cells, HUVEC were transiently transfected by AMAXA with a TL1A expression vector (pcDNA3.1/TL1A). Expression of TL1A was verified by Western blot (Figure 2A, left). Subcellular localization of TL1A was probed by indirect immunofluorescence using two fixation methods (methanol/acetone and paraformaldehyde). Both fixation methods revealed the accumulation of TL1A at the plasma membrane, with additional staining of a perinuclear structure, presumably the Golgi apparatus (Figure 2A, right). It is known that the extracellular part of some transmembrane proteins, in particular the ligands of the TNF family, is proteolytically cleaved and thereby secreted into the extracellular space (50), a process called ectodomain shedding. To test whether such mechanism applies to TL1A, U-2OS cells and HUVEC were transiently transfected with pcDNA3.1/TL1A. Twenty-four hours after transfection, cells were washed and the medium was replaced. After incubation for an additional 24 hours, cell extract and supernatants were prepared and analyzed by Western blot (Figure 2B and C). In cell extracts, a main protein species with an apparent MW of 36 kDa was detected, along with several bands of lower MW. Two bands of about 30 and 25 kDa were detected in the supernatant of transfected cells. This observation implies that the 36-kDa band in the cell extract represents the uncleaved transmembrane form of TL1A, which is converted to smaller extracellular fragments. This process, referred to as ectodomain shedding, usually involves enzymes of the ADAM (A Disintegrin And Metalloprotease) family (51), and many members of the TNF receptor family are proteolytically cleaved by TACE (TNF-α converting enzyme/ADAM17). To test whether ectodomain shedding of TL1A involves ADAM metalloproteases, cells were treated with TAPI-1, a specific inhibitor of ADAM17. The shedding assay was validated by showing the inhibition of Prion protein (PrP) shedding, which depends on ADAM17 (52). An 11.5 kDa fragment of PrP referred to as N1 was detected in the supernatant of U-2OS cells overexpressing PrP, and the generation of this fragment was successfully inhibited by the addition of 10 μM TAPI-1 (data not shown).
Figure 2.
Ectodomain shedding of transmembrane tumor necrosis factor–like cytokine 1A (TL1A) in human umbilical vein endothelial cells (HUVEC) and U-2OS cells. (A) HUVEC were transfected with TL1A cDNA or empty vector (Mock) by AMAXA electroporation, immunostained for TL1A, and examined by confocal fluorescence microscopy. Nuclei were stained with TO-PRO 3 (right). Expression of TL1A was verified by Western blot (left). (B) HUVEC were transfected with either empty vector (Mock) or a TL1A overexpression vector by AMAXA electroporation. HUVEC cell extracts and supernatant were examined by Western blot using antibodies specific for TL1A. (C) U-2OS cells were transiently transfected with TL1A cDNA and Mock using Lipofectamine 2000. After antibiotic selection for transfected cells, they were seeded at a density of 3 × 105 per well in a six-well plate. Ten micromolars of TAPI-1 and the equivalent volume of DMSO as a control were added as indicated and incubated for 24 hours. Cell extracts and speed-vac concentrated supernatants were examined by Western blot using anti-TL1A antibody. (D) U-2OS cells were treated for 2 hours with 50 ng/mL phorbol-12-myristat-13-acetate (PMA) and 5 mM H2O2, as indicated. Micrographs of treated cells are shown. (E and F) U-2OS cells were transfected with TL1A cDNA and seeded as described previously. Cells were either untreated (NT) or treated with increasing concentrations of PMA (E) or H2O2 (F) in the presence or absence of TAPI-1 (20 μM), as indicated. After 2 hours, supernatants were harvested, concentrated by speed-vac evaporation, and subjected to Western blot analysis. (G) Supernatant of U-2OS cells overexpressing TL1A was concentrated via speed-vac evaporation and treated with the indicated enzymes to remove all N- and O-linked oligosaccharides. The enzymes were added sequentially, in a cumulative manner, so that in the end all five enzymes are added to the reaction (from left to right on the indicated Western blot). TL1A fragments were separated on a large sodium dodecyl sulfate–polyacrylamide gel electrophoresis for higher resolution and analyzed by Western blot using anti-TL1A antibody.
The amount of soluble fragments in the supernatant of cells overexpressing TL1A was strongly reduced by the addition of 10 μM TAPI-1 (Figure 2C), indicating that the inhibition of ADAMs reduces the constitutive release of soluble TL1A in U-2OS cells. It is known that PMA can stimulate the shedding activity of cells (53) and that ROS production is involved in PMA-induced ectodomain shedding (54). To address the question if TL1A ectodomain shedding can also be activated via this pathway, cells were treated for 2 hours with PMA and H2O2, respectively. Whereas both treatments did not induce visible differences in cell size or cell number (Figure 2D), we observed enhanced TL1A ectodomain shedding when cells were treated with PMA (Figure 2E) or H2O2 (Figure 2F).
Two major bands with apparent MW of 30 and 25 kDa were found in the supernatant of cells expressing TL1A. It is known that both secreted proteins and membrane proteins (facing toward the extracellular space) are subject to glycosylation. Accordingly, the TL1A fragments observed in conditioned media may result from differential glycosylation, as was suggested by others before (23). Prediction of N-glycosylation sites using bioinformatics tools indicated two potential glycosylation sites at N133 and N229 of the full-length TL1A sequence (55). To test this possibility, supernatant of TL1A overexpressing cells was treated with N-glycosidase F to remove N-linked saccharides, which shifted both fragments toward lower MW (Figure 2G). As a result of extended glycosidase treatment, a third fragment of further reduced MW was observed in some experiments, which may result from proteolytic degradation; this was not further investigated. The addition of four additional enzymes, which would remove possible O-linked saccharides, had no significant impact on the apparent MW of the TL1A fragments (Figure 2G), suggesting that the extracellular soluble fragments of TL1A are N-glycosylated but not O-glycosylated. There seems to be no differential posttranslational modification of the two TL1A fragments, which indicates that both fragments most likely differ by their amino acid composition.
Role of TL1A in CEP Senescence
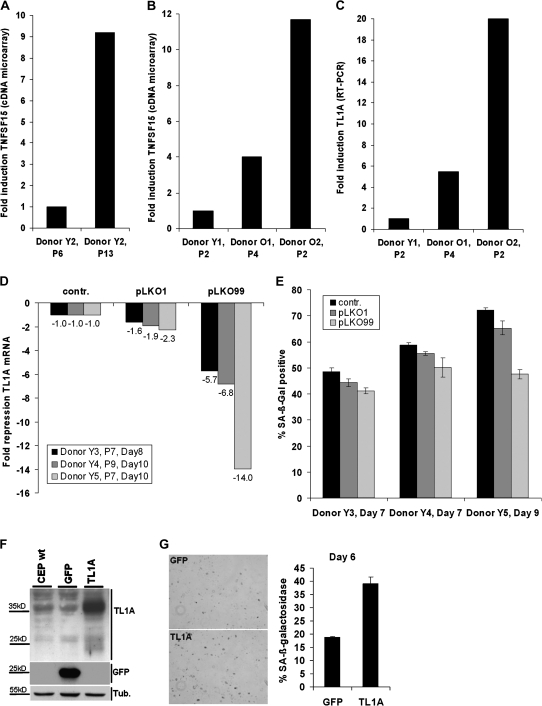
To investigate the regulation of TL1A during senescence of human endothelial cells, human CEP were isolated from peripheral blood of young and old human donors. To approve their endothelial identity, CD31, an adhesion molecule expressed mainly on endothelial cells, and von Willebrand factor, another endothelial cell–specific molecule, were stained by indirect immunofluorescence and evaluated by fluorescence microscopy. The isolated CEP were positive for von Willebrand and CD31, which supports their endothelial nature (data not shown). CEP isolated from a young donor (Y2) were serially passaged until they reached senescence, as monitored by positive staining for SA-β-gal. According to the established protocol, CEP cultures are derived from single or very few cells, which emerged from the selection procedure, selecting cells for growth in endothelial growth medium and adherence to collagen-coated dishes. These colonies derive from one single cell or at best a few cells. Senescence experiments were started when about a million cells were available. This means that at the beginning of the experiments the cells already underwent roughly 18 population doublings on average. From this point, cells could be grown for another five to six passages before they reached senescence. Senescence was defined as the point, when more than 80% of the cells were positive for staining with SA-β-gal. Total mRNA was isolated from CEP at early and late passage and subjected to cDNA microarray analysis using Affymetrix microarray HG-U133Plus2.0, revealing differential regulation of about 1000 genes in cellular senescence (data not shown). TL1A mRNA was upregulated ninefold in senescent CEP (Figure 3A). To investigate if regulation of TL1A by replicative senescence is also reflected in vivo, TL1A expression levels of CEP isolated from two old donors (O1 and O2) were compared with levels of young donor 1 (Y1). A 4- to 12-fold upregulation of TL1A in CEP derived from old donors was observed (Figure 3B). Regulation of TL1A gene expression shown in Figure 3B was confirmed by RTq-PCR with TL1A isoform-specific primers (Figure 3C).
Figure 3.
Upregulation of tumor necrosis factor–like cytokine 1A (TL1A) messenger RNA (mRNA) in replicative senescent circulating endothelial progenitor cells (CEP) as well as in elderly donors. (A) CEP of a young donor (Y2 = 35 years) were serially passaged until they reached senescence. mRNA was prepared and subjected to cDNA microarray analysis. Microarray results were normalized, and the regulation for TNFSF15 probe set (TL1A) is shown. (B) In addition, CEP of young (Y1 = 30 years) and old donors (O1 = 70 years and O2 = 79 years) were isolated and analyzed by cDNA microarray. (C) The mRNA expression observed by cDNA microarray analysis was confirmed by real-time-quantitative-PCR (RTq-PCR) using primers specific for the detection of TL1A. Results are shown as fold change compared with “young” CEP regarding replicative age or donor age, respectively. (D) CEP derived from three young donors (Y3, Y4, and Y5) were transduced with either control lentivirus or three lentiviral knockdown vectors targeting TL1A (pLKO1, pLKO99). RTq-PCR showing the lentiviral knockdown of TL1A in CEP was performed 8 and 10 days after transduction at the indicated passage numbers. Results are shown as fold change compared with control. (E) Quantification of senescence-associated β-galactosidase (SA-β-gal)–positive cells after knockdown of TL1A. CEP of young donors (Y3, Y4 and Y5) were fixed and stained for SA-β-gal 7 or 9 days after transduction. Phase contrast microscopic pictures were taken at 400× magnification. The percentage of SA-β-gal–positive cells was calculated by counting about 1,500 cells per experiment. (F) CEP were transduced with lentiviral vectors expressing green fluorescent protein (GFP) or TL1A, and cell extracts were subjected to Western blot using GFP or TL1A-specific antibodies, respectively. Wild-type CEP were used as a control. (G) Six days after transduction, cells were fixed and stained for SA-β-gal. Pictures are representative of at least three experiments.
The amount of TL1A and the VEGI-174 isoform in senescent CEP was tested by isoform-specific real-time-quantitative PCR. As a measure of the template starting amount in the PCR reaction, the threshold cycle (ct value) was analyzed. The ct values for TL1A in three senescent CEP cDNA samples were 27.3 ± 1.1, suggesting a significant amount of TL1A transcript in senescent CEP. The same cDNA used with isoform-specific primers for VEGI-174 showed ct values of 34.9 ± 1.6, which is at the limit of detection. For normalization of the isoform-specific RTq-PCR, levels of β-2 microglobulin were quantified in parallel, showing a ct value of 19.54 ± 0.0 (data not shown). These results confirm that the dominant isoform in senescent CEP is TL1A. Detection of isoform VEGI-192 was not attempted because it is reported to be even less abundant than VEGI-174 (23).
To address a potential contribution of TL1A to CEP senescence, lentiviral knockdown of TL1A was performed in CEP from three different young donors with knockdown constructs targeting two different regions of the TL1A mRNA. CEP from donors Y3, Y4, and Y5 were transduced with 8 multiplicity of infection. CEP isolated from peripheral blood generally reach senescence quite early because of extensive proliferation at the beginning of the culture (46), that is, in passage 10–13, and CEP in passage 7 therefore represent CEP that are already near senescence. Figure 3D shows the efficiency of the two knockdown constructs to downregulate endogenous TL1A versus control. TL1A knockdown cells were stained for SA-β-gal 7 and 9 days after transduction, and the amount of stained cells was quantified (Figure 3E). For both TL1A-specific shRNAs, the amount of SA-β-gal–positive cells was decreased compared with control.
The role of TL1A in senescence of CEP was also studied by lentiviral overexpression of TL1A. Overexpression of TL1A was performed in early passage cells from a young donor with green fluorescent protein as a control and confirmed by Western blot (Figure 3F). Six days after transduction, CEP were stained for SA-β-gal, and the amount of SA-β-gal–positive cells was quantified (Figure 3G). TL1A overexpression increased the amount of SA-β-gal–positive cells by about 20% compared with CEP overexpressing green fluorescent protein.
Effects of TL1A Expression in HUVEC
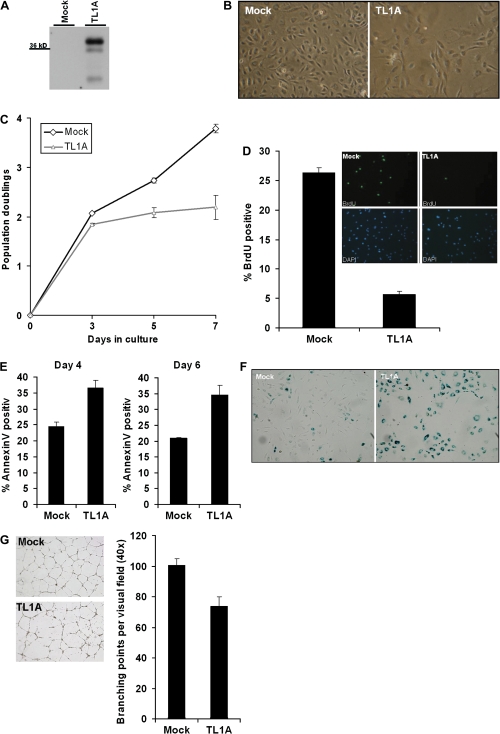
The ability of TL1A to influence the senescence response in young HUVEC was tested by overexpression. To this end, a lentiviral system was established to efficiently transduce primary cells with low toxicity. Young HUVEC in passage 7 were transduced with five multiplicity of infection of empty vector (Mock) and TL1A overexpression vector, respectively. Figure 4A shows the overexpression of TL1A 7 days after transduction of young HUVEC, evaluated by TL1A-specific Western blot. Investigating the cells by phase contrast microscopy 6 days after transduction revealed diminished cell numbers and an enlarged phenotype of HUVEC overexpressing TL1A compared with Mock (Figure 4B). Three days after transduction, TL1A-transduced HUVEC stopped proliferation (Figure 4C). To investigate the amount of cells in S phase, cells were incubated with bromodeoxyuridine for 1.5 hours and stained for incorporation. TL1A overexpression severely reduced the amount of cells in S phase (Figure 4D). Annexin V staining of Mock and TL1A-transduced cells 4 and 6 days after transduction shows an increase in apoptosis of TL1A overexpressing cells (Figure 4E). The earlier observations are typical signs for the induction of a senescent-like phenotype in HUVEC (56) by TL1A overexpression. To further confirm this effect, cells were stained for SA-β-gal 7 days after transduction, and staining was documented by phase contrast microscopy (Figure 4F). This experiment revealed a strong increase in staining for SA-β-gal in TL1A overexpressing cells. The ability of endothelial cells to differentiate on Matrigel to form tubes within 24 hours is a common assay for angiogenesis (57). Cells were placed on Matrigel incubated over night and then the branching points per visual field were assessed. Figure 4G shows a diminished rate of tube formation of HUVEC overexpressing TL1A.
Figure 4.
Phenotypic consequences of lentiviral tumor necrosis factor–like cytokine 1A (TL1A) overexpression in young human umbilical vein endothelial cells (HUVEC). (A) Lentiviral overexpression of TL1A was confirmed 7 days after transduction of young HUVEC in passage 7 by Western blot using antibodies specific for TL1A. Lentiviral empty vector (Mock) was used as a control. (B) Six days after transduction, morphology of transduced cells was observed by phase contrast microscopy. (C) Population doubling time was assessed via CASY cell counting. (D) Six days after transduction, the amount of cells in S phase was determined using a bromodeoxyuridine assay. 4′-6-diamidino-2-phenylindole was used to stain cell nuclei. Pictures shown are representative of at least three experiments. (E) Four and six days after lentiviral transduction of young HUVEC with either empty vector (Mock) or TL1A, the cells were stained with Annexin V and monitored for apoptosis by FACS. (F) Seven days after lentiviral transduction of HUVEC with either empty vector (Mock) or TL1A, cells were fixed and stained for senescence-associated β-galactosidase (SA-β-gal). Pictures are representative of at least three experiments. (G) Five days after lentiviral transduction of young HUVEC with either empty vector (Mock) or TL1A, 5 × 104 cells each were seeded on 300 μL Matrigel in a 24-well plate. On the next day, tube formation was documented by phase contrast microscopy. Three experiments were performed and representative pictures are shown.
Differential Induction of Endothelial Cell Apoptosis by TL1A Fragments
When young HUVEC were incubated with conditioned medium from TL1A expressing U-2OS cells, we observed a slight increase in the rate of apoptosis and a reduction in the number of viable cells (data not shown), suggesting a potential contribution of extracellular TL1A fragments to the proapoptotic phenotype obtained by ectopic expression of TL1A (see previously, Figure 4E). However, the detectable concentration of TL1A in conditioned medium was very low (less than 0.1 ng/mL; data not shown), which may limit the efficiency of the conditioned medium. The low TL1A concentration in conditioned media also prevented our attempts to purify TL1A fragments from conditioned medium.
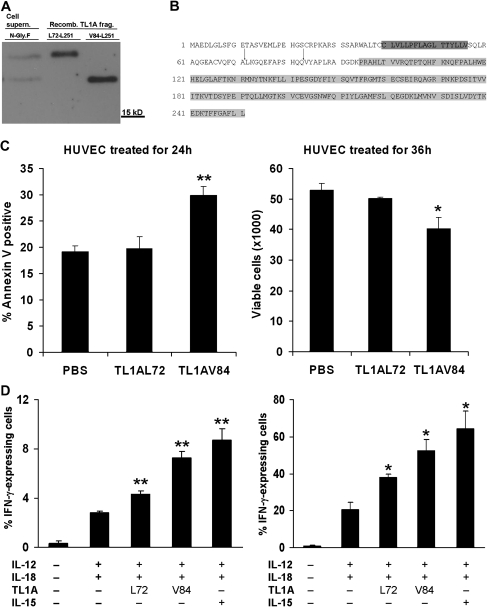
In an alternative strategy to test the role of extracellular TL1A fragments, TL1A fragments of the predicted size were produced in E coli. Fragments were designed according to the already described TL1A fragment TL1AL72-L251 (21), and a second fragment corresponding to the shorter TL1A fragment obtained after complete deglycosylation (Figure 5A). Taking into account, the MW difference between both fragments, the shorter fragment, referred to as TL1AV84-L251, was designed to start at amino acid 84 of TL1A. Recombinant TL1A fragments were run side by side with deglycosylated TL1A fragments from conditioned medium and analyzed by Western blot. As predicted, the larger TL1A fragment from conditioned media comigrated with recombinant TL1AL72-L251 (Figure 5A), whereas the smaller TL1A fragment comigrated with recombinant TL1AV84-L251 (Figure 5A), suggesting the occurrence of one additional ADAM cleavage site between Q83 and V84 of full-length TL1A. Both the canonical and the newly identified cleavage site are located between the transmembrane region of TL1A and the TNF-homology domain (Figure 5B).
Figure 5.
Identity and biologic activity of tumor necrosis factor (TNF)–like cytokine 1A (TL1A) fragments. (A) Concentrated cell supernatant of TL1A overexpressing U-2OS cells was deglycolysed with N-glycosidase F and compared with the molecular weight of Escherichia coli recombinant TL1A fragments TL1AL72-L251 and TL1AV84-L251 by Western blot using TL1A-specific antibody. (B) Sites for ADAM-mediated cleavage of TL1A. Cleavage between alanine71 and leucine72 (indicated by an arrow) would lead to the described TL1AL72-L251 (21). The newly described TL1AV84-L251 would be cleaved between glutamine83 and valine84. Putative transmembrane region is marked in dark grey. TNF-homology domain is marked in light grey. Both domains were predicted by the Human Protein Reference Database. (C) About 50,000 young human umbilical vein endothelial cells (HUVEC) in passage 7 were seeded in six-wells treated with 20 μg of TL1AL72-L251 or TL1AV84-L251 for 24 or 36 hours and subjected to Annexin V FACS (left) and cell counting (right), respectively. *p < .05 and **p < .01, phosphate-buffered saline (PBS) versus TL1AV84 (Student’s t test; n = 3). (D) TL1A fragments induced interferon (IFN)-γ production in human peripheral blood mononuclear cells (PBMC). (Left) Percentage of IFN-γ-expressing PBMC. (Right) Percentage of IFN-γ-expressing natural killer cells. *p < .05 and **p < .01, IL-12+IL-18 versus L72, V84 and IL-15 (Student’s t test; n = 3).
Whereas we show in this communication that TL1A is a transmembrane protein that releases two soluble ectodomain fragments by shedding (Figure 2), it was reported in the literature that recombinant TL1AL72-L251 showed no effect on HUVEC proliferation or apoptosis but had strong effects on cells of the immune system like PBMCs (21,58). Conversely, shorter recombinant fragments showed proapoptotic properties on endothelial cells (59,60). To test if the two soluble TL1A fragments identified in this communication have divergent biologic effects on HUVEC and PBMCs, recombinant fragments were incubated with HUVEC and PBMC, respectively. TL1AV84-L251 but not TL1AL72-L251 induced apoptosis in HUVEC after 24 hours of treatment, as assessed by Annexin V FACS (Figure 5C, left) and strongly reduced cell viability after 36 hours of treatment as assayed by CASY cell counting (Figure 5C, right). In PBMCs, TL1AV84-L251 was more effective than TL1AL72-L251 to induce IFN-γ secretion after stimulation with IL-12/IL-18 (Figure 5D, left). Within the PBMC pool, the NK cells was the predominant population of cells that produced IFN-γ upon TL1A fragment treatment (Figure 5D, right). TL1AV84-L251 was just as effective in inducing IFN-γ secretion as IL-15.
DISCUSSION
In this study, we clarified the role of TL1A and its suspected splice variants in human endothelial cells. We formally showed that full-length TL1A is inserted into the cell membrane and provide new data suggesting that two extracellular TL1A fragments with nonidentical functions are generated by differential ectodomain shedding. We found that the expression of TL1A increased with donor age in CEP, and upregulation of TL1A was also found with in vitro senescence of CEP. To study the role of TL1A in senescence and apoptosis of endothelial cells, we established a lentiviral system to overexpress and knockdown TL1A in HUVEC and CEP and found that TL1A induces cellular senescence in both model systems. Together, these observations establish TL1A as a novel regulator of cellular senescence in human endothelial cells.
TL1A/VEGI-251 is the Predominant RNA Species in Endothelial Cells
Three cDNAs derived from the VEGI/TL1A gene have been described, namely VEGI-174, VEGI-192, and VEGI-251, the latter being also referred to as TL1A. These cDNA species differed in their N-terminal region and therefore can be distinguished by PCR with isoform-specific primer pairs. TL1A was found to be quantitatively by far the main isoform in both HUVEC and CEP, whereas levels of both VEGI-174 and VEGI-192 were very low in HUVEC, in agreement with other studies (23), and in CEP.
Initially, VEGI-174 had been described as the principal full-length gene product (19), and high amounts of VEGI-174 were reported in placenta mRNA (23), suggesting that it might be an authentic form of VEGI RNA. Indeed, we could readily isolate and clone a VEGI-174 DNA fragment from a commercially available placenta mRNA (data not shown). However, the placenta mRNA had been isolated with a modified guanidinium thiocyanate method (61), which is known to yield considerable amounts of genomic DNA contamination (62). BLASTing the putative mRNA of VEGI-174 (GenBank AF039390) against the human genome revealed that the putative mRNA is coded by a continuous genomic region (data not shown). Therefore, it is highly likely that the VEGI-174 DNA clone derived from genomic DNA rather than cDNA.
TL1A is a Transmembrane Protein Subjected to Differential Ectodomain Shedding by ADAM Metalloproteases
The subcellular localization of the uncleaved TL1A precursor protein was not known before our study. The hydrophobicity analysis of TL1A shows a typical N-terminal peak indicating hydrophobic amino acids that can be inserted into the plasma membrane (data not shown). We show here that TL1A localizes to the cell membrane and the Golgi apparatus, essential for secretion or for insertion into the membrane (63). Accordingly, a prominent TL1A protein species of 36 kDa was obtained in cell extracts, probably representing the membrane inserted uncleaved TL1A. Conflicting results were reported concerning the localization and mode of secretion of TL1A. TL1A was predicted to be secreted because the hydrophobic N-terminus was interpreted as a secretion signal (23), whereas others used FACS analysis of antibody decorated cells to conclude that TL1A is a transmembrane protein, in line with known properties of other TNF ligands (21,35). Data shown here clearly support the latter explanation and identify TL1A as a transmembrane protein anchored into the plasma membrane with its N-terminus, a characteristic feature of type II transmembrane proteins. The data shown here highlight the involvement of ADAM metalloproteases in the proteolytic cleavage of transmembrane TL1A for the first time.
Processing of TL1A into secreted protein fragments was described before, and the N-terminal amino acid of one such fragment was determined as L72 (21), which was subsequently referred to as TLAL72-L251. TLAL72-L251 was reported as the only secreted form of TL1A and recombinant protein produced in E coli was used to demonstrate its biologic effects on PBMCs and T cells leading to a proinflammatory response (21,58). In the current study, two distinct fragments of TL1A were obtained in the supernatant of U-2OS and HUVEC after TL1A expression, whereas other TNF ligands are generally reported to release just one distinct fragment. Two potential N-glycosylation sites were predicted at N133 and N229 of TL1A (55), raising the possibility that altered glycosylation patterns were responsible for differences in the apparent MW of the fragments (23). This possibility could be excluded by our studies because after removing all N- and O-bound saccharides, two fragments with different MW (20 and 25 kDa) remained. Because the formation of both fragments was inhibited to the same extend by TAPI-1, it seems that two distinct fragments are produced from TL1A by differential ectodomain shedding. Data shown here suggest the occurrence of an additional cleavage site for TL1A located between Q83 and V84 of the full-length protein. Both cleavage sites are located between the transmembrane region of TL1A and the TNF-homology domain (Figure 5B) as expected. Data shown here support the novel concept that membrane inserted TL1A is differentially processed, presumably via ectodomain shedding at two different cleavage sites, resulting in two soluble TL1A fragments.
TL1A: A Novel Regulator of Cellular Senescence in Human Endothelial Cells
TL1A mRNA was upregulated in human CEP from old versus young human volunteers (Figure 3A); moreover, TL1A was also upregulated upon extended passaging of CEP, suggesting a novel role of TL1A in the senescence of endothelial cells in vitro and in vivo. Supporting a functional role of TL1A in CEP senescence, we found that depletion of TL1A extended the replicative life span of CEP cultures. When full-length TL1A was overexpressed in young HUVEC or CEP, this led to the enlarged phenotype typical of senescent cells, strong staining for SA-β-gal, an inhibition of cell proliferation, and an increased rate of apoptosis, representing characteristic features of senescent endothelial cells (56). However, CEP seem to be more resistant to apoptosis compared with HUVEC, in line with the known increased sensitivity of HUVEC toward proapoptotic stimuli, such as oxidative stress (64). In conclusion, overexpression of full-length TL1A in young HUVEC and CEP induced premature senescence, and senescence was delayed in CEP when TL1A was depleted. These novel findings suggest a causal role of TL1A in senescence of endothelial cells.
The mechanisms by which TL1A regulates endothelial cell senescence remain to be established. TL1A is known to interact with death receptor 3 (DR3), and this interaction can be modulated by DcR3. Both DR3 and DcR3 are expressed in HUVEC and CEP (data not shown), suggesting that these receptors may be involved in the phenotypic alterations obtained by TL1A overexpression. Accordingly, it was reported that DcR3, a known decoy for TL1A, induces HUVEC proliferation (34), and effects of DcR3 on endothelial cell proliferation were conferred via blocking the negative regulatory role of TL1A (34). The authors suggested that DcR3 might act as an angiogenic factor via blocking the negative regulator TL1A. This hypothesis is consistent with our finding that knockdown of TL1A in human CEP induces cell proliferation.
Biologic Activities of Extracellular TL1A Fragments
According to the literature, the fragment TL1AL72-L251 has immunomodulatory activities. The signal induced by this fragment on T cells via DR3 interactions results in increased responsiveness to IL-2 and in the secretion of proinflammatory cytokines like IFN-γ and granulocyte macrophage colony-stimulating factor (21). Furthermore, TL1AL72-L251 was shown to synergize with IL-12 and IL-18 to enhance IFN-γ production in human T cells and NK cells (33). Although DR3 contains a death domain, TL1AL72-L251 did not induce significant caspase activation or apoptosis in primary T cells (21,65). Besides T cells, the fragment stimulated monocytes/macrophages to secrete IL-8 via DR3-mediated nuclear factor kappa B activation (66). The secretion of proinflammatory IFN-γ was stimulated in lamina propria mononuclear cells, indicating that TL1A may have a role in inflammatory bowel disease (26,29). The TL1A fragment also increased IL-2 production in PBMCs (58). These results show a strong effect of recombinant TL1AL72-L251 on cells of the immune system, which results in a proinflammatory response. In line with these data, we show here that TL1AL72-L251 induced IFN-γ secretion in human PBMCs stimulated with IL-12 and IL-18, and most probably NK cells are the main source of IFN-γ production (Figure 5D).
A major unresolved question in research on TL1A concerns the point that TL1A overexpression leads to antiproliferative and proapoptotic effects in endothelial cells [(21,23), this report], but the underlying mechanisms remained elusive. Recombinant TL1AL72-L251 did not influence proliferation and survival in HUVEC (Figure 5C), as was also observed by others (58). In addition, TL1AL72-L251 had no significant antiangiogenic activity in vitro or in vivo (21,58). Whereas, as pointed out previously, the only so far known extracellular fragment of TL1A, TL1AL72-L251, is devoid of any proapoptotic activity, our finding of a second shorter TL1A fragment, which has proapoptotic activity, can resolve this question. Novel data shown here suggest that a second soluble fragment of TL1A, most probably TL1AV84-L251, is generated by ectodomain shedding of membrane bound TL1A (Figures 2 and 5A). In contrast to TL1AL72-L251, the novel fragment induced apoptosis in HUVEC (Figure 5C). Indeed, several previous reports described that artificially created fragments shorter than TL1AL72-L251 can induce apoptosis in endothelial cells. Since the discovery of a partial TL1A cDNA in 1997, the majority of studies focused on the application of diverse extracellular fragments of TL1A that were produced as recombinant proteins in E coli. The strong focus on research with recombinant proteins derived from the TNF family is explained by their potential therapeutic value for treating cancer (67).
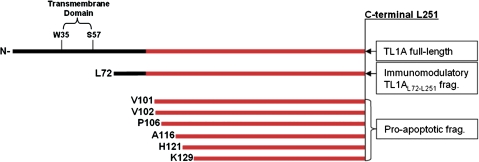
However, interpretation of these data is hampered by use of differential nomenclature, as follows: the amino acid sequences of VEGI-174 and TL1A overlap in the C-terminal part, starting at the V24 residue of VEGI-174. The V24 residue in VEGI-174 corresponds to the V101 residue in full-length TL1A (Figure 6). Therefore, for the following section, the nomenclature of the VEGI-174 fragments is adapted to TL1A. The application of recombinant TL1AV101-L251 (VEGIV24-L174) led to the time- and concentration-dependent apoptosis of endothelial cells (59,60). TL1AV102-L251 (VEGIV25-L174) also markedly inhibited the growth of endothelial cells and induced apoptosis (59). Recombinant TL1AP106-L251 (VEGIP29-L174) induced growth arrest in endothelial cells, and cells that had entered the growth cycle underwent apoptosis via the activation of caspase-3 (68). TL1AA116-L251 (VEGIA39-L174) was only applied on cancer cell lines where it induced apoptosis. Interestingly, the same fragment induced proliferation in human diploid fibroblasts (69). TL1AH121-L251 (VEGIH44-L174) showed significant inhibitory effects on growth of HUVEC, and TL1AK129-L251 (VEGIK52-L174) inhibited angiogenesis (70).
Figure 6.
Overview of tumor necrosis factor (TNF)–like cytokine 1A (TL1A) fragments. TL1A fragments used in this communication and in the previous literature are shown. On top, the schematic drawing of the TL1A full-length sequence with the putative transmembrane domain and the TNF-homology domain (gray) is shown. Below the recombinant fragments used in different studies are indicated with their N-terminal amino acid and their biologic function.
In summary, the data shown here suggest that TL1A is a transmembrane protein that can be processed into a large fragment mainly involved in immunomodulation (TL1AL72-L251) and to a smaller fragment (putative TL1AV84-L251) that is able to induce growth arrest and apoptosis in endothelial cells, however, has also strong effects on immune cells. The two fragments are probably produced via ADAM17/10-mediated alternative cleavage of membrane inserted TL1A. It might be that within an organismic context, soluble TL1AL72-L251 may exert its effects mainly in a paracrine manner to influence cells of the immune system, whereas the TL1AV84-L251 fragment would act in an autocrine way to induce proliferation inhibition and apoptosis in endothelial cells.
FUNDING
This work was supported by grants from the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung-National Research Network S93) and the European Community (Integrated Projekt Proteomage, LSHM-CT-2005-518230) to C.M. Further the European Future Leaders in Ageing Research in Europe fellowship funded by the Austrian Federal Ministry of Science and Research to D.H.-B.
References
- 1.Campisi J, Sedivy J. How does proliferative homeostasis change with age? What causes it and how does it contribute to aging? J Gerontol A Biol Sci Med Sci. 2009;64:164–166. doi: 10.1093/gerona/gln073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ressler S, Bartkova J, Niederegger H, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 6.Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 7.Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65:510–520. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 8.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh’s dilemma. Am J Physiol Heart Circ Physiol. 2006;290:H1729–H1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 10.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 11.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 13.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 14.Ferber A, Chang C, Sell C, et al. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J Biol Chem. 1993;268:17883–17888. [PubMed] [Google Scholar]
- 15.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jacob NK, Ladner KJ, et al. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 2009;10:1272–1278. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan KB, Harrop J, Reddy M, et al. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene. 1997;204:35–46. doi: 10.1016/s0378-1119(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhai Y, Ni J, Jiang GW, et al. VEGI, a novel cytokine of the tumor necrosis factor family, is an angiogenesis inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J. 1999;13:181–189. doi: 10.1096/fasebj.13.1.181. [DOI] [PubMed] [Google Scholar]
- 21.Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 22.Ware CF. The TNF superfamily-2008. Cytokine Growth Factor Rev. 2008;19:183–186. doi: 10.1016/j.cytogfr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chew LJ, Pan H, Yu J, et al. A novel secreted splice variant of vascular endothelial cell growth inhibitor. FASEB J. 2002;16:742–744. doi: 10.1096/fj.01-0757fje. [DOI] [PubMed] [Google Scholar]
- 24.Jin T, Guo F, Kim S, Howard A, Zhang YZ. X-ray crystal structure of TNF ligand family member TL1A at 2.1A. Biochem Biophys Res Commun. 2007;364:1–6. doi: 10.1016/j.bbrc.2007.09.097. [DOI] [PubMed] [Google Scholar]
- 25.Al-Lamki RS, Wang J, Tolkovsky AM, et al. TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol. 2008;19:953–960. doi: 10.1681/ASN.2007060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamias G, Martin C, III, Marini M, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 27.Kang YJ, Kim WJ, Bae HU, et al. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005;29:229–235. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Prehn JL, Mehdizadeh S, Landers CJ, et al. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178:4033–4038. doi: 10.4049/jimmunol.178.7.4033. [DOI] [PubMed] [Google Scholar]
- 30.Chinnaiyan AM, O’Rourke K, Yu GL, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 31.Screaton GR, Xu XN, Olsen AL, et al. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci U S A. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Lamki RS, Wang J, Thiru S, et al. Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am J Pathol. 2003;163:401–411. doi: 10.1016/S0002-9440(10)63670-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadakis KA, Prehn JL, Landers C, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 34.Yang CR, Hsieh SL, Teng CM, Ho FM, Su WL, Lin WW. Soluble decoy receptor 3 induces angiogenesis by neutralization of TL1A, a cytokine belonging to tumor necrosis factor superfamily and exhibiting angiostatic action. Cancer Res. 2004;64:1122–1129. doi: 10.1158/0008-5472.can-03-0609. [DOI] [PubMed] [Google Scholar]
- 35.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 36.Takedatsu H, Michelsen KS, Wei B, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bull MJ, Williams AS, Mecklenburgh Z, et al. The death receptor 3-TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. J Exp Med. 2008;205:2457–2464. doi: 10.1084/jem.20072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bamias G, Siakavellas SI, Stamatelopoulos KS, Chryssochoou E, Papamichael C, Sfikakis PP. Circulating levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3 (DcR3) in rheumatoid arthritis. Clin Immunol. 2008;129:249–255. doi: 10.1016/j.clim.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meylan F, Davidson TS, Kahle E, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappu BP, Borodovsky A, Zheng TS, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SH, Lee WH, Kwon BS, Oh GT, Choi YH, Park JE. Tumor necrosis factor receptor superfamily 12 may destabilize atherosclerotic plaques by inducing matrix metalloproteinases. Jpn Circ J. 2001;65:136–138. doi: 10.1253/jcj.65.136. [DOI] [PubMed] [Google Scholar]
- 43.Kim WJ, Kang YJ, Suk K, Park JE, Kwon BS, Lee WH. Comparative analysis of the expression patterns of various TNFSF/TNFRSF in atherosclerotic plaques. Immunol Invest. 2008;37:359–373. doi: 10.1080/08820130802123139. [DOI] [PubMed] [Google Scholar]
- 44.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 45.Hampel B, Fortschegger K, Ressler S, et al. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp Gerontol. 2006;41:474–481. doi: 10.1016/j.exger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Untergasser G, Koeck R, Wolf D, et al. CD34+/CD133- circulating endothelial precursor cells (CEP): characterization, senescence and in vivo application. Exp Gerontol. 2006;41:600–608. doi: 10.1016/j.exger.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Unterluggauer H, Hampel B, Zwerschke W, Jansen-Durr P. Senescence-associated cell death of human endothelial cells: the role of oxidative stress. Exp Gerontol. 2003;38:1149–1160. doi: 10.1016/j.exger.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rainer J, Sanchez-Cabo F, Stocker G, Sturn A, Trajanoski Z. CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Res. 2006;34:W498–W503. doi: 10.1093/nar/gkl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 51.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent B, Paitel E, Saftig P, et al. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J Biol Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- 53.Althoff K, Mullberg J, Aasland D, et al. Recognition sequences and structural elements contribute to shedding susceptibility of membrane proteins. Biochem J. 2001;353:663–672. doi: 10.1042/0264-6021:3530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Oliver P, Lancaster JJ, et al. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 55.Kim S, Zhang L. Identification of naturally secreted soluble form of TL1A, a TNF-like cytokine. J Immunol Methods. 2005;298:1–8. doi: 10.1016/j.jim.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol. 2001;36:1327–1347. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
- 57.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 58.Yao JJ, Zhang M, Miao XH, et al. Isoform of vascular endothelial cell growth inhibitor (VEGI72-251) increases interleukin-2 production by activation of T lymphocytes. Acta Biochim Biophys Sin (Shanghai) 2006;38:249–253. doi: 10.1111/j.1745-7270.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 59.Yue TL, Ni J, Romanic AM, et al. TL1, a novel tumor necrosis factor-like cytokine, induces apoptosis in endothelial cells. Involvement of activation of stress protein kinases (stress-activated protein kinase and p38 mitogen-activated protein kinase) and caspase-3-like protease. J Biol Chem. 1999;274:1479–1486. doi: 10.1074/jbc.274.3.1479. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Pan W, Zhu FL, et al. Cloning, expression and biological activity of VEGI(151), a novel vascular endothelial cell growth inhibitor. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2000;32:485–489. [PubMed] [Google Scholar]
- 61.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 62.Siebert PD, Chenchik A. Modified acid guanidinium thiocyanate-phenol-chloroform RNA extraction method which greatly reduces DNA contamination. Nucleic Acids Res. 1993;21:2019–2020. doi: 10.1093/nar/21.8.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 64.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 65.Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003;278:39251–39258. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]
- 66.Su WB, Chang YH, Lin WW, Hsieh SL. Differential regulation of interleukin-8 gene transcription by death receptor 3 (DR3) and type I TNF receptor (TNFRI) Exp Cell Res. 2006;312:266–277. doi: 10.1016/j.yexcr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Ruegg C, Hasmim M, Lejeune FJ, Alghisi GC. Antiangiogenic peptides and proteins: from experimental tools to clinical drugs. Biochim Biophys Acta. 2006;1765:155–177. doi: 10.1016/j.bbcan.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Tian S, Metheny-Barlow L, et al. Modulation of endothelial cell growth arrest and apoptosis by vascular endothelial growth inhibitor. Circ Res. 2001;89:1161–1167. doi: 10.1161/hh2401.101909. [DOI] [PubMed] [Google Scholar]
- 69.Haridas V, Shrivastava A, Su J, et al. VEGI, a new member of the TNF family activates nuclear factor-kappa B and c-Jun N-terminal kinase and modulates cell growth. Oncogene. 1999;18:6496–6504. doi: 10.1038/sj.onc.1203059. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M, Wang L, Wang HW, Pan X, Pan W, Qi ZT. [Effect of N-terminal deletion on biological activity of vascular endothelial cell growth inhibitor] Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:133–137. [PubMed] [Google Scholar]