Abstract
The RING finger ubiquitin ligase Siah2 controls the stability of various substrates involved in stress and hypoxia responses, including the PHD3, which controls the stability of HIF-1α. In the present study we determined the role of Siah2 phosphorylation in the regulation of its activity toward PHD3. We show that Siah2 is subject to phosphorylation by p38 MAPK, which increases Siah2-mediated degradation of PHD3. Consistent with these findings, MKK3/MKK6 double-deficient cells, which cannot activate p38 kinases, exhibit impaired Siah2-dependent degradation of PHD3. Phosphopeptide mapping identified T24 and S29 as the primary phospho-acceptor sites. Phospho-mutant forms of Siah2 (S29A or T24A/S29A) exhibit impaired degradation of PHD3, particularly after hypoxia. Conversely, a phospho-mimic form of Siah2 (T24E/S29D) exhibits stronger degradation of PHD3, compared with wild type Siah2. Whereas phospho-mutant Siah2 exhibits weaker association with PHD3, phospho-mimic Siah2 associates as well as wild type and is localized within the perinuclear region, suggesting that phosphorylation of Siah2 affects its subcellular localization and, consequently, the degree of its association with PHD3. In all, our findings reveal the phosphorylation of Siah2 by p38 and the implications of such phosphorylation for Siah2 activity toward PHD3.
Regulation of protein stability is largely mediated by ubiquitin ligases, which mark their substrates for efficient proteasome-dependent degradation (1). The conjugation of ubiquitin moieties to substrates requires coordinated action of the ubiquitin-activating enzyme (E1),2 the ubiquitin-conjugating enzyme E2, and the E3 ligase, which associates with the substrate and thereby determines the specificity in degradation of various substrates (2). Among the major types of E3 ligases are the RING finger proteins, which contain a characteristic cysteine-rich zinc binding domain defined by a pattern of conserved cysteine and histidine residues and catalyze polyubiquitination (3). Characteristic of RING finger ubiquitin ligases is their ability to mediate degradation of their substrates as well as their own availability by promoting their own ubiquitination-dependent degradation (4). Siah is a member of the RING finger E3 ligases that was originally identified and characterized in Drosophila as sina (seven in absentia) and was shown to affect degradation of Tramtrack, a transcriptional repressor of the R7 cell fate during eye development (5,6). Siah2-dependent degradation was reported for more than a dozen substrates including the transcriptional co-activator (OBF-1 (6)), nuclear receptor co-repressor (N-CoR (7)), the signaling regulatory module for c-Jun NH2-terminal kinase, p38, and IκB kinase (IKK), tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) (8), the anti-apoptosis protein (BAG-1 (9)), the surface receptor DCC (10), the cyclin-dependent kinase activator RINGO/speedy (11), the Krebs cycle regulatory protein α-ketoglutarate dehydrogenase (12), and the rate-limiting factor in HIF-1α stability, PHD3 (13, 14).
Utilizing oxygen and 2-oxoglutarate as co-factors, PHDs hydroxylate HIF-1α in normoxia and mild (physiological) hypoxia condition (15). Hydroxylation of HIF-1α is prerequisite to its association with the von Hippel-Landau tumor suppressor gene product (pVHL), which results in HIF-1α ubiquitination and subsequent proteasome-dependent degradation (16). Through these substrates, Siah2 affects major cellular regulatory processes such as those affecting stress kinases, cell cycle distribution, transcriptional control, and apoptosis. In most cases Siah2 activity is associated with cellular stress, suggesting its role as a stress-activated ligase.
Activity of Siah2 is subject to extensive regulation. At the RNA level, Siah2 transcription is increased upon changes in oxygen tension, as was shown in mild hypoxia (13). At the protein level, Siah2 is subject to extensive self (auto)-ubiquitination, which limits its availability under certain conditions (17). Furthermore, Siah2 activity as a ubiquitin ligase requires cooperation with adaptor proteins. Siah-interacting protein was shown to be important in regulation of Siah2 ligase activity toward β-catenin (18). More recent studies pointed to the role of another adaptor protein, POSH, in regulation of Siah2 activity along the c-Jun NH2-terminal kinase signaling pathway (19). These observations point to the tight regulation of Siah2 activity.
Whereas the role of post-translational modification in regulation of substrate recognition by E3 ligases is well documented, only a handful cases have demonstrated the role of ligase phosphorylation in regulation of its activity. Among those, the E3 ligase Itch was phosphorylated by c-Jun NH2-terminal kinase-1 after T cell receptor engagement that enhanced its enzymatic activity (20). In contrast, Nedd4–2 was found to be phosphorylated by serum glucocorticoid-inducible protein kinase 1 (SGK-1) and thereby inactivated (21). Here we demonstrate that phosphorylation of Siah2 by p38, a MAPK activated by cytokines, reactive oxygen species, and DNA damage as well as hypoxia, increases its effect on PHD3.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
HeLa cells, wild type mouse embryonic fibroblasts (MEFs), p38−/− MEFs, and MKK3/6−/− MEFs were maintained in Dulbecco’s modified Eagles medium supplemented with fetal bovine serum (10%) and 100 µg/ml penicillin/streptomycin. 293T cells were maintained in Dulbecco’s modified Eagles medium supplemented with bovine serum (10%) and antibiotics. Cell cultures were maintained at 37 °C in 5% CO2. Various kinase inhibitors were purchased from Calbiochem. Recombinant active p38 MAPKα was purchased from Upstate Inc. Antibodies against FLAG epitope, phospho-p38 MAPK, anti-β-actin, and anti-α-tubulin were purchased from Sigma. Anti-HA antibody, anti-p38 MAPK, was purchased from Santa Cruz. Anti-HIF-1α antibody was purchased from Novus Biologicals. Polyclonal anti-HIF-1α antibody was a generous gift from Dr. Gary Chiang.
Lipofectamine Plus reagent was purchased from Invitrogen. Cells were maintained under hypoxia using an in vivo2 Hypoxia 400 work station from Biotrace International Inc.
Plasmids and Transfection
The following mutants were generated with the indicated primers in site-directed mutagenesis (QuikChange) using Siah2 plasmid as a template: Siah2 T24A (5′-CCGCCGCCGCCTCAGGCCCCGCACGCCCCGTCC and antisense), Siah2 S29A (5′-GGACGGGGCGTGCGGGGCCTGAGGCGGCGGCGG and antisense), Siah2 T24A S29A (5′-CCGCACGCCCCGGCCCCGGCTGCGCCC and antisense), Siah2 S29D (5′-CCGCACGCCCCGGACCCGGCTGCGCCC and antisense), Siah2 T24ES29D (5′-CCGCCGCCTCAGGAACCGCACGCCCCGGACCCGGCTGCGCCC and antisense), Siah2 S69A (5′-GGGGCCGACCCGGTGGCCCCGCAGCACCACGAG and antisense), Siah2 T120A (5′-TGCAGGGGCGCCCTAGCGCCCAGCATCAGGAAC and antisense), Siah2 D-mut-HA (R272A, R273A, R274A) (5′-GAGTTGAATGGGAACGCGGCGGCACTGACCTGGGAGGCC and antisense). All mutations were confirmed by DNA sequencing. GST-Siah2 and its phospho mutants were expressed in Escherichia coli BL21 and purified using immobilized glutathione beads as described previously (8). 293T cells were transfected using the calcium phosphate precipitation method. HeLa, NIH3T3, and MEF cells were transfected with Lipofectamine PLUS reagent.
In Vitro Ubiquitination Assay
In vitro translated 35S-labeled Siah2-HA was subjected to one or three rounds of phosphorylation with recombinant p38 MAPK (or mock phosphorylation) followed by an in vitro ubiquitination assay in ubiquitination buffer (50 mm Tris-HCl, pH 8.0, 5 mm MgCl2, 0.5 mm dithiothreitol, 2 mm NaF, and 3 µm okadaic acid) supplemented with purified HA-ubiquitin (0.5 µg), 2 mm ATP, E1 (Affinity Research, Exeter, UK), purified E2 (UbcH5b) (0.5 µg), and/or purified Siah2 for 25 min at 37 °C. Reaction mixtures were then separated on 7% SDS-PAGE followed by immunoblotting with anti-HA antibody.
Immunoblotting
Cells were lysed using lysis buffer containing 350 mm NaCl, 0.25% Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 1 mm dithiothreitol, 1 mm glycerol phosphate, 1 mm sodium orthovanadate, and 30% glycerol with protease inhibitor mixture (Sigma). Protein concentrations were determined using Coomassie protein assay reagent (Pierce). Proteins were boiled in SDS sample buffer and separated on SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane. The blots were blocked with 5% nonfat dry milk in TBST (50 mm Tris-Cl, pH 8.0, 10 mm NaCl, and 0.1% Tween 20) at room temperature for 1 h followed by incubation with the indicated antibodies at 4 °C overnight followed by washes in TBST and incubation with either anti-mouse or anti-rabbit secondary antibody tagged to a fluorophore emitting at either 680 or 800 nm (Amersham Biosciences) for 1 h at room temperature. Immunoprecipitation was performed by incubating 1.0 mg of proteins with 1 µg of anti-FLAG or anti-HA or anti-Siah2 antibodies overnight and then further incubating them for 2 h with 20 µl of protein G beads (Invitrogen). Beads were washed three times with lysis buffer, and proteins were solubilized in 2× Laemmli buffer and subjected to Western blot analysis as detailed above. After washes in Tris-buffered saline Tween, blots were visualized using a Li-COR machine and analyzed using the Odyssey software (Version 2.0), which was also used for quantification, as indicated under the respective blots.
In Vitro p38 MAPK Assay
GST-Siah2 was incubated with active recombinant p38 MAPKα (20 ng) in the presence of reaction buffer containing 12 mm Tris-HCl (pH 7.5, 0.1 mm EGTA) supplemented with 1 µCi of [γ-32P]ATP diluted in 40 mm MgCl2 and 250 mm ATP. The reaction was carried out at 30 °C for 15 min. The reaction was stopped by adding Laemmli buffer, and samples were then boiled and subjected to SDS-PAGE analysis. Phosphorylation of substrates was measured by autoradiography. In some experiments (i.e. Fig. 2B) p38 MAPK immunoprecipitated from UV-treated or untreated cell lysates was used as a source of enzyme and GST-ATF2 (amino acids 1–105) was used as a substrate.
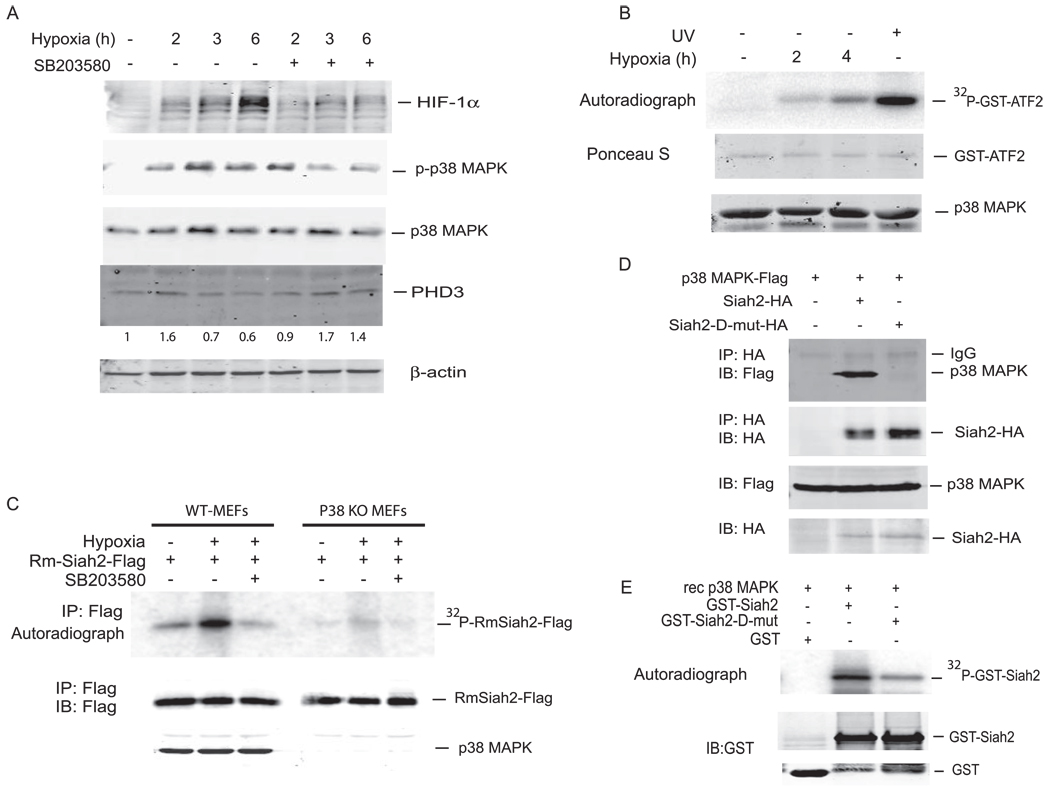
FIGURE 2. Hypoxia induces p38 MAPK activity and Siah2 phosphorylation.
A, MEFs were subjected to 1% hypoxia for the indicated time intervals in the absence or presence of SB203580 (5 µm), and cell lysates were subjected to immunoblot analysis using anti-HIF-1α, anti-phospho p38 MAPK, anti-p38 MAPK, anti-PHD3, and anti-β-actin antibodies. B, MEFs were treated with 1% hypoxia and UV (40J/m2) for the indicated time intervals. P38 MAPK was immunoprecipitated, and an in vitro kinase assay was performed using GST-ATF2 as a substrate in the presence of [γ-32P]ATP. Phosphorylation of ATF2 was monitored by autoradiography, and p38 levels were detected by immunoblotting with anti-p38 MAPK antibody. C, WT MEFs and P38 KO MEFs were transfected with RING mutant (Rm) Siah2-FLAG. 48 h later cells were subjected to 1% hypoxia in the absence or presence of p38 MAPK inhibitor, SB203580, for 4 h in the presence of [32P]orthophosphate as indicated. Cells were harvested, and lysates were immunoprecipitated (IP) with anti-FLAG antibody and subjected to SDS-PAGE analysis. Phosphorylation was monitored by autoradiography followed by immunoblotting (IB) with anti-FLAG antibody. Cell lysates from WT MEFs and p38 KO MEFs were subjected to immunoblot analysis using anti-p38 MAPK antibody. D, 293T cells were transfected with Siah2-HA, Siah2-D mut-HA mutant, and p38 MAPK-FLAG. Cell lysates were subjected to immunoprecipitation with anti-HA antibody followed by immunoblotting with anti-FLAG antibody and anti-HA antibody, respectively. Total cell lysates were also subjected to analysis using antibodies to FLAG to monitor p38 MAPK levels. E, GST-Siah2 and a GST-Siah2-D mut-HA mutant were subjected to an in vitro kinase assay using recombinant p38 MAPK followed by SDS-PAGE analysis. Phosphorylation of Siah2 was monitored by autoradiography.
Phosphopeptide Analysis
For in vivo 32P metabolic labeling wild type (WT)-MEFs and p38 knock-out (KO) MEFs were transfected with Rm-Siah2 and 24 h later incubated with phosphate-free medium containing 10% dialyzed fetal bovine serum for 2 h. The medium was removed, reconstituted with 4 mCi/ml [32P]orthophosphate, and added to cells followed by hypoxia treatment for 4 h. Cells were washed and harvested. Cell lysates were then subjected to immunoprecipitation with anti-FLAG antibody followed by SDS-PAGE analysis. 1 µg of recombinant Siah2 proteins were phosphorylated by recombinant p38 kinase in the presence of 10 µCi of [γ-32P]ATP in kinase buffer as described (22), resolved by SDS-PAGE, and transferred to nitrocellulose. Radiolabeled Siah2 was excised and analyzed by tryptic peptide mapping using the protocol of Luo and co-workers (23).
Immunofluorescence Microscopy
Transfected or untransfected cells grown on glass coverslips were fixed with 3% paraformaldehyde and 2% sucrose in phosphate-buffered saline (PBS) for 20 min at room temperature and washed twice with PBS followed by incubation for 5 min in permeabilization buffer (0.3% Triton X-100, 3 mm MgCl2, and 6.8% sucrose). After blocking the cells with 3% bovine serum albumin/phosphate-buffered saline for 30 min, cells were incubated with anti-HA antibody (1:100) for 1 h, washed, and further incubated with fluorescein isothiocyanate-labeled secondary antibody (1:500 dilutions) for 1 h. Coverslips were inverted and mounted on slides with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) and affixed with nail polish. Fluorescence was monitored using a fluorescence microscope (IX71; Olympus).
RESULTS
Siah2 Enhances Degradation of PHD3 in Hypoxia and Is Phosphorylated by p38 MAPK in Vitro
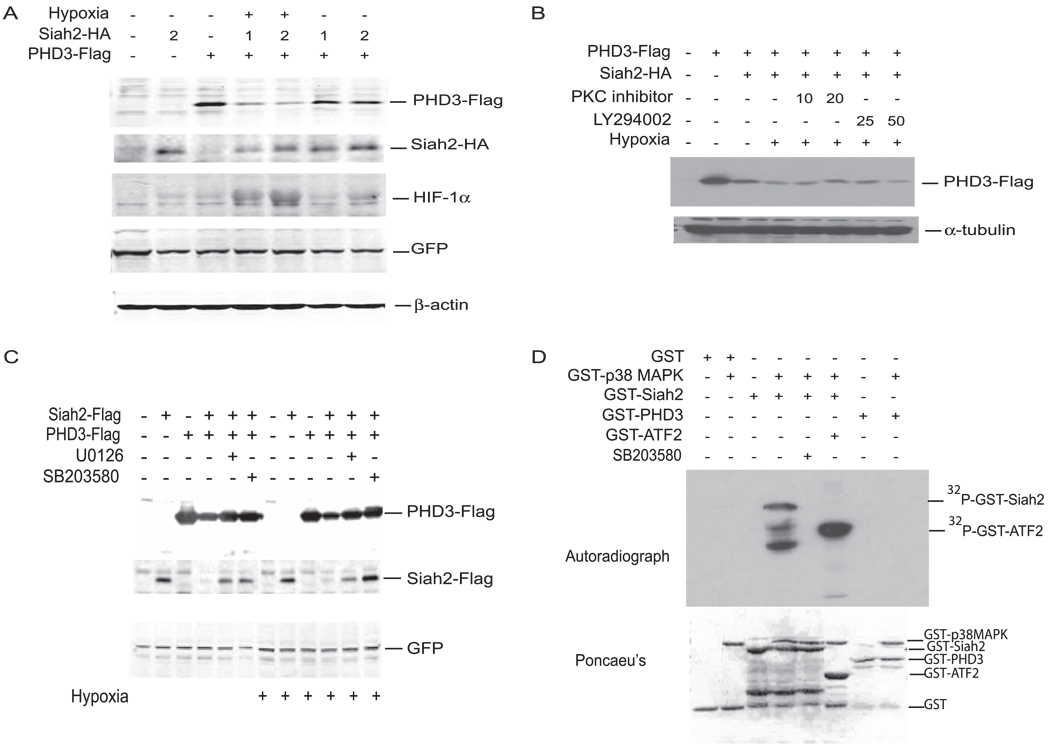
To determine the effect of hypoxia on Siah2-mediated degradation of PHD3, MEF cells were co-transfected with Siah2-HA and PHD3-FLAG followed by growth under normoxia (21%) or hypoxia (1%). Immunoblot analysis revealed that Siah2-dependent degradation of PHD3 is more pronounced under hypoxia (Fig. 1A). The effect of Siah2 on PHD3 degradation was concomitantly seen at the level of HIF-1α, which inversely correlated with that of PHD3. These data are consistent with our earlier finding that Siah2 targets PHD3 for degradation and that the Siah2 effect on PHD3 is better seen in hypoxia (13, 14). Because hypoxia triggers activation of several protein kinases implicated in the hypoxia response, we sought to determine whether Siah2 activity increases in hypoxia could be attributed to altered phosphorylation by a hypoxia-activated kinase. To this end, we compared the effects of pharmacological inhibitors of major signaling pathways on Siah2-mediated degradation of PHD3. Inhibition of protein kinase C did not have a major effect on altered the degree of PHD3 degradation by Siah2 (Fig. 1B). Inhibition of phosphatidylinositol 3-kinase slightly increased Siah2-mediated degradation of PHD3 (Fig. 1B). In contrast, inhibition of p38 MAPK and extracellular signal-regulated kinase 1/2 attenuated Siah2 activity in both normoxia and hypoxia (Fig. 1C). To test whether p38 MAPK and extracellular signal-regulated kinase (ERK) can directly phosphorylate Siah2, we carried out in vitro kinase reactions using purified bacterially produced and recombinant p38 MAPKα and ERK-2. These assays revealed that whereas ERK did not phosphorylate Siah2 (data not shown), p38 efficiently phosphorylated Siah2 but not PHD3 in vitro (Fig. 1D). These data suggest that Siah2 is phosphorylated by p38 and provide the initial indication that such phosphorylation affects Siah2 activity. Subsequent studies, therefore, focused on the role of p38 MAPK in Siah2 phosphorylation.
FIGURE 1. Siah2 phosphorylation by p38 MAPK.
A, MEFs were transfected with Siah2-HA and PHD3 FLAG. 48 h later cells were either left untreated or treated with 1% hypoxia for 4 h. Cell lysates were subjected to immunoblot analysis using anti-FLAG, anti-HA, anti-HIF-1α, anti-green fluorescent protein (GFP), and anti-β-actin antibodies. B, 293T cells were transfected with Siah2-FLAG and PHD3-FLAG as indicated. Cells were either left untreated or treated with 1% hypoxia for 4 h in the presence or absence of protein kinase C (PKC) inhibitor (10 and 20 µm, 4 h) and phosphatidylinositol 3-kinase inhibitor (LY294002, 25 and 50 µm, 4 h). C, 293T cells were transfected with Siah2-FLAG and PHD3-FLAG as indicated. Cells were either left untreated or treated with 1% hypoxia for 4 h in presence or absence of p38 MAPK (SB203580, 5 µm for 4 h) and extracellular signal-regulated kinase inhibitors (U0126, 20 µm for 4 h). Cell lysates were subjected to immunoblot analysis using anti-FLAG, anti-HA, and anti-green fluorescent protein antibodies. D, in vitro kinase assay. Bacterially purified GST-Siah2, GST-PHD3, and GST-ATF2 were incubated with active recombinant p38α in the presence of [γ-32P]ATP for 15 min. The reaction mixture was than subjected to SDS-PAGE analysis. Phosphorylation of substrates was monitored by autoradiography.
Hypoxia Activates p38 MAPK and Stabilizes HIF-1α in a p38 MAPK-dependent Manner
To assess the relevance of p38 to the hypoxia response, we assessed possible changes in the activity of p38 MAPK during hypoxia. Hypoxia treatment (for 2 or 6 h) resulted in activation of p38 MAPK, which coincided with stabilization of HIF-1α. The relation between p38 activation in hypoxia and stabilization of HIF-1α was demonstrated by using the pharmacological inhibitor of p38, which abolished HIF-1α stability (Fig. 2A). This observation is consistent with earlier reports that linked p38 to stabilization of HIF-1α (24, 25). Of note, inhibition of p38 activity also caused a mild increase in PHD3 levels (Fig. 2A), which is compatible with the predicted effect of p38 on Siah2 activity and consequently PHD3 stability. The activation of p38 MAPK by hypoxia was further confirmed by immuno-kinase assays in which p38 was immunoprecipitated from hypoxia-treated cells used for the in vitro kinase reaction of GST-ATF2. Of note, the degree of p38 activation was lower than seen upon treatment with UV irradiation (Fig. 2B). Additional confirmation of the role of p38 in Siah2 phosphorylation came from in vivo labeling experiments in which cells were metabolically labeled with [32P]orthophosphate followed by immunoprecipitation and analysis of exogenously expressed Siah2 (RING mutant was used in these experiments to allow sufficient accumulation of Siah2 since WT Siah2 is subject to an extensive degree of autoubiquitination and degradation, even when expressed as an exogenous protein). Immunoprecipitation of Siah2 followed by autoradiography revealed its phosphorylation in cells after hypoxia treatment; such phosphorylation was abolished after exposure to the p38 pharmacological inhibitor (SB203580; 5 µm, which specifically affects p38 kinases) (Fig. 2C). Further support for the role of p38 in Siah2 phosphorylation comes from the analysis of p38 KO MEFs in which Siah2 phosphorylation was vastly attenuated (Fig. 2C). These data establish that Siah2 is a substrate for p38 MAPK and that its phosphorylation by p38 is induced in hypoxia.
p38 phosphorylation of its substrates was often shown to require association through a conserved D-domain containing a docking site as shown for mitogen-activated protein phosphatase-1 (MKP-1), MKP-2, MKK1, MKK7, and PAC-1 (supplemental Fig. S1). Siah2 sequence analysis identified such a docking site, which is conserved among mouse, human, and Xenopus Siah2 (supplemental Fig. S1). To test the possible importance of the D domain for p38 association and phosphorylation of Siah2, we mutated key residues within this domain (Arg-272, Arg-273, and Arg-274 were converted to Ala; Siah2-D-mut). Expression of WT or D-domain-mutant Siah2 revealed that association with p38 is impaired in the D-mutant form (Fig. 2D). Consistent with this observation, kinase reactions using recombinant p38α as a kinase and bacterially produced and purified WT or D domain-mutant Siah2 as substrates revealed that the phosphorylation of the D domain-mutant form of Siah2 was reduced compared with phosphorylation of the WT protein by p38 (Fig. 2E). These data suggest that p38 association is important for its phosphorylation of Siah2.
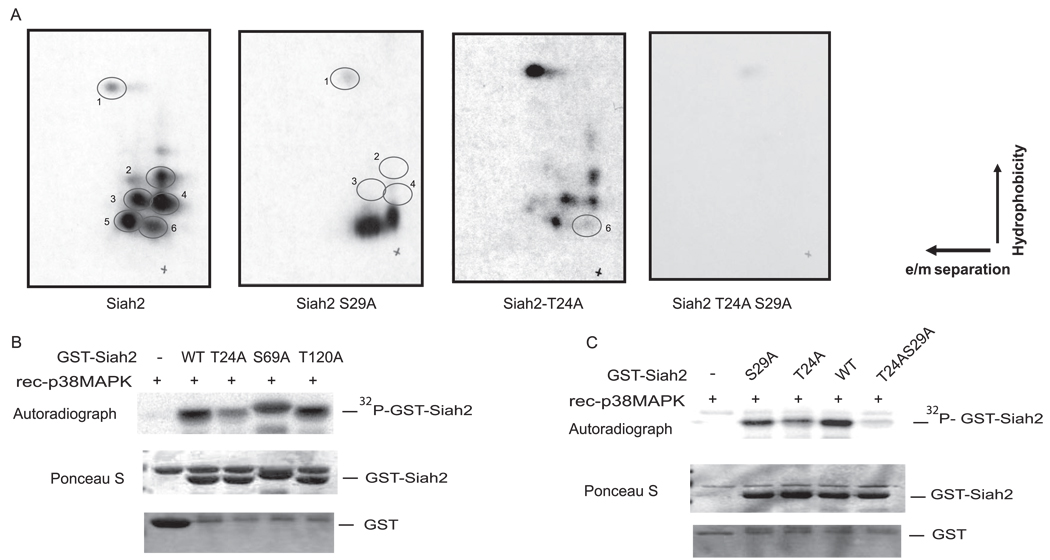
p38 MAPK Phosphorylates Siah2 at Threonine 24 and Serine 29
To identify putative sites for Siah2 phosphorylation of p38, we performed phosphopeptide mapping analysis of GST-Siah2. This analysis identified several peptides phosphorylated in vitro by p38 (Fig. 3A). On the basis of the relative position of the phosphorylated peptides, we predicted possible phosphorylation on amino-terminal residues of Siah2, which were likely to include Thr-24 and Ser-29. To determine whether these are indeed the sites of p38 phosphorylation of Siah2, we monitored change in the peptides phosphorylated on mutant Siah2 using GST-Siah2-T24A, GST-Siah2-S29A, and GST-Siah2-T24A/S29A. This analysis revealed that both Thr-24 and Ser-29 on Siah2 are phosphorylation sites for p38 MAPK as mutation of either site caused the loss of the corresponding peptides (Fig. 3A). Significantly, the double mutant form of Siah2 was no longer phosphorylated by p38 (Fig. 3A). We cannot exclude the possibility that Siah2 may be subject to phosphorylation on other MAPK acceptor sites, which is below the detection limits under the experimental conditions used here.
FIGURE 3. Phosphopeptide mapping for Siah2 phosphorylation by p38.
A, GST-Siah2 and point mutants GST-Siah2-T24A, GST-Siah2 S29A, and GST-Siah2 T24AS29A were phosphorylated in vitro using active recombinant p38 MAPK and then subjected to tryptic digestion. Peptides were separated by chromatography, and phosphorylation was monitored by autoradiography. B, GST-Siah2 and GST-Siah2 mutated on threonine 24, serine 69, and serine 120 to alanine were subjected to an in vitro kinase assay using recombinant (rec) p38 MAPK in the presence of [γ-32P]ATP. The reaction mixture was than subjected to SDS-PAGE analysis, and Siah2 phosphorylation was monitored by autoradiography. C, analysis was performed similar to that shown in panel B, except that single or double phospho-mutant of Siah2 were used.
Further confirmation of the peptide mapping results was obtained upon comparison of all possible p38 phosphorylation sites on Siah2. Mutation of Siah2 on threonine 24 and serine 29 to alanine decreased phosphorylation of Siah2 by p38 MAPK, whereas mutation of serine 69 or threonine 120 did not affect the degree of p38 phosphorylation (Fig. 3B). Consistent with the peptide mapping analysis, mutation on both threonine 24 and serine 29 abolished p38-mediated phosphorylation of GST-Siah2 (Fig. 3C). These data suggest that threonine 24 and serine 29 are the primary sites of phosphorylation on Siah2 by p38 MAPK.
Serine 29 Mutation on Siah2 Attenuates Its Ability to Degrade PHD3
To determine the possible role of Siah2 phosphorylation by p38, we monitored in vitro ubiquitination of Siah2 (self ubiquitination) in vitro and its ability to cause PHD3 degradation in vivo. To this end in vitro translated Siah2-HA was subjected to 1 or 3 rounds of phosphorylation (thereby resulting in a low and a highly phosphorylated form of Siah2) using recombinant p38 followed by analysis of self-ubiquitination in vitro. As shown in Fig. 4A, in vitro ubiquitination of Siah2 was not affected upon its phosphorylation by p38. These data indicate that p38 phosphorylation is not required for its E3 ubiquitin ligase activity per se, as reflected here by its degree of autoubiquitination.
FIGURE 4. Degradation of PHD3 by phospho-mutant forms of Siah2.
A, in vitro ubiquitination (Ub) of WT and mutant Siah2. In vitro translated (IVT) 35S-labeled Siah2-HA was subjected to in vitro kinase assay (or control mock reaction) using recombinant p38 MAPK and either cold or [γ32P]ATP once (lane 5) or three times (lane 6). The reaction mixture was washed extensively and subjected to an in vitro ubiquitination reaction in the presence of E1, E2 (UbcH5c), and Ub-HA for 25 min at 37 °C. The reaction mixture was then separated on SDS-PAGE, and ubiquitination of GST-Siah2 was monitored by immunoblots with anti-HA antibody. The degree of phosphorylation was monitored by autoradiograph, and total levels of GST-Siah2 are shown (middle panel). Levels of Siah2-HA was monitored by immunoblot analysis with anti-HA antibody (lower panel). B, NIH3T3 cells were transfected with Siah2-HA, Siah2 T24A-HA, Siah2S29A-HA, and PHD3-FLAG. Cell lysates were subjected to immunoblot analysis and probed with the antibodies indicated in the figure. C, 293T cells were co-transfected with 1 or 2 µg of Siah2-HA, Siah2 S29A-HA, Siah2 S29D-HA, and PHD3-FLAG. Cell lysates were subjected to immunoblot analysis and probed with anti-FLAG, anti-HA, and β-actin antibodies. D, HeLa cells were transfected with Siah2-HA, Siah2S29A-HA, and Siah2S29D-HA. Cells were either left untreated or treated with 1% hypoxia. Cell lysates were subjected to immunoblot analysis and probed with anti-PHD3, anti-HA, and anti-β-actin antibodies. E, WT MEFs and MKK3/6 knock-out MEFs were transfected with PHD3-FLAG and various amounts (µg) of Siah2-HA as indicated. 48 h later cells were either untreated or treated with 1% hypoxia for 4 h. Cell lysates were subjected to immunoblot analysis using anti-FLAG antibody and anti-β-actin antibody.
To assess possible effects of Siah2 phosphorylation on the degradation of PHD3, we transfected NIH3T3 cells with Siah2-HA, Siah2-T24A-HA, or Siah2-S29A-HA together with PHD3-FLAG. Immunoblot analysis revealed efficient degradation of PHD3 by the WT and the T24A mutant form of Siah2, whereas the S29A mutant of Siah2 was less efficient in mediating PHD3 degradation (Fig. 4B). These data suggest that Ser-29 may be more important in the Siah2 ability to mediate PHD3 degradation. To confirm this possibility, we generated and tested the E3 ligase activity of a phospho-mimic mutant of Ser-29 by mutating it into a negatively charged residue, aspartic acid. Siah2-S29D activity was compared with that of WT and of mutant S29A using PHD3 as a substrate. This analysis revealed that Siah2S29D was more efficient in degradation of PHD3 than the WT Siah2 (Fig. 4C). To verify the effect of WT and mutant forms of Siah2 on degradation of endogenous PHD3, we utilized HeLa cells, which express higher levels of endogenous PHD3. Basal levels of endogenous PHD3 increases under hypoxia (Fig. 4D) due to a HIF-1α-dependent increase in PHD3 transcription (26). Consistent with the changes seen in exogenously expressed PHD3, expression of Siah2-S29A-HA did not affect PHD3 stability, whereas the phospho-mimic mutant Siah2-S29D was more efficient than WT Siah2 in degrading PHD3 (Fig. 4D, compare the second, fourth, sixth, and eighth lanes, respectively).
Siah2-dependent Degradation of PHD3 Is Impaired in MKK3/6 Double KO Cells
To provide direct support for the role of p38 in regulating Siah2 activity toward PHD3 degradation, we used MEFs obtained from MKK3/6 mice, which lack the p38 upstream kinases (27). To this end, WT and MKK3/6 double KO MEFs were co-transfected with Siah2 and PHD3, and the changes in PHD3 stability were analyzed in normoxia and hypoxia. Whereas in WT MEFs Siah2-mediated degradation of PHD3 was seen in normoxia and further increased in hypoxia, such increases were attenuated in MKK3/6 double KO MEFs (Fig. 4E). These data provide genetic support for the role of p38 MAPK in Siah2-dependent degradation of PHD3.
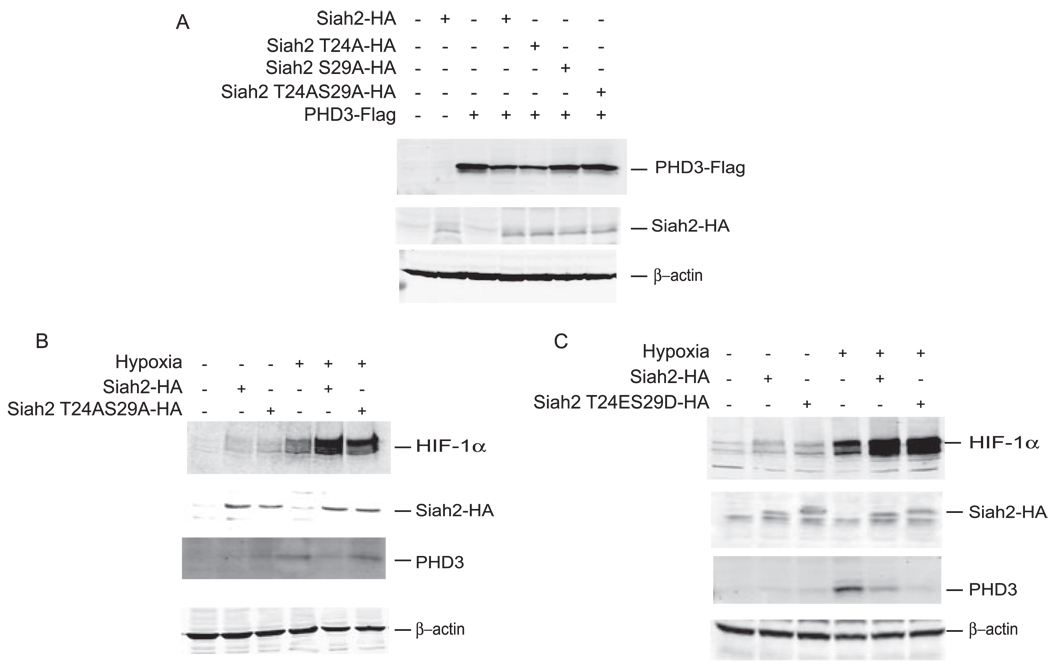
Effect of Phospho-mutant Siah2 on HIF-1α Stabilization
We next compared Siah2-HA, Siah2 T24A-HA, Siah2 S29A-HA, and Siah2 T24AS29A-HA for their ability to degrade PHD3. Whereas Siah2-HA and Siah2 T24A-HA degrades PHD3 efficiently, Siah2 S29A-HA and Siah2 T24AS29A-HA were not effective (Fig. 5A). To further assess the consequences of differential degradation of PHD3 by phospho-mutant and phospho-mimic Siah2-HA for HIF-1α, 293T cells were transfected with Siah2-HA, Siah2 mutated on both phospho-acceptor sites (T24A/S29A-HA), and PHD3-FLAG and subsequently subjected to normoxia or hypoxia. Analysis of HIF-1α stability revealed that hypoxia (5%) induced stabilization of HIF-1α and that such stabilization was increased further upon exogenous expression of WT Siah2 but to a lesser degree upon expression of Siah2 mutated on both p38 phospho-acceptor sites (Fig. 5B). In contrast, HIF-1α levels induced by hypoxia were further elevated upon expression of the phospho-mimic mutant of Siah2, which coincided with effective degradation of endogenous PHD3 (Fig. 5C). These data reveal that elevated expression of Siah2 contributes to stabilization of HIF-1α under hypoxia and that the phosphorylation of Siah2 by p38 on T24S29 is important for the Siah2-dependent effect on both PHD3 and consequently HIF-1α under hypoxia.
FIGURE 5. Effect of Siah2 phospho-mutants on the stabilization of HIF-1α.
A, 293T cells were transfected with PHD3-FLAG, Siah2-HA, Siah2 T24A-HA, and Siah2 S29A-HA. Cell lysates were subjected to immunoblot analysis using anti-FLAG, anti-HA, and anti-β-actin antibodies. B, HeLa cells were transfected with Siah2-HA and Siah2 double phospho-mutant T24AS29A-HA. Cells were either left untreated or treated with 5% hypoxia. Cell lysates were subjected to immunoblotting and probed with anti-HIF-1α, anti-HA, anti-PHD3, and anti-β-actin antibodies. C, HeLa cells were transfected with Siah2-HA and double phospho-mutant Siah2 T24ES29D-HA. Cells were either left untreated or treated with 5% hypoxia. Cell lysates were subjected to immunoblotting and probed with anti-HIF-1α, anti-HA, anti-PHD3, and anti-β-actin antibodies.
PHD3 Association with Siah2 Is Affected by p38 Phosphorylation
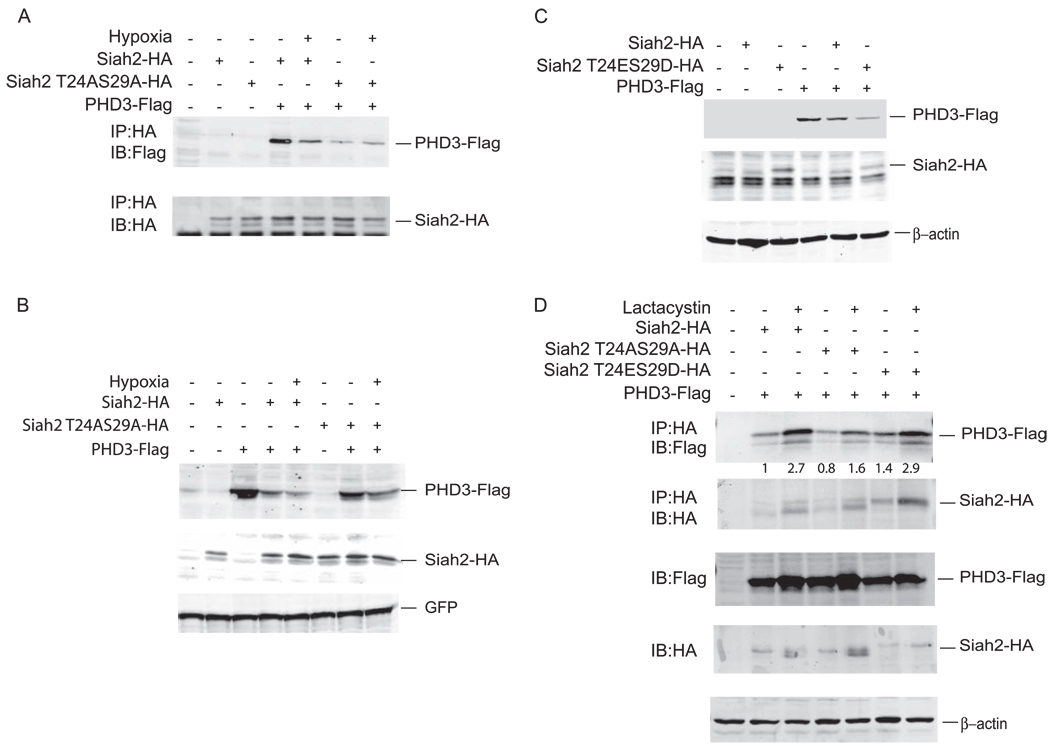
In earlier studies we demonstrated that PHD3 associates with Siah2 and that this association is mediated by the carboxyl-terminal domain of PHD3 (14). Because this association is prerequisite for the Siah2 ability to ubiquitinate PHD3, which results in its degradation by the proteasome, we monitored possible changes in the association between WT and phospho-mutant forms of Siah2 and PHD3. Expression of Siah2-HA, Siah2-T24A/S29A-HA, and PHD3-FLAG in 293T cells revealed that phospho-mutant Siah2 exhibits lower association with PHD3 compared with the WT form (Fig. 6A). The association between WT Siah2 and PHD3 was reduced after hypoxia (due to degradation of PHD3 by Siah2), whereas it remained unchanged in the case of the phospho-mutant form of Siah2, evidently because of impaired E3 ligase activity, which requires p38 phosphorylation in response to hypoxia treatment. Under the same conditions, Siah2 mutated on both phospho-acceptors sites lost most of its ability to cause degradation of PHD3 (Fig. 6B). Similar analysis of the phospho-mimic form of Siah2 revealed its ability to elicit increased PHD3 degradation compared with the WT form of Siah2 (Fig. 6C).
FIGURE 6. Interaction of Siah2 phospho-mutants with PHD3.
A, 293T cells were transfected with PHD3-FLAG, Siah2-HA, and Siah2-T24AS29A. 48 h later cells were either untreated or treated with 1% hypoxia. Cell lysates were subjected to immunoprecipitation (IP) with anti-HA antibody followed by immunoblot (IB) analysis with anti-FLAG antibody followed by anti-HA antibody. B, 293T cells were transfected with Siah-HA, double phospho-mutant Siah2 T24AS29A-HA, and PHD3-FLAG. Cells were either left untreated or treated with 1% hypoxia. Cell lysates were subjected to immunoblot analysis with anti-FLAG, anti-HA, and anti-green fluorescent protein antibodies. C, 293T cells were transfected with Siah2-HA, double phospho-mimetic mutant Siah2 T24ES29D-HA, and PHD3-FLAG. Cell lysates were subjected to immunoblot analysis with anti-FLAG, anti-HA, and anti-β-actin antibodies. D, 293T cells were transfected with Siah2-HA, Siah2 T24AS29A-HA, and Siah2 T24ES29D-HA, and PHD3-FLAG. Cell lysates were prepared after treatment with 20 µm lactacystin (4 h) or control mock treatment followed by immunoprecipitation with anti-HA antibody and immunoblot analysis using anti-FLAG and anti-HA antibodies. Cell lysates were subjected to immunoblotting and probed with anti-FLAG, anti-HA, and anti-β-actin antibodies.
Thus, we next monitored the interaction between PHD3-FLAG, Siah2-T24A/S29A-HA, and Siah2-T24ES29D-HA in the presence of proteasome inhibitor. Treatment with lactacystin increased the degree of PHD3 association with WT and phospho-mutant forms of Siah2, although in both cases relative binding of the phospho-mutant form of Siah2 was noticeably reduced (Fig. 6D). Unlike the reduced association with phospho-mutants, binding with the phospho-mimic form of Siah2 was indistinguishable from the WT form of Siah2 and was enhanced by the addition of proteasome inhibitor (Fig. 6D). These data suggest that phosphorylation of Siah2 on threonine 24 and serine 29 is important for its binding with PHD3.
Phosphorylation of Siah2 by p38 Affects Its Subcellular Localization
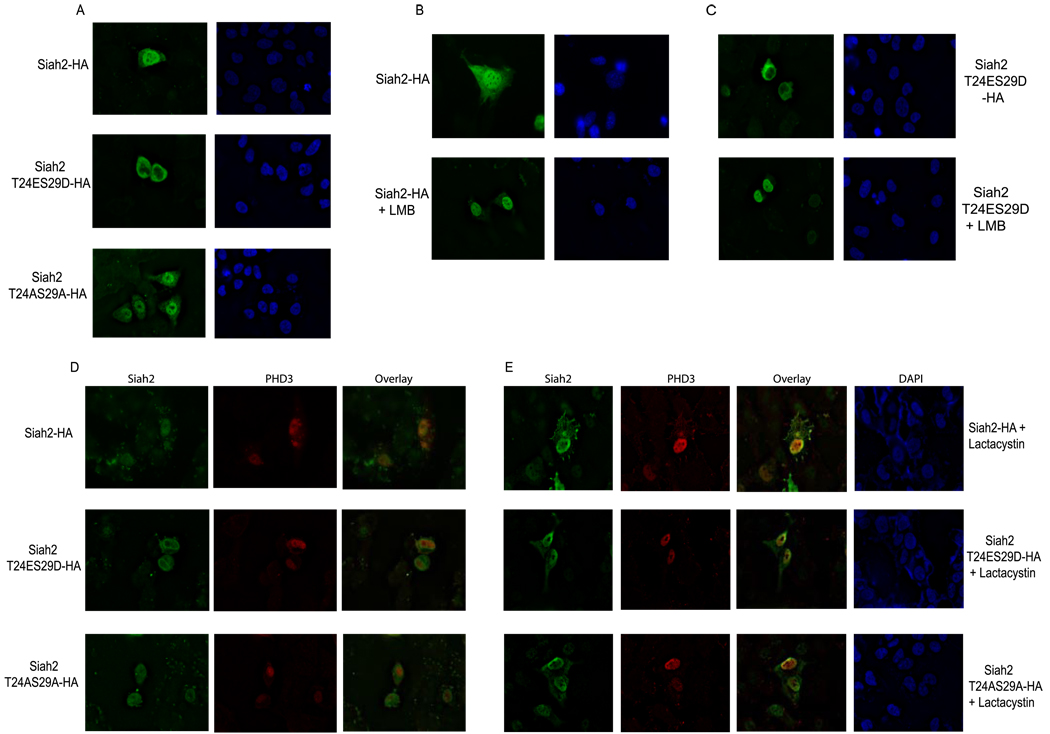
Given the changes in the association of phospho-mutant Siah2 and PHD3, we tested the possibility that phosphorylation of Siah2 affects its subcellular localization, which would contribute to lesser association. HeLa cells were transfected with Siah2-HA, Siah2 T24AS29A-HA, and Siah2 T24ES29D-HA and subjected to immunofluorescence microscopy using anti-HA antibody to detect Siah2 and its phospho-mutants. Both the WT and phospho-mutant (Siah2-T24AS29A-HA) forms of Siah2 were found in both nuclear and cytosolic fractions; however, the phospho-mimic form of Siah2 (Siah2-T24ES29D-HA) was predominantly found within the perinuclear region (Fig. 7A). These data suggest that phosphorylation of Siah2 on Thr-24/Ser-29 results in its exclusion from the nucleus and that its activity after such modification may be enhanced toward proteins that colocalize within this fraction.
FIGURE 7. Subcellular localization of Siah2 phospho-mutants.
A, HeLa cells were transfected with Siah2-HA, Siah2 T24AS29A-HA, and Siah2 T24ES29D-HA. Cells were fixed and subjected to immunofluorescence with anti-HA antibody. B and C, HeLa cells were transfected with Siah2-HA (panel B) and Siah2 T24ES29D-HA (panel C). Cells were treated with nuclear export inhibitor, leptomycin B (100 nm), for 3 h as indicated. Cells were fixed and subjected to immunofluorescence with anti-HA antibody. D, cells were transfected with the indicated HA-Siah2 WT and mutant forms and FLAG PHD3 and processed for immunostaining using antibodies to HA (green) or FLAG (red). Representative pictures are shown for each antibody as well as their overlay. E, cells were transfected with Siah2-HA, SiahT24S29A-HA, Siah2 T24ES29D-HA, and FLAG PHD3 and treated with 20 µm lactacystin for 4 h. Cells were then processed for immunostaining using antibodies to HA (green) or FLAG (red). Representative pictures are shown for each antibody as well as their overlay.
To further determine whether changes in Siah2 localization upon its phospho-mimic mutation can be attributed to export from the nucleus, we tested possible changes in localization of Siah2 after treatment with leptomycin B, a nuclear export inhibitor. Leptomycin B caused accumulation of both Siah2-HA and Siah2-HA T24E/S29D-HA in the nucleus (Fig. 7, B and C). This finding suggests that localization of Siah2-T24E/S29D within the perinuclear region is due to active export from the nucleus, suggesting that p38 phosphorylation of Siah2 facilitates its export from the nucleus.
We next determined which of the Siah2 forms co-localizes with PHD3. As expected, WT Siah2 colocalized with PHD3, primarily within the nuclei (Fig. 7D, upper panel). However, Siah2 mutated on its phospho-acceptor sites did not co-localize with PHD3 despite the fact that both proteins are located within the nuclei, suggesting that subnuclear localization is important for Siah2-PHD3 interaction. Surprisingly, phospho-mimic of Siah2 did not colocalize with PHD3, as it retained its perinuclear staining, whereas PHD3 retained its nuclear staining (Fig. 7D, middle panel). Because our biochemical data revealed increased binding between Siah2 and PHD3 after lactacystin treatment, we further tested their localization when proteasome activity is inhibited, therefore increasing the possibility for capturing association between PHD3 and Siah2 before degradation of PHD3. In the presence of lactacystin Siah2-HA co-localizes with PHD3 in the nucleus and cytosol to a greater degree than seen with Siah2 T24AS29A-HA (Fig. 7E). Co-localization pattern of phospho-mimic Siah2 with PHD3 was found to be increased after exposure with lactacystin, similar to what was seen with WT, which is consistent with our biochemical findings. These data suggest that selective subnuclear localizations are required for Siah2 ability to cause degradation of PHD3 and that the constitutive signal for export of Siah2 to the cytosol, as seen for the phospho-mimic Siah2, is suffice to cause efficient degradation of PHD3 within the nuclei before its export to the cytoplasm. These data further confirm the importance of Siah2 phosphorylation in the regulation of its subcellular localization and degradation of PHD3.
Effect of UV Irradiation on PHD3 Degradation by Siah2
Because p38 is activated by diverse stress stimuli, we have tested the impact of UV irradiation, a potent activator of p38 (Fig. 2B), on Siah2-dependent degradation of PHD3. To this end 293T cells were co-transfected with Siah2-HA and PHD3-FLAG followed by mock or UV treatment. Siah2 ability to reduce the level of PHD3 was less pronounced after UV treatment (supplemental Fig. S2). The lower impact of Siah2 on degradation of PHD3 after UV exposure can be attributed to additional changes elicited by this treatment on Siah2 or PHD3 protein. Indeed, UV irradiation causes an increase in expression of exogenous PHD3, which can be attenuated upon expression of Siah2 (supplemental Fig. S3). Inhibition of p38 activity by dominant negative form did not affect the level of PHD3 expression, implying that the level of PHD3 after UV irradiation is less affected by p38 (supplemental Fig. S4). The effect of Siah2 on PHD3 degradation is distinct from its effect on TRAF2 degradation, which is enhanced after UV irradiation (supplemental Fig. S5 and Ref. 8). These data suggest that p38 phosphorylation of Siah2 is important but not always sufficient for determining the overall level of its substrates.
DISCUSSION
The regulation of E3 ligases is central in the control of their ability to ubiquitinate their substrates. Such regulation has been shown to include modification by deubiquitinating enzymes and sumoylation and association with scaffold proteins as well as partner RING finger proteins. It is, therefore, surprising that only a handful reports address the role of phosphorylation of ubiquitin ligases. In the present study we demonstrate that phosphorylation of Siah2 on Thr-24 and Ser-29 is mediated by p38 MAPK and that such phosphorylation increases Siah2 activity toward PHD3 and consequently toward the levels of HIF-1α. Our findings are consistent with the report that HIF-1α stabilization in hypoxia is dependent on p38 kinases as MKK3/6 double KO cells exhibit impaired level of HIF-1α expression (24).
The notion that p38 is important for Siah2 activity toward degradation of PHD3 is explained by the effect of p38 on (i) subcellular localization of Siah2 and (ii) Siah2 affinity to PHD3. That phosphorylation of a ubiquitin ligase can alter its subcellular localization has been shown for Mdm2, which is phosphorylated by protein kinase B/AKT to result in its localization in the nucleus (29). In the case of Siah2 such phosphorylation causes its exclusion from the nucleus and its localization in the perinuclear region, as revealed by the phospho-mimic form of Siah2. However, co-localization with PHD3 was primarily seen with the WT and not with the phospho-mutant form of Siah2. Of note, co-localization of PHD3 with the phospho-mimic form as well as with the WT but not phospho-mutant form of Siah2 was seen in the nuclear domain upon treatment of cells with proteasome inhibitor, suggesting that the Siah2 effect on PHD3 is carried out within the nuclear domain. This observation in conjunction with our biochemical data indicating strong association and efficient degradation of PHD3 by phospho-mimic Siah2 suggest that (a) Siah2-mediated PHD3 degradation takes place within defined subnuclear fractions (since phospho-mutant Siah2, which generally localized within the nuclei did not colocalize with PHD3) and (b) p38 phosphorylation of Siah2 causes its nuclear exclusion after its degradation of PHD3 in the nucleus.
Because Siah2 substrates are both nuclear and cytosolic, such exclusion is expected to affect the selectivity of Siah2 toward its substrates. Preliminary data suggest that the nuclear co-repressor N-CoR, which is among the nuclear substrates for Siah2, is less affected by Siah2 mutated to resemble phosphorylation by p38 than by Siah2 mutated on these phospho-acceptor sites, suggesting that substrate recognition can be affected by phosphorylation-dependent localization of the Siah2 protein and perhaps serving as a molecular switch to determine the substrate specificity. Along these lines, our study revealed that p38 phosphorylation of Siah2 takes place in response to hypoxia but also after cytokine (tumor necrosis factor α) stimuli or UV irradiation. Surprisingly, the activation of p38 under each of these conditions does not imply increased efficiency in the degradation of PHD3 after each of these stimuli. As found for UV irradiation, Siah2-dependent degradation of PHD3 is less efficient when compared with hypoxia response, probably due to additional changes elicited by UV irradiation on the ligase or the substrate. Kinetics and efficiency of Siah2 degradation of its substrates is expected to vary depending other modifications, localization, and activity of Siah2 and its substrates.
It is possible that phosphorylation on Siah2 can be also mediated by other stress kinases, including c-Jun NH2-terminal kinase, which are equally capable of phosphorylating similar phosphorylation motifs. Consistent with this possibility is the notion that different forms of stress cause Siah2 phosphorylation by different MAPK, which would be expected to have equal impact on its localization and kinase activity. Common to MAPK substrates is the D-domain, which is also found on Siah2 and which serves as a docking site for different members of the MAPK family (30). Such a domain is usually required for p38 association-dependent phosphorylation and possible translocation from one cellular compartment to another. Given the analysis performed with leptomycin B, we conclude that p38 phosphorylation is likely to occur within the nucleus and to promote the export of the phosphorylated ligase to the cytosol, where it concentrates along the perinuclear region.
Constitutive activation of Siah2 by p38 is expected to increase in its degradation of PHD3 with consequential stabilization of HIF-1α. “Hypoxia like phenotypes,” where HIF-1α is stabilized under normoxic conditions, were reported in cases where there is constitutively active AKT signaling (associated with Pten mutation in tumors (31)), suggesting that additional signals are required for stabilization of HIF-1α in addition to increased Siah2 phosphorylation. The latter coincides with the observation that AKT also contributes to transcriptional activation of Siah2,3 suggesting that both transcription and phosphorylation of Siah2 are required for mediating an impact on PHD3 sufficient to result in HIF-1α stabilization.
Of the two sites phosphorylated by p38, Ser-29 appears to have greater impact on Siah2 ability to elicit degradation of PHD3. However, because T24 mutation also decreased Siah2 phosphorylation, it is plausible that phosphorylation of Thr-24 regulates the degree or duration of Ser-29 phosphorylation rather than the changes in Siah2 localization and ligase activity. Phosphorylation of adjacent sites has been reported for several substrates. Often an initial phosphorylation recruits regulatory proteins that alter conformation of the substrate, in turn enabling phosphorylation on a second site, as shown for c-Myc phosphorylation on Ser-58/62 sites (32) or c-Jun phosphorylation by glycogen synthase kinase 3β (28).
In all, the present study provides new insight into regulation of Siah2 activity through its phosphorylation by p38 MAPK. Such regulation offers another layer in the regulation of Siah2 by its interacting proteins POSH and Siah-interacting protein. One would expect that in concert with p38 phosphorylation Siah2 may be subjected to phosphorylation by different kinases, possible control by protein phosphatases, other post-translational modifications, and the activity of deubiquitinating enzymes.
Supplementary Material
Acknowledgments
We thank Angel Nebrada for providing p38 KO cells, David Bowtell for antibodies against Siah2, and Bob Abraham and Gary Chiang for providing the antibodies to HIF-1α. We thank members of the Ronai laboratory for advice and discussions.
Footnotes
This work was supported by NCI, National Institutes of Health Grant RO1-CA111515 (to Z. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
The abbreviations used are: E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; E3 ligase, ubiquitin-protein isopeptide ligase; MAPK, mitogen-activated protein kinase; PHD3, prolyl hydroxylase domain containing 3; MEF, mouse embryonic fibroblast; HA, hemagglutinin; GST, glutathione S-transferase; WT, wild type; KO, knock out; HIF-1α, hypoxia-inducible factor 1α; ERK, extracellular signal-regulated kinase; MKK, mitogen-activated kinase kinase.
K. Nakayama and Z. Ronai, unpublished data.
Supplemental Material can be found at: http://www.jbc.org/content/suppl/2006/11/13/M606568200.DC1.html
REFERENCES
- 1.Hershko A, Ciechanover A. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joazeiro CA, Weissman AM. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 4.Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Li Y, Carthew RW, Lai ZC. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- 6.Boehm J, He Y, Greiner A, Staudt L, Wirth T. EMBO J. 2001;20:4153–4162. doi: 10.1093/emboj/20.15.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Guenther MG, Carthew RW, Lazar MA. Genes Dev. 1998;12:1775–1780. doi: 10.1101/gad.12.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habelhah H, Frew IJ, Laine A, Janes PW, Relaix F, Sassoon D, Bowtell DD, Ronai Z. EMBO J. 2002;21:5756–5765. doi: 10.1093/emboj/cdf576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzawa S, Takayama S, Froesch BA, Zapata JM, Reed JC. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu G, Zhang S, Vidal M, Baer JL, Xu T, Fearon ER. Genes Dev. 1997;15:2701–2714. doi: 10.1101/gad.11.20.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez GJ, Vogtlin A, Castro A, Ferby I, Salvagiotto G, Ronai Z, Lorca T, Nebreda AR. Nat. Cell Biol. 2006;10:1084–1094. doi: 10.1038/ncb1472. [DOI] [PubMed] [Google Scholar]
- 12.Habelhah H, Laine A, Erdjument-Bromage H, Tempst P, Gershwin ME, Bowtell DD, Ronai Z. J. Biol. Chem. 2004;279:53782–53788. doi: 10.1074/jbc.M410315200. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, Bowtell DD, Ronai Z. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama K, Gazdoiu S, Abraham R, Pan ZQ, Ronai Z. Biochem. J. 2006 doi: 10.1042/BJ20061135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield CJ, Ratcliffe PJ. Nat. Rev. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 16.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 17.Hu G, Fearon ER. Mol. Cell. Biol. 1999;1:19724–19732. doi: 10.1128/mcb.19.1.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzawa S, Reed JC. Mol. Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Sproul A, Wang W, Kukekov N, Greene LA. J. Biol. Chem. 2006;281:303–312. doi: 10.1074/jbc.M509060200. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher E, Gao M, Liu YC, Karin M. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1717–1722. doi: 10.1073/pnas.0510664103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. J. Biol. Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- 22.Saxena M, Williams S, Brockdorff J, Gilman J, Mustelin T. J. Biol. Chem. 1999;274:11693–11700. doi: 10.1074/jbc.274.17.11693. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Hurley TR, Sefton BM. Oncogene. 1990;5:921–923. [PubMed] [Google Scholar]
- 24.Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mol. Cell. Biol. 2005;12:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shemirani B, Crowe DL. Oral Oncol. 2002;38:251–257. doi: 10.1016/s1368-8375(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 26.Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. J. Cell. Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- 27.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Mayo LD, Donner DB. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanoue T, Nishida E. Cell. Signal. 2003;5:455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 31.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. Cancer Cell. 2005;6:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, Counter CM, Nevins JR, Means AR, Sears R. Nat. Cell Biol. 2004;4:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.