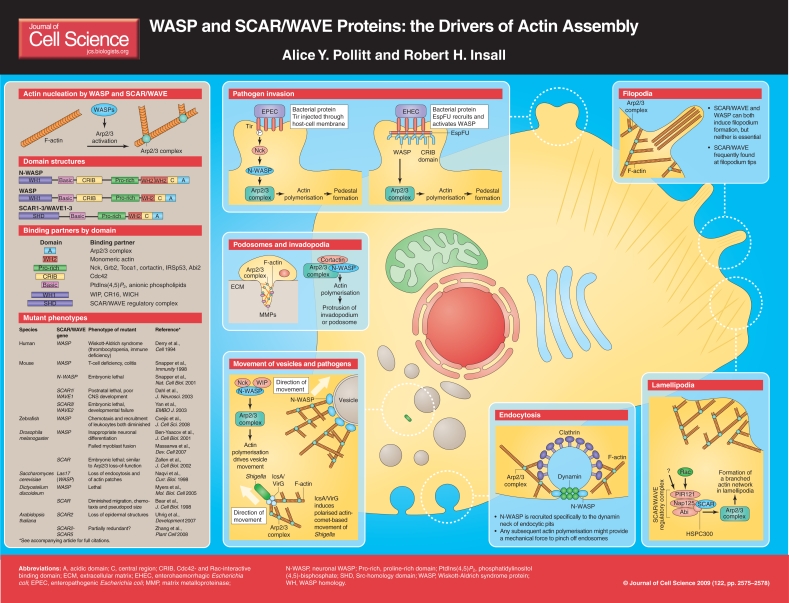
The actin cytoskeleton plays an essential role in numerous aspects of cell biology, such as cell morphology and motility. Actin's role in these processes is tightly regulated, in particular through the Arp2/3 complex (Goley and Welch, 2006), a major initiator of actin polymerisation that promotes the formation of branched actin-filament networks. When the Arp2/3 complex was first purified completely, it became clear that the complex was not sufficient to initiate new actin filaments on its own (Machesky et al., 1999). Members of the Wiskott-Aldrich syndrome protein (WASP) family were subsequently identified as the major regulators of the Arp2/3 complex (Millard et al., 2004); these proteins activate the Arp2/3 complex to nucleate new actin filaments. The importance of WASP proteins was immediately recognised when it became clear that they were involved in linking signalling events to the regulation of the actin cytoskeleton.
The WASP family consists of two principal classes of protein – WASPs and SCAR/WAVEs. WASPs are named after Wiskott-Aldrich syndrome, in which mutations in the gene encoding WASP cause immune and blood deficiencies, whereas the dual name of SCAR/WAVE emerged because the same protein was discovered independently by two groups – `SCAR' through Dictyostelium discoideum genetics (Bear et al., 1998) and `WAVE' by homology with WASP (Miki et al., 1998). SCAR was the first name to be used, for both the Dictyostelium protein and its mammalian homologues, but in mammalian cells WAVE is now more commonly used.
Figure 1.
Both WASPs and SCAR/WAVEs are present throughout evolutionary history. Drosophila melanogaster, Dictyostelium and Caenorhabditis elegans possess one of each. Yeasts possess WASP but no SCAR/WAVE, whereas the opposite is true in plants (Uhrig et al., 2007). Mammals typically have two WASPS – the haematopoietic-specific WASP and the ubiquitous N-WASP – and three SCAR/WAVE proteins. Other members of the WASP family are beginning to emerge – WASH (Linardopoulou et al., 2007) (which appears to be as universally expressed as WASP and SCAR/WAVE, although its physiological role has not yet been studied), and WHAMM (Campellone et al., 2008) and JMY (Zuchero et al., 2009) (which are recently discovered proteins that are specific to metazoa and have roles in vesicle traffic). In this poster article, we describe the range of physiological functions that have been established for WASP and SCAR/WAVE proteins. WASH, WHAMM and JMY are not discussed further as too little is currently known about their roles.
Domain structure and interacting proteins
Members of the WASP family of proteins are characterised by a conserved domain arrangement (see poster) (Innocenti et al., 2004). The C-terminus is responsible for binding to and activating the Arp2/3 complex. It comprises one or two WASP homology 2 (WH2) domains, which bind to monomeric actin, followed by a short central (C) region and an acidic (A) domain, which interacts with the Arp2/3 complex. Polyproline repeats within the proline-rich region provide possible sites for the binding of numerous Src-homology 3 (SH3)-domain-containing proteins (see poster) (Takenawa and Suetsugu, 2007). It remains to be determined which of these interactors are physiologically important and how they regulate WASP-family proteins in vivo (see below for a discussion of WASP and SCAR/WAVE regulation).
The organisation of the N-terminal region, which contains domains that are thought to provide a connection with regulatory proteins, differs among WASP-family members. WASP and N-WASP contain a WASP homology 1 (WH1) domain, also known as an Ena/VASP homology 1 (EVH1) domain, and a CRIB domain, which binds to the small GTPase Cdc42. The WH1 domain interacts with the WASP-interacting protein (WIP) family of proteins, and this interaction is thought to suppress the activity of WASP or N-WASP. By contrast, the SCAR/WAVE proteins contain a SCAR homology domain (SHD), and do not possess any type of GTPase-binding domain. Both WASPs and SCARs/WAVEs contain a basic region, which might bind to phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] and other anionic phospholipids and localise the proteins to the plasma membrane. Thus, the N-terminal domains define the way in which both WASP and SCAR/WAVE proteins are regulated, and also to a large extent their subcellular localisation.
Regulation of WASP and SCAR/WAVE activity
WASP and N-WASP are predominantly found in an autoinhibited conformation in which the C-terminus of the protein is occluded through its interaction with the N-terminus. This autoinhibition is released by the competitive binding of the small GTPase Cdc42 and the phospholipid PtdIns(4,5)P2 (Kim et al., 2000), although it is not clear whether the lipid acts as a signalling molecule or merely as an anionic marker of the plasma membrane (Insall and Weiner, 2001). Other proteins are thought to bind to WASPs and regulate their activity – WIP is a frequently observed binding partner (Ramesh et al., 1997) and TOCA1, which (like WASP) binds to Cdc42, might add selectivity and cooperation to the Cdc42-dependent activation of WASPs (Ho et al., 2004). The role of SH3-domain adaptors such as Nck and Grb2 in WASP activation remains a mystery; early papers reported that they bound to the proline-rich domains of both WASP and SCAR, but these have been relatively unsupported by recent data (Buday, 1999).
Unlike WASPs, SCAR/WAVE proteins are not autoinhibited and they form part of a larger regulatory complex that contains four other proteins – PIR121, Nap1, Abi and HSPC300 (Eden et al., 2002). The small GTPase Rac interacts with the complex via the PIR121 subunit, and other as-yet-unknown proteins are likely to interact with the other complex members (Ibarra et al., 2006). There has been controversy about the dynamics and basal activity of the complex. Eden et al. found that the intact complex was inactive, but that it released an active subcomplex containing SCAR/WAVE and HSPC300 when stimulated (Eden et al., 2002). Recent work has not reproduced the splitting of the complex, but does indicate that the pure complex has no activity until it is stimulated by upstream signals that include Rac and probably additional inputs (Ismail et al., 2009).
Phosphorylation is emerging as an important mode of regulating the WASP family of proteins. Tyrosine phosphorylation of WASP leads to an increase in its ability to activate the Arp2/3 complex without apparent need for Cdc42 or PtdIns(4,5)P2, and serine/threonine phosphorylation can stimulate actin polymerisation in vitro; however, the physiological significance of phosphorylation in vivo has yet to be demonstrated (Cory et al., 2003). In mammals, tyrosine phosphorylation of WAVE2 by the Abl kinase might be involved in its localisation to the leading edge, whereas tyrosine phosphorylation of WAVE1 by Src increases its affinity for the Arp2/3 complex in vitro (Ardern et al., 2006). WAVE1 is also basally serine/threonine phosphorylated (Kim et al., 2006), which inhibits its ability to activate the Arp2/3 complex in vitro and in vivo. cAMP signalling reduces WAVE1 phosphorylation, which apparently increases the ability of WAVE1 to activate the Arp2/3 complex (Kim et al., 2006).
The Arp2/3 complex and actin assembly
Actin monomers are present in the cytoplasm at extremely high levels – actin is the most abundant protein in most eukaryotic cells, and more than half of it is typically monomeric in living cells. Actin does not spontaneously polymerise because of a high kinetic barrier. Once filaments are initiated, they extend rapidly until they are actively stopped, but a catalyst is required to start a new filament. There are two principal classes of such catalysts, which are usually called `nucleators' because they nucleate new filaments – formins and the Arp2/3 complex. Formins generate single actin filaments that are typically oriented orthogonally to the membrane. However, the Arp2/3 complex – when it has been activated by a WASP-family protein – nucleates new actin at a 70° angle from the side of pre-existing filaments, generating a crosslinked, anisotropic meshwork of actin (see poster).
Exactly how members of the WASP family of proteins interact with the Arp2/3 complex to result in its activation remains to be fully elucidated. The actin-related subunits of the Arp2/3 complex, Arp2 and Arp3, are proposed to form a `pseudo-actin dimer' that nucleates actin polymerisation but, in the open (inactive) conformation, Arp2 and Arp3 are too far apart to do this, suggesting that a conformational change is required. Studies using electron microscopy suggest that the Arp2/3 complex is in equilibrium between an open (inactive) and a closed (active) conformation, and that binding of WASP-family members locks it into its active state (Rodal et al., 2005). In addition, the WH2 domain seems to recruit a new actin monomer to the activated Arp2/3 complex, completing the nucleation of a new branch (Boczkowska et al., 2008).
Biological functions of WASP-family proteins
The activity of the WASP family of proteins has been implicated in numerous biological processes that involve the reorganisation of the actin cytoskeleton (see poster). Their activation can lead to the formation of numerous actin-based structures, such as lamellipodia, filopodia, podosomes and plant trichomes. In addition to these structural roles, WASP-family proteins have central roles in membrane trafficking, and are manipulated during infection by intracellular pathogens. Other emerging roles include cell-substrate adhesion (Ibarra et al., 2006) and cytokinesis (Pollitt and Insall, 2008); others will no doubt continue to emerge.
Actin-based structures
Lamellipodia are sheet-like structures at the leading edge of the cell, in which actin filaments are arranged into a crosslinked network (Pollard and Borisy, 2003). Electron microscopy reveals that these filaments are branched at 70°, which is characteristic of Arp2/3-complex activity. Multiple studies in a range of cell types have shown that SCAR/WAVE proteins are required for lamellipodium formation and typically act downstream of the small GTPase Rac (Kunda et al., 2003; Yan et al., 2003). By contrast, the role of the WASP family in the formation of filopodia – long, finger-like cell-membrane protrusions that contain bundles of straight actin filaments – is much less clear. Earlier work suggested that N-WASP localises to these structures and that activation of N-WASP by the small GTPase Cdc42 leads to their formation. However, filopodia can still be formed in WASP-deficient cells (Snapper et al., 2001) and the parallel orientation of filopodial actin filaments does not suggest a role of activation of the Arp2/3 complex in their formation. One alternative hypothesis is that SCAR/WAVE proteins induce filopodia after branched actin filaments are reorganised by parallel actin-binding proteins (Biyasheva et al., 2004). The relative contributions of the WASP family to this and other pathways, in particular those that involve formins, remain controversial.
Podosomes are structures formed by haematopoietic cells and osteoclasts, and invadopodia are related structures that protrude into the matrix from certain types of invasive cancer cells. The formation of these structures is mediated via actin reorganisation and the activation of matrix metalloproteinases, which degrade the extracellular matrix (Buccione et al., 2004). Activation of the Arp2/3 complex by N-WASP has been shown to be essential for the formation of podosomes and might also be essential for invadopodia (Mizutani et al., 2002).
Vesicle trafficking and pathogen infection
Along with the Arp2/3 complex, WASP and N-WASP are recruited to sites of phagocytosis. There is also evidence that these proteins are involved in clathrin-mediated endocytosis (Qualmann and Kelly, 2000). Several proteins have been shown to interact with both dynamin (a key endocytic mediator) and N-WASP, and these might bridge the two proteins during endocytosis. N-WASP is recruited specifically to the dynamin neck of endocytic pits, where it might lead to actin polymerisation; this could provide a mechanical force to pinch off endosomes. N-WASP is also implicated in the formation of the actin-comet tails that are sometimes found behind vesicles and that appear to propel them though the cytoplasm (Innocenti et al., 2004).
The same mechanisms that result in vesicle movement are also thought to be exploited by bacterial pathogens to allow them to move through the cytoplasm of the infected cell using the cell's own actin. Pathogens such as Shigella and mycobacteria achieve this by expressing proteins that recruit and activate WASP-family proteins in the host cell. This induces actin polymerisation at the cell surface of the bacterium through activation of the Arp2/3 complex. Actin-based motility of Shigella is mediated by the bacterial protein IcsA/VirG, which is localised in a polarised manner on the bacterial surface. IcsA/VirG recruits and activates host N-WASP by mimicking Cdc42, which can bind to the regulatory domains of N-WASP to promote a conformational change that relieves autoinhibition (Egile et al., 1999).
The recruitment of proteins that activate N-WASP is also exploited by enteropathogenic and enterohaemorrhagic Escherichia coli (EPEC and EHEC, respectively) to induce the formation of actin-based pedestals in the host-cell plasma membrane (Caron et al., 2006). EPEC inject a protein called Tir through the host-cell membrane. Tir is phosphorylated by host-cell kinases and recruits the adapter protein Nck, which activates N-WASP, leading to actin polymerisation and the formation of a pedestal, which seems to allow EPEC to maintain unusually strong adhesion and thus to remain in the host intestine. EHEC use a different mechanism, in which they inject two proteins, Tir and EspFU (also known as TccP). EspFU binds to and activates the host WASP through a repeated amino acid sequence that mimics the central region of the WASP C-terminal tail and releases autoinhibition (Cheng et al., 2008; Sallee et al., 2008).
Conclusion
Our understanding of WASP and SCAR/WAVE proteins is growing fast, but there remains a great deal left to discover. Newly discovered WASP-family members such as WASH and WHAMM have broadened the range of physiological roles of the family, interacting proteins and pathways are only beginning to be discovered, and the mechanisms by which physiological events control activation of the Arp2/3 complex through the WASP family are only partially understood. What is clear is that most functions of the actin cytoskeleton, and probably several other aspects of cell physiology, depend crucially on WASP and its relatives. This is an exciting field.
Acknowledgments
We are very grateful to the MRC for a Senior Fellowship to R.H.I. and project grant support for A.Y.P., to Laura Machesky for comments, and to the anonymous referees for constructive comments. Deposited in PMC for release after 6 months.
References
- Ardern, H., Sandilands, E., Machesky, L. M., Timpson, P., Frame, M. C. and Brunton, V. G. (2006). Src-dependent phosphorylation of Scar1 promotes its association with the Arp2/3 complex. Cell Motil. Cytoskeleton 63, 6-13. [DOI] [PubMed] [Google Scholar]
- Bear, J. E., Rawls, J. F. and Saxe, C. L., 3rd (1998). SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J. Cell Biol. 142, 1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaacov, S., Le Borgne, R., Abramson, I., Schweisguth, F. and Schejter, E. D. (2001). Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J. Cell Biol. 152, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyasheva, A., Svitkina, T., Kunda, P., Baum, B. and Borisy, G. (2004). Cascade pathway of filopodia formation downstream of SCAR. J. Cell Sci. 117, 837-848. [DOI] [PubMed] [Google Scholar]
- Boczkowska, M., Rebowski, G., Petoukhov, M. V., Hayes, D. B., Svergun, D. I. and Dominguez, R. (2008). X-ray scattering study of activated Arp2/3 complex with bound actin-WCA. Structure 16, 695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccione, R., Orth, J. D. and McNiven, M. A. (2004). Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5, 647-657. [DOI] [PubMed] [Google Scholar]
- Buday, L. (1999). Membrane-targeting of signalling molecules by SH2/SH3 domain-containing adaptor proteins. Biochim. Biophys. Acta 1422, 187-204. [DOI] [PubMed] [Google Scholar]
- Campellone, K. G., Webb, N. J., Znameroski, E. A. and Welch, M. D. (2008). WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134, 148-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron, E., Crepin, V. F., Simpson, N., Knutton, S., Garmendia, J. and Frankel, G. (2006). Subversion of actin dynamics by EPEC and EHEC. Curr. Opin. Microbiol. 9, 40-45. [DOI] [PubMed] [Google Scholar]
- Cheng, H. C., Skehan, B. M., Campellone, K. G., Leong, J. M. and Rosen, M. K. (2008). Structural mechanism of WASP activation by the enterohaemorrhagic E. coli effector EspF(U). Nature 454, 1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory, G. O., Cramer, R., Blanchoin, L. and Ridley, A. J. (2003). Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell 11, 1229-1239. [DOI] [PubMed] [Google Scholar]
- Cvejic, A., Hall, C., Bak-Maier, M., Flores, M. V., Crosier, P., Redd, M. J. and Martin, P. (2008). Analysis of WASp function during the wound inflammatory response-live-imaging studies in zebrafish larvae. J. Cell Sci. 121, 3196-3206. [DOI] [PubMed] [Google Scholar]
- Dahl, J. P., Wang-Dunlop, J., Gonzales, C., Goad, M. E., Mark, R. J. and Kwak, S. P. (2003). Characterization of the WAVE1 knock-out mouse: implications for CNS development. J. Neurosci. 23, 3343-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry, J. M., Ochs, H. D. and Francke, U. (1994). Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 78, 635-644. [DOI] [PubMed] [Google Scholar]
- Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M. and Kirschner, M. W. (2002). Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790-793. [DOI] [PubMed] [Google Scholar]
- Egile, C., Loisel, T. P., Laurent, V., Li, R., Pantaloni, D., Sansonetti, P. J. and Carlier, M. F. (1999). Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146, 1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley, E. D. and Welch, M. D. (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713-726. [DOI] [PubMed] [Google Scholar]
- Ho, H. Y., Rohatgi, R., Lebensohn, A. M., Le Ma, Li, J., Gygi, S. P. and Kirschner, M. W. (2004). Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118, 203-216. [DOI] [PubMed] [Google Scholar]
- Ibarra, N., Blagg, S. L., Vazquez, F. and Insall, R. H. (2006). Nap1 regulates Dictyostelium cell motility and adhesion through SCAR-dependent and -independent pathways. Curr. Biol. 16, 717-722. [DOI] [PubMed] [Google Scholar]
- Innocenti, M., Zucconi, A., Disanza, A., Frittoli, E., Areces, L. B., Steffen, A., Stradal, T. E., Di Fiore, P. P., Carlier, M. F. and Scita, G. (2004). Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat. Cell Biol. 6, 319-327. [DOI] [PubMed] [Google Scholar]
- Insall, R. H. and Weiner, O. D. (2001). PIP3, PIP2, and cell movement: similar messages, different meanings? Dev. Cell 1, 743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail, A. M., Padrick, S. B., Chen, B., Umetani, J. and Rosen, M. K. (2009). The WAVE regulatory complex is inhibited. Nat. Struct. Mol. Biol. 16, 561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. S., Kakalis, L. T., Abdul-Manan, N., Liu, G. A. and Rosen, M. K. (2000). Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404, 151-158. [DOI] [PubMed] [Google Scholar]
- Kim, Y., Sung, J. Y., Ceglia, I., Lee, K. W., Ahn, J. H., Halford, J. M., Kim, A. M., Kwak, S. P., Park, J. B., Ho Ryu, S. et al. (2006). Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442, 814-817. [DOI] [PubMed] [Google Scholar]
- Kunda, P., Craig, G., Dominguez, V. and Baum, B. (2003). Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr. Biol. 13, 1867-1875. [DOI] [PubMed] [Google Scholar]
- Linardopoulou, E. V., Parghi, S. S., Friedman, C., Osborn, G. E., Parkhurst, S. M. and Trask, B. J. (2007). Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 3, e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky, L. M., Mullins, R. D., Higgs, H. N., Kaiser, D. A., Blanchoin, L., May, R. C., Hall, M. E. and Pollard, T. D. (1999). Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA 96, 3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwa, R., Carmon, S., Shilo, B. Z. and Schejter, E. D. (2007). WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell 12, 557-569. [DOI] [PubMed] [Google Scholar]
- Miki, H., Suetsugu, S. and Takenawa T. (1998). WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard, T. H., Sharp, S. J. and Machesky, L. M. (2004). Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome protein): family proteins and the Arp2/3 complex. Biochem. J. 380, 1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani, K., Miki, H., He, H., Maruta, H. and Takenawa, T. (2002). Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 62, 669-674. [PubMed] [Google Scholar]
- Myers, S. A., Han, J. W., Lee, Y., Firtel, R. A. and Chung, C. Y. (2005). A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis. Mol. Biol. Cell 16, 2191-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi, S. N., Zahn, R., Mitchell, D. A., Stevenson, B. J. and Munn, A. L. (1998). The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr. Biol. 8, 959-962. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D. and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. [DOI] [PubMed] [Google Scholar]
- Pollitt, A. Y. and Insall, R. H. (2008). Abi mutants in Dictyostelium reveal specific roles for the SCAR/WAVE complex in cytokinesis. Curr. Biol. 18, 203-210. [DOI] [PubMed] [Google Scholar]
- Qualmann, B. and Kelly, R. B. (2000). Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148, 1047-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh, N., Antón, I. M., Hartwig, J. H. and Geha, R. S. (1997). WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. USA 94, 14671-14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal, A. A., Sokolova, O., Robins, D. B., Daugherty, K. M., Hippenmeyer, S., Riezman, H., Grigorieff, N. and Goode, B. L. (2005). Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat. Struct. Mol. Biol. 12, 26-31. [DOI] [PubMed] [Google Scholar]
- Sallee, N. A., Rivera, G. M., Dueber, J. E., Vasilescu, D., Mullins, R. D., Mayer, B. J. and Lim, W. A. (2008). The pathogen protein EspF(U) hijacks actin polymerization using mimicry and multivalency. Nature 454, 1005-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper, S. B., Rosen, F. S., Mizoguchi, E., Cohen, P., Khan, W., Liu, C. H., Hagemann, T. L., Kwan, S. P., Ferrini, R., Davidson, L. et al. (1998). Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9, 81-91. [DOI] [PubMed] [Google Scholar]
- Snapper, S. B., Takeshima, F., Anton, I., Liu, C. H., Thomas, S. M., Nguyen, D., Dudley, D., Fraser, H., Purich, D., Lopez-Ilasaca, M. et al. (2001). N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat. Cell Biol. 3, 897-904. [DOI] [PubMed] [Google Scholar]
- Takenawa, T. and Suetsugu, S. (2007). The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37-48. [DOI] [PubMed] [Google Scholar]
- Uhrig, J. F., Mutondo, M., Zimmermann, I., Deeks, M. J., Machesky, L. M., Thomas, P., Uhrig, S., Rambke, C., Hussey, P. J. and Hulskamp, M. (2007). The role of Arabidopsis SCAR genes in ARP2-ARP3-dependent cell morphogenesis. Development 134, 967-977. [DOI] [PubMed] [Google Scholar]
- Yan, C., Martinez-Quiles, N., Eden, S., Shibata, T., Takeshima, F., Shinkura, R., Fujiwara, Y., Bronson, R., Snapper, S. B., Kirschner, M. W. et al. (2003). WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. EMBO J. 22, 3602-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen, J. A., Cohen, Y., Hudson, A. M., Cooley, L., Wieschaus, E. and Schejter, E. D. (2002). SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 156, 689-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., Mallery, E. L., Schlueter, J., Huang, S., Fan, Y., Brankle, S., Staiger, C. J. and Szymanski, D. B. (2008). Arabidopsis SCARs function interchangeably to meet actin-related protein 2/3 activation thresholds during morphogenesis. Plant Cell 20, 995-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero, J. B., Coutts, A. S., Quinlan, M. E., Thangue, N. B. and Mullins, R. D. (2009). p53-cofactor JMY is a multifunctional actin nucleation factor. Nat. Cell Biol. 11, 451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]