Abstract
Although large interindividual differences in pain exist, the underlying factors that contribute to these variations remain poorly understood. Consequently, being able to accurately explain variability in pain ratings in terms of its contributing factors could provide insights into developing a better understanding of individual differences in pain experience. In the present investigation, we show that a significant portion of the variability in experimental heat pain ratings may be predicted using simple quantitative sensory testing and a series of psychological questionnaires including State Trait and Anxiety Inventory (STAI), Center for Epidemiologic Studies – Depression Scale (CES-D), and Positive and Negative Affect Schedule – Expanded form (PANAS-X). A factor analysis was used to reduce individual predictors into sets of composite predictive factors. A multifactorial model that was generated from these factors can reliably predict a significant amount of the variability in heat pain sensitivity ratings (r2 = 0.537, p=0.027). Moreover, individual variables including heat pain thresholds and self-assessment of pain sensitivity were found to be poor predictors of heat pain sensitivity. Taken together, these results suggest that a variety of factors underlie individual differences in pain experience, and that a reliable model for predicting pain should be constructed from a combination of these factors.
Perspective
The present study provides a way to predict subjects’ experimental heat pain sensitivity using a multifactorial model generated from a combination of sensory and psychological factors. Future application of such a model in the studies of clinical pain could potentially improve the quality of care provided for patients in pain.
Keywords: pain prediction, psychological factors, model, heat pain, psychophysics
Introduction
A complete subjective experience of pain is uniquely personal and varies significantly from one individual to the next 7, 40. Twin studies indicate that a large portion of interindividual variations in pain experience cannot be accounted for by genetic factors alone, but may arise either from direct environmental influences or by interactions between genetic and epigenetic factors. Although partially determined by both genetic makeup and environment, psychological and cognitive factors are unique to each individual and play an important role in shaping one’s pain experience 35, 40, 41. However, little is known about factors that contribute to interindividual differences in pain sensitivity. For this reason, providing care for patients in pain has proven to be to a difficult and intricate task, and many patients report being unsatisfied with pain relief and care that they have received 31, 34.
To date, many studies have attempted to predict pain experienced by postoperative patients using various predictors ranging from pain thresholds, anxiety scores, blood pressure, and age 13, 25, 32, 36, 65. Most find statistically reliable, but weak correlations between these predictors and important outcome variables. Nevertheless, across studies, suprathreshold experimental pain ratings have been shown to be highly correlated with clinical pain intensity ratings, analgesic use, and other important outcome variables 19, 23, 42, 63. Granot et al. 19, for instance, reported 48°C suprathreshold noxious thermal stimulation as being useful in predicting postoperative pain intensity rating during both rest and activity, while pain thresholds were not.
More recently, many studies have suggested that psychological factors can also significantly influence one’s subjective pain experience 20, 23, 32, 37, 42, 58, 59. For instance, in a postcesarean pain study by Pan et al. 42, a significant portion of the variability in total analgesic requirement can be explained by variability in STAI (State Trait Anxiety Inventory) scores. Furthermore, multiple lines of evidence indicate that emotional states and attitudes of patients can have a profound impact on pain associated with chronic diseases 4, 22, 54. Consistent with these findings, a study by Nielsen et al. 39 examining the reliability of heat pain ratings found that a larger portion of the variance in pain ratings is accounted for by individual differences than variation in stimulus temperature. Nevertheless, the exact contribution of various psychological factors including anxiety, depression, and personality to interindividual variations in pain sensitivity remains poorly understood. In addition, variables such as one’s self-assessment of pain sensitivity may influence treatment in clinical situations. However, the reliability of such variables as predictors of pain sensitivity remains to be established.
Since experimental pain ratings of suprathreshold noxious stimuli may account for much of variability of clinical pain intensity ratings, analgesic use, and other important outcome variables 19, 23, 42, being able to identify reliable predictors of experimental pain ratings may provide useful insights to better understand the factors that may be important in contributing to individual differences in pain experience. Furthermore, in experimental settings, pain thresholds are often used to determine individualized stimulus intensities to minimize effects of individual variation. However, the relationship between pain thresholds and suprathreshold ratings is poorly characterized. In order to investigate these questions and generate reliable models that can be used to predict experimental pain ratings, we performed detailed quantitative sensory testing and psychological assessments on a group of healthy volunteers.
Methods
Subjects
Twenty-one healthy volunteers (eleven male and ten female), 21–38 years old (mean 26.7), participated in this study. All subjects gave informed consent acknowledging that they understood: (1) that they would experience experimental painful stimuli, (2) that all methods and procedures were clearly explained, and (3) that they were free to withdraw from the experiment at any time without prejudice. All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine.
Assessment of psychological factors
Prior to quantitative sensory testing and thermal stimulation, subjects were asked to rate their self-assessment of pain sensitivity using a Visual Analog Scale (VAS). The scale has ‘not at all sensitive to pain’ anchored on one end and ‘extremely sensitive to pain’ on the other. Subsequently, they were asked to complete the State Trait and Anxiety Inventory (STAI), Center for Epidemiologic Studies – Depression Scale (CES-D), and Positive and Negative Affect Schedule – Expanded form (PANAS-X). STAI is a questionnaire that assesses trait (20 questions) and state (20 questions) anxiety. CES-D is a 20-item self-report scale designed to measure presence and severity of depressive symptoms 50. Both STAI and CES-D have been used in many pain studies to assess anxiety and depression, respectively 1, 21, 26, 28, 42, 55, 66. PANAS-X is a 60-item questionnaire designed to measure different inner states and emotions. The PANAS-X has two higher dimensions that assess negative and positive affect, and another dimension for specific affects that is divided into basic negative emotions, basic positive emotions, and other affective states. Among the 60 words presented, ten are in the negative affectivity and ten are in positive affectivity dimensions. Each word is rated on a scale from one to five, as to whether the word fits the habitual or current state of the individual 62. In this study, the habitual state was requested.
Psychophysical data collection
Subjects rated pain using a 0–10 range mechanical (15 cm) visual analog scale (VAS) that has been widely used to assess pain because of ease of use while providing quantifiable measurements of pain intensity and pain unpleasantness (Parisian Novelty Co., Chicago, IL; 48). The minimum was anchored with ‘No pain sensation’ or ‘Not at all unpleasant’, while the maximum was anchored with ‘Most intense pain imaginable’ or ‘Most unpleasant imaginable’. Using an audio analogy, subjects were instructed to distinguish between pain intensity and pain unpleasantness 47. All thermal stimuli were applied to their nondominant ventral forearem via a 16×16-mm2 peltier device (Medoc TSA II, Ramat Yishai, Israel) secured with a Velcro strap. Baseline temperature was maintained at 35°C, and stimulus temperatures were delivered with rise and fall rates of 6°C/s and were feedback controlled. During a training session, subjects rated 32 noxious heat stimuli applied to their non-dominant ventral forearm (35, 43–49°C, 5 s duration) using the VAS in order to gain experience rating pain 6. These data are not reported further.
Heat pain stimulation
Subjects provided post-stimulus pain intensity and pain unpleasantness VAS ratings of fifteen stimuli of five different temperatures (35, 43, 45, 47 or 49°C, 3 repetitions for each temperature) delivered at 5 s duration in a pseudo-random fashion on the non-dominant ventral forearm. To minimize sensitization, habituation or hyperalgesia, all trials were separated by a minimum of 30 s and were systematically distributed over the forearm to minimize repetitive stimulation of the same skin site 43, 44. Stimulus-response curves were generated for each subject using the logarithmic equation:
Where t represents stimulus temperature
The coefficient and intercept generated for heat pain intensity and heat pain unpleasantness were both used as outcome variables.
Cold pain stimulation
During cold pain stimulation, thermal stimuli were delivered to the ventral surface of the non-dominant forearm via a 32×32-mm2 peltier device (Medoc TSA II, Ramat Yishai, Israel). Subjects provided post-stimulus pain intensity and pain unpleasantness VAS ratings of eighteen stimuli of six different temperatures (35, 20, 15, 10, 5 or 0°C, 3 repetitions for each temperature) delivered at 5 s duration in a pseudo-random fashion on the non-dominant ventral forearm. To minimize sensitization habituation or hyperalgesia, all stimuli were delivered as described above.
Quantitative testing of sensory thresholds
Thermal thresholds
Heat pain threshold, cold pain threshold, warm detection threshold, and innocuous cool detection threshold were determined by the method of limits. For each of the four modalities of interest, the 32×32-mm2 thermode was applied to the non-dominant ventral forearm. For warm detection and heat pain thresholds, the temperature was increased at 1°C/s from 35 to 50°C. Subjects were then asked to indicate either the point at which the baseline temperature transitions into a warm sensation (warm detection) or when nonpainful warm sensation changed into a painful heat sensation (heat pain) by pressing a button. For innocuous cool and cold pain thresholds, temperature was decreased at 1°C/s from 35 to 0°C. Subjects were subsequently asked to indicate the point at which the baseline temperature changed into a cool sensation (innocuous cool) or when nonpainful cool sensation transitions into a painful cold sensation (cold pain). For each of the modalities measured, the test was repeated successively six times and the mean threshold temperature was calculated. To minimize sensitization habituation or hyperalgesia, all stimuli were delivered as described above.
Cold pain tolerance
After the thermal stimulation, subjects were requested to immerse their dominant hand in a container of ice-saturated water (approximately 1–2°C) up to the level of the wrist and keep it submerged until the pain became intolerable 27, 38, 45, 56. While their hands were immersed, subjects were instructed to continuously flex and extend their fingers to minimize boundary layer warming. Time from the start of the immersion of the hand until withdrawal were recorded. Our unpublished observations with this method of determining cold tolerance suggest that this method is sufficiently difficult for subjects to tolerate, therefore no upper time limit was set.
Statistical analysis
Statistical analyses were conducted using SPSS software version 13.0 (SPSS Inc., Chicago, IL). Prior to analysis, descriptive statistics were calculated for all variables (i.e., histograms, mean, SD, and range) to confirm that the assumptions for the conducted analyses were satisfied. For all analyses, two-tailed testing at p< 0.05 was used for statistical significance.
To reduce the number of considered predictors in a regression model, principal component factor analysis with varimax rotation was used for data reduction 42. Factor analysis is often used as a data reduction technique to mathematically identify meaningful subgroups of items based on their relationship to each other. Care was taken to create a solution of item groups (factors) that were very similar to each other and accounted for at least as much variance as single predictor (i.e, Eigenvalues > 1.0), but were relatively uncorrelated across factors. Because factor analysis is typically conducted on much larger samples than that of the current exploratory study, a minimally acceptable factor loading of 0.80 or more was used to identify the defining predictors and to better ensure the stability of identified factors 42. Identified predictors were then combined to form factor scores by summing (or subtracting) the individual items in the factor (i.e., unit weighting; taking into consideration the sign of the factor loading). Outcomes were also combined to form factor scores by summing (or subtracting) the individual items in the factor. In some instances, these factors involved combinations of items that would not intuitively thought to be related, but nevertheless exhibited substantial shared variability.
In addition, some independent variables that accounted for a large portion of the variability in the data set, but did not exhibit covariation with other independent variables were also used independently as factors. Next, multiple regression analyses were used to examine if a group of generated predictors (factors) could be used in conjunction to reliably predict each outcome factor. For each of these models, all of the identified factor scores were forced into the model and no attempt was made to further calibrate the model after estimation. Finally, Pearson correlations were used to determine if individual predictors of heat pain threshold and self-assessment of pain sensitivity can predict heat pain sensitivity.
Results
Predictors assessment
The complete details for predictor and outcome variables are shown in table 1. The mean (SD) heat and cold pain thresholds were 47.2 (1.59)°C and 8.5 (8.09)°C, respectively. The mean (SD) warm and cool detection thresholds were 36.8 (0.68)°C and 31.9 (1.28)°C, respectively. The mean self-assessment of pain sensitivity was 3.20 (1.46). The mean state and trait anxiety scores were 33.33 (6.98) and 37.76 (9.73), respectively. The mean negative and positive affect dimensions of PANAS-X were 15.71 (5.85) and 34.24 (4.88), respectively. The mean CES-D score was 7.62 (4.82). It is important to note that none of our subjects had depression scores that would fall in the range of clinical depression (greater than or equal to 21 on CES-D).
Table 1.
Descriptive Statistics of Main Predictor Variables and Outcome Variables
| n | Range | Mean | SD | |
|---|---|---|---|---|
| Predictor variables | ||||
| Sensory modalities threshold | ||||
| Cool detection threshold temperature (°C) | 21 | 27.417 – 33.3 | 31.913 | 1.283 |
| Cold pain threshold temperature (°C) | 21 | 0.433 –28.35 | 8.544 | 8.085 |
| Warm detection threshold temperature (°C) | 21 | 36 – 38.5 | 36.849 | 0.6795 |
| Heat pain threshold temperature (°C) | 21 | 42.633 – 49.433 | 47.157 | 1.590 |
| Psychological factors assessment | ||||
| Self-assessment of pain sensitivity (VAS) | 21 | 1 – 5.7 | 3.195 | 1.455 |
| STAI | ||||
| State anxiety | 21 | 20 – 46 | 33.333 | 6.938 |
| Trait anxiety | 21 | 25 – 55 | 37.762 | 9.731 |
| Total anxiety# | 21 | 47–96 | 71.095 | 13.141 |
| CES-D score | 21 | 1 – 20 | 7.619 | 4.822 |
| PANAS-X | ||||
| Negative affect | 21 | 10 – 32 | 15.714 | 5.849 |
| Positive affect | 21 | 21 – 43 | 34.238 | 4.878 |
| Total affectΦ | 21 | 4 – 33 | 18.524 | 6.392 |
| Outcome variables | ||||
| Noxious stimuli VAS ratings | ||||
| Intensity rating at 0 °C | 21 | 0.133 – 7.267 | 1.452 | 1.559 |
| Unpleasantness rating at 0 °C | 21 | 0 – 6.567 | 0.960 | 1.471 |
| Intensity rating at 49 °C | 21 | 0.4 – 6.167 | 2.322 | 1.466 |
| Unpleasantness rating at 49 °C | 21 | 0 – 3.2 | 1.413 | 1.022 |
| Cold tolerance (seconds) | 19 | 9.93 – 440.53 | 80.05 | 100.694 |
| Coefficient of intensity* | 21 | 0.802 – 8.074 | 4.136 | 1.926 |
| Intercept of intensity* | 21 | −20.003 to (−1.398) | −10.453 | 4.714 |
| Coefficient of unpleasantness& | 21 | −0.5 –12.596 | 4.650 | 3.415 |
| Intercept of unpleasantness& | 21 | −32.372 – 0.725 | −12.26 | 8.486 |
Sum of state anxiety and trait anxiety,
Positive affect minus negative affect,
Variables derived from generating temperature vs. heat pain intensity ratings stimulus-response curve,
Variables derived from generating temperature vs. heat pain unpleasantness ratings stimulus-response curve.
Outcomes assessment
The mean (SD) VAS pain intensity and unpleasantness ratings for 49°C noxious heat stimuli were 2.32 (1.47) and 1.41 (1.02), respectively (Table 1). The mean (SD) VAS pain intensity and unpleasantness ratings for 0°C noxious cold stimuli were 1.45 (1.56) and 0.96 (1.47), respectively. The mean (SD) cold pain tolerance duration was 80.1 (100.7) seconds. It is important to note that there is a large interindividual variability in the range of pain ratings provided by the subjects. This finding is consistent with those of other experimental pain studies that also report large interindividual variations in pain ratings and highlights the large interindividual differences that exist in the pain experience 7, 53.
Predictive and outcome factors using factor analysis
The analysis resulted in five newly formed predictive factors (groups) that accounted for 90% of the total observed variance in the predictors (Table 2). The analysis also yielded five newly formed outcome factors (groups) that accounted for 90% of the total observed variances in outcomes (Table 3). The analysis allows independent variables that exhibited substantial covariation to be combined to form a single factor (thus taking up less room in the regression model). Each factor was then given a name consistent with the several independent measured component variables (Table 2 and 3). For example, negative affect score, depression score, and anxiety scores exhibited substantial covariation and were combined to form a single predictor factor - negative mood (Table 2). Similarly, the outcome variables heat pain intensity ratings and heat pain unpleasantness ratings also exhibited a great deal of covariation and were combined to form the heat pain sensitivity outcome factor (Table 3). These predictor factors were then used in conjunction to generate multiple regression models for predicting each of the composite outcome factors.
Table 2.
The Five Predictive Factors (Groups) and Their Corresponding Component Predictor Variables
| Factor Number | Factor Name | Description |
|---|---|---|
| Factor 1 | Thresholds difference | This represents the difference between heat pain and cold pain thresholds |
| Factor 2 | Warm detection | Threshold temperature for detecting warm sensation |
| Factor 3 | Cool detection | Threshold temperature for detecting cool sensation |
| Factor 4 | Negative mood | This consists of the sum of CES-D, negative affect dimension of PANAS-X, and STAI anxiety scores |
| Factor 5 | Pain positivity | This consists of the sum of positive affect dimension of PANAS-X and self-assessment of pain sensitivity scores |
Table 3.
The Five Outcome Factors (Groups) and Their Corresponding Component Outcome Variables
| Factor Number | Factor Name | Description |
|---|---|---|
| Factor 1 | Heat pain sensitivity | This consists of the sum of mean intensity and mean unpleasantness VAS rating of 49°C noxious heat stimuli |
| Factor 2 | Cold pain sensitivity | This consists of the sum of mean intensity and mean unpleasantness VAS rating of noxious cold stimuli |
| Factor 3 | Heat pain intensity stimulus-response | This represents the difference between coefficient and intercept of heat pain intensity stimulus-response curve |
| Factor 4 | Heat pain unpleasantness stimulus-response | This represents the difference between coefficient and intercept of heat pain unpleasantness stimulus-response curve |
| Factor 5 | Cold tolerance | This represents the time duration (seconds) in which subjects were able to keep their hand submerged in ice/water mixture. |
Final predictive model with multiple regression analysis
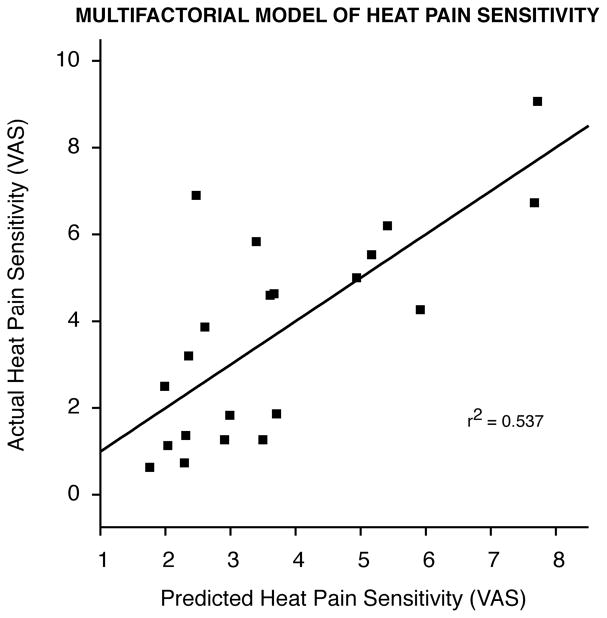
Final predictive models with multiple regression analysis for each composite outcome factor are shown in Table 4. Each of the models was generated using five composite predictive factors (negative mood, pain thresholds, pain positivity, cool detection, and warm detection). Out of the five multiple regression models generated, only models for heat pain and cold pain sensitivity reached statistical significance (Table 4). The five composite predictors provided the best predictive model for heat pain sensitivity (r2 = 0.537, p=0.027)(Fig. 1 and Table 5). Out of the five composite predictors, negative mood and cool detection significantly carried a majority of the weight in this model (β = −0.469, p=0.033 and β = −0.591, p=0.006, respectively)(Table 5). Thus, these factors may contribute importantly to the individual differences in pain experience during experimental heat pain. The model generated for cold pain sensitivity (r2 = 0.614, p=0.008), although statistically significant, was largely driven by an outlier. After exclusion of the outlier, a model generated from the five predictors no longer predicted cold pain sensitivity and was thus excluded from our results. The models generated for cold tolerance, heat pain intensity stimulus-response, and heat pain unpleasantness stimulus-response were not statistically significant, although there appears to be a trend towards significance for heat pain intensity stimulus-response (r2 = 0.442, F=2.372, p=0.089) and heat pain unpleasantness stimulus-response (r2 =0.410, F=2.087, p=0.124) models (Table 4).
Table 4.
Models for Outcome Factors as Predicted by Predictor Factors
| Outcome | F | P Value | r2 | Adjusted r2 |
|---|---|---|---|---|
| Heat Pain Sensitivity | 3.483 | 0.027 | 0.537 | 0.383 |
| Cold Pain Sensitivity * | 4.766 | 0.008 | 0.614 | 0.485 |
| Heat Pain Intensity Stimulus Response | 2.372 | 0.089 | 0.442 | 0.255 |
| Heat Pain Unpleasantness Stimulus Response | 2.087 | 0.124 | 0.410 | 0.214 |
| Cold Tolerance | 0.374 | 0.857 | 0.135 | −0.226 |
Model significance influenced by an outlier. After the outlier was removed, the model was not significant.
F = F statistic, which evaluates the model; r2 = variance in outcome accounted for by the predictors
Figure 1.
Multifactorial model of heat pain sensitivity. The multifactorial model of heat pain sensitivity can account for a significant portion of the variability in actual heat pain sensitivity ratings (r2=0.537; p=0.027). The graph shows heat pain sensitivity ratings as predicted by our multifactorial model (regression line) vs. subjects’ actual heat pain sensitivity ratings.
Table 5.
Multiple Regression Analyses for Each Outcome (Final Model)
| Outcome | Predictors | b | β | t | P Value | Model |
|||
|---|---|---|---|---|---|---|---|---|---|
| F | P Value | r2 | Adjusted r2 | ||||||
| Heat pain sensitivity | 3.483 | 0.027 | 0.537 | 0.383 | |||||
| Negative mood | −0.054 | −0.469 | −2.355 | 0.033 | |||||
| Pain thresholds | −0.044 | −0.163 | −0.843 | 0.413 | |||||
| Pain positivity | 0.048 | 0.109 | 0.603 | 0.555 | |||||
| Cool detection | −1.113 | −0.591 | −3.166 | 0.006 | |||||
| Warm detection | −0.160 | −0.045 | −0.243 | 0.811 | |||||
b = unstandardized regression coefficient; β = standardized regression coefficient; F = F statistic, which evaluates the model; r2 = variance in outcome accounted for by the predictors; t = t statistic, which evaluates the predictor
Pearson correlations between heat pain sensitivity and pain thresholds and self-assessment of pain sensitivity
In addition, we found that heat pain thresholds accounted for a very small variability in heat pain sensitivity (r2=0.044; p=0.362). Self-assessment of pain sensitivity also did not account for a significant portion of variability in heat pain sensitivity (r2=0.033; p=0.429). These results suggest that both heat pain threshold and self-assessment of pain sensitivity are not reliable predictors of the pain experience evoked by noxious stimuli at suprathreshold temperatures.
Discussion
The subjective experience of pain is a uniquely individual and personal sensory experience that involves much more than a simple relay of incoming nociceptive information. To date, the factors that contribute to interindividual differences in the pain experience remain poorly understood. In the present investigation, we show that self-assessment of pain sensitivity and pain thresholds were found to be poor predictors of an individual’s pain sensitivity (r2=0.033; p=0.429, r2=0.044; p=0.362, respectively). However, factor analysis with a combination of meaningful predictors can produce a model that can accurately predict heat pain sensitivity (r2 = 0.537, p=0.027)(Fig. 1 and Table 5). Out of the five composite predictors for heat pain sensitivity, negative mood and cool detection significantly carried a majority of the weight in this model (β = −0.469, p=0.033 and β = −0.591, p=0.006, respectively). The finding that acute heat pain sensitivity cannot be easily predicted by any single variable is consistent with evidence that pain is a complex subjective experience that is constructed from a variety of factors unique to each individual.
Psychological factors and pain sensitivity
Previously, many studies have suggested that the pain experience may be greatly influenced by many psychological factors, including anxiety and emotional state 20, 23, 32, 37, 42, 58, 59. The prevalence of depression in the chronic pain patient population suggests that pain experience and psychological state such as mood may be closely linked 16, 60, 61. Similarly, catastrophizing about pain and clinical depression have been shown to negatively affect the pain experienced by patients 1–3, 11, 13, 17, 18, 20, 22, 26, 60. However, most of these studies were done in clinical populations (e.g. clinically depressed patients or patients undergoing major surgical procedures). Moreover, many studies done in non-clinical settings that reported increased pain sensitivity from anxiety actually tested the effects of induced anticipatory anxiety of an impending event on pain as opposed to the effects of baseline anxiety levels on pain 8, 46, 51. In the present investigation, negative mood (sum of negative affect, depression, and anxiety scores) was inversely correlated with heat pain sensitivity (β = −0.469, p=0.033)(Table 5). In addition, since none of our subjects met the criteria for clinical depression, these findings likely reflect how a combination of negative psychological factors and emotional state can influence nociceptive processing in general population in a non-clinical setting. Thus, even in normal healthy subjects, a combination of psychological test scores may provide useful information for predicting one’s pain sensitivity. It is possible that the observed inverse relationship between negative mood and pain sensitivity may reflect the contribution of brain areas involved in processing cognitive information in priming anti-nociceptive systems through top-down modulation. Such cognitive and psychological factors may be importantly involved in both placebo and nocebo effects. Moreover, studies investigating the placebo effect and the cognitive and psychological influences on pain suggest that brain areas including the prefrontal cortex, anterior cingulate cortex, and the insular cortex may be importantly involving in mediating these processes 9, 10, 29, 49, 57, 64.
Predicting one’s own pain sensitivity
Self-assessment of pain sensitivity is often used as supplemental information in determining analgesic/anesthetic requirements of patients before an acute surgical procedure. For example, patients who believe that they are highly sensitive to pain may strongly prefer conscious sedation over a local anesthetic block when undergoing 3rd molar tooth extraction. However, self-assessments of pain sensitivity have previously been shown to be poor predictors of relatively complex correlates of actual pain sensitivity such as acute pain tolerance, temporal summation of pain, and clinical pain 14, 52. Consistent with these findings, we found that an insignificant portion of the variability in experimental heat pain sensitivity was accounted for by the variability in self-assessment of pain sensitivity (r2=0.033; p=0.429). Taken together, these findings suggest that obtaining self-assessments of pain sensitivity may not provide practically useful information for pain management.
Relationship of thermal thresholds and pain sensitivity
Pain thresholds, together with the slope of the stimulus-response curve, form the fundamental elements of psychophysical equations that predict the magnitude of pain evoked by a given stimulus. Accordingly, individual variations in pain thresholds would be expected to predict a substantial portion of the variability in pain sensitivity. Studies of post-operative pain, however, have produced mixed results on the ability of pain thresholds to predict pain. One study suggests that both resting and evoked pain can be predicted to some extent by pain thresholds 42 while other studies support no such relationship 19. However, these studies necessarily use a threshold stimulus modality that is distinct from that of the post-operative pain. Accordingly, the mismatch between the threshold and the suprathreshold stimulus modalities may contribute to the variability of the findings. In the present study, even when the modality of the threshold stimulus is the same as the modality of the suprathreshold stimulus, we found that no significant amount of variability in experimental heat pain sensitivity was accounted for by the variability in heat pain thresholds (r2=0.044; p=0.362). Furthermore, since it seems intuitive to assume that subjects with lower pain thresholds may exhibit higher pain ratings at suprathreshold noxious temperatures, studies of experimental pain have frequently used subjects’ pain thresholds to determine appropriate suprathreshold noxious temperatures 24, 30. The present findings raise substantial questions about the validity of this strategy. These findings suggest that pain threshold may be more closely related to the ability of the primary nociceptive afferents to detect a noxious stimulus, where as heat pain ratings of a suprathreshold noxious stimulus depend not only sensitivity of each subject’s primary nociceptive afferents to a clearly noxious stimulus but also other cognitive factors unique to each individual (a supraspinal effect) that contribute to how a noxious stimulus is perceived. Although a definitive conclusion regarding a lack of relationship between pain threshold and heat pain sensitivity may not be drawn from a negative finding, our finding is in agreement with similar other studies with significantly larger sample size 19.
In contrast to pain thresholds, cool detection threshold was also a major predictor of heat pain sensitivity in our model. The inverse correlation between cool detection and heat pain sensitivity seen in our model (β = −0.591, p=0.006)(Table 5) may suggest that cool-sensitive afferents could play a role in anti-nociception during noxious stimulation. Cool stimuli have been shown to inhibit pain 5, 15 and cool-sensitive primary afferent nerve fibers have been reported to paradoxically respond to painful heat stimuli 12, 33. Thus, although highly speculative, it may be possible that subjects with either larger numbers of cool fibers or more easily activated cool afferents can recruit pain modulation more readily. However, the exact roles that cool-sensitive afferents play in heat pain modulation require further investigation. Nonetheless, measurement of cool detection threshold can provide useful predictive information about experimental heat pain sensitivity.
Prediction of pain with multifactorial models
Even though the sample size in the present investigation was relatively small, and the ratio of predictors to sample size almost certainly over-fit the available measurements (even with the data reduction techniques), we observed statistical significance in predictive utility as well as in the correlations in several instances. Nevertheless, due to a relatively small sample size, the study is meant to be exploratory in nature, and the applicability of our findings must be applied and interpreted with caution. Our model, generated from relatively simple and brief questionnaires and sensory testing, accounted for over half of the variability observed in suprathreshold noxious pain ratings (r2 = 0.537, p=0.027)(Fig. 1 and Table 5). These results suggest that a multifactorial model can provide an accurate method to predict an individual’s thermal pain sensitivity. Additionally, since suprathreshold heat pain stimuli ratings have been reported to have good predictive value because they closely mirror the clinical pain experience 19, 42, 63, this multifactorial model may potentially be beneficial in screening for subjects that may suffer from severe postoperative pain and need additional pain management. Nevertheless, a significant part of the variability in pain ratings cannot be explained by our existing multifactorial predictive model. In addition, our study only attempts to explain variability in experimental pain. Clinical pain is a complex experience influenced by many factors and is impossible to be simulated in a laboratory setting. Thus, applying these results to predict treatments for clinical pain may not be appropriate. However, future studies examining these factors in both experimental and clinical pain may be of great utility. The refinement and eventual application of multifactorial predictive models of experimental pain, clinical pain, and analgesic requirements holds the potential to significantly improve the quality of care provided to these patients.
Acknowledgments
This study was supported by NIH R01 NS39426 and DA20168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alschuler KN, Theisen-Goodvich ME, Haig AJ, Geisser ME. A comparison of the relationship between depression, perceived disability, and physical performance in persons with chronic pain. Eur J Pain. 2008;12:757–64. doi: 10.1016/j.ejpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Auerbach SM, Laskin DM, Frantsve LM, Orr T. Depression, pain, exposure to stressful life events, and long-term outcomes in temporomandibular disorder patients. J Oral Maxillofac Surg. 2001;59:628–33. doi: 10.1053/joms.2001.23371. discussion 634. [DOI] [PubMed] [Google Scholar]
- 3.Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson LM, Wegener ST. Chronic pain in neurorehabilitation populations: Prevalence, severity and impact. NeuroRehabilitation. 2000;14:127–137. [PubMed] [Google Scholar]
- 5.Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjork E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–48. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- 6.Coghill RC, Mayer DJ, Price DD. Wide dynamic range but not nociceptive-specific neurons encode multidimensional features of prolonged repetitive heat pain. J Neurophysiol. 1993;69:703–16. doi: 10.1152/jn.1993.69.3.703. [DOI] [PubMed] [Google Scholar]
- 7.Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100:8538–42. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornwall A, Donderi DC. The effect of experimentally induced anxiety on the experience of pressure pain. Pain. 1988;35:105–13. doi: 10.1016/0304-3959(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 9.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–9. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craggs JG, Price DD, Perlstein WM, Nicholas Verne G, Robinson ME. The dynamic mechanisms of placebo induced analgesia: Evidence of sustained and transient regional involvement. Pain. 2008 doi: 10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–75. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- 12.Dubner R, Sumino R, Wood WI. A peripheral “cold” fiber population responsive to innocuous and noxious thermal stimuli applied to monkey’s face. J Neurophysiol. 1975;38:1373–89. doi: 10.1152/jn.1975.38.6.1373. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RR, Fillingim RB, Maixner W, Sigurdsson A, Haythornthwaite J. Catastrophizing predicts changes in thermal pain responses after resolution of acute dental pain. J Pain. 2004;5:164–70. doi: 10.1016/j.jpain.2004.02.226. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Fillingim RB. Self-reported pain sensitivity: lack of correlation with pain threshold and tolerance. Eur J Pain. 2007;11:594–8. doi: 10.1016/j.ejpain.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gammon GD, Starr I. Studies On The Relief Of Pain By Counterirritation. J Clin Invest. 1941;20:13–20. doi: 10.1172/JCI101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisser ME, Roth RS, Theisen ME, Robinson ME, Riley JL., 3rd Negative affect, self-report of depressive symptoms, and clinical depression: relation to the experience of chronic pain. Clin J Pain. 2000;16:110–20. doi: 10.1097/00002508-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 18.Graff-Guerrero A, Pellicer F, Mendoza-Espinosa Y, Martinez-Medina P, Romero-Romo J, de la Fuente-Sandoval C. Cerebral blood flow changes associated with experimental pain stimulation in patients with major depression. J Affect Disord. 2008;107:161–8. doi: 10.1016/j.jad.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Granot M, Lavee Y. Psychological factors associated with perception of experimental pain in vulvar vestibulitis syndrome. J Sex Marital Ther. 2005;31:285–302. doi: 10.1080/00926230590950208. [DOI] [PubMed] [Google Scholar]
- 21.Harden RN, Bruehl S, Stanos S, Brander V, Chung OY, Saltz S, Adams A, Stulberg SD. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106:393–400. doi: 10.1016/j.pain.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Haythornthwaite JA, Benrud-Larson LM. Psychological aspects of neuropathic pain. Clin J Pain. 2000;16:S101–5. doi: 10.1097/00002508-200006001-00017. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YW, Somma J, Hung YC, Tsai PS, Yang CH, Chen CC. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103:613–8. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Huber MT, Bartling J, Pachur D, Woikowsky-Biedau S, Lautenbacher S. EEG responses to tonic heat pain. Exp Brain Res. 2006;173:14–24. doi: 10.1007/s00221-006-0366-1. [DOI] [PubMed] [Google Scholar]
- 25.Jamison RN, Taft K, O’Hara JP, Ferrante FM. Psychosocial and pharmacologic predictors of satisfaction with intravenous patient-controlled analgesia. Anesth Analg. 1993;77:121–5. [PubMed] [Google Scholar]
- 26.Jones DA, Rollman GB, White KP, Hill ML, Brooke RI. The relationship between cognitive appraisal, affect, and catastrophizing in patients with chronic pain. J Pain. 2003;4:267–77. doi: 10.1016/s1526-5900(03)00630-8. [DOI] [PubMed] [Google Scholar]
- 27.Jones SF, McQuay HJ, Moore RA, Hand CW. Morphine and ibuprofen compared using the cold pressor test. Pain. 1988;34:117–22. doi: 10.1016/0304-3959(88)90156-X. [DOI] [PubMed] [Google Scholar]
- 28.Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–23. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 29.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci. 2007;18:173–90. doi: 10.1515/revneuro.2007.18.3-4.173. [DOI] [PubMed] [Google Scholar]
- 30.Lautenbacher S, Roscher S, Strian F. Tonic pain evoked by pulsating heat: temporal summation mechanisms and perceptual qualities. Somatosens Mot Res. 1995;12:59–70. doi: 10.3109/08990229509063142. [DOI] [PubMed] [Google Scholar]
- 31.Liu SS, Wu CL. The effect of analgesic technique on postoperative patient-reported outcomes including analgesia: a systematic review. Anesth Analg. 2007;105:789–808. doi: 10.1213/01.ane.0000278089.16848.1e. [DOI] [PubMed] [Google Scholar]
- 32.Logan HL, Gedney JJ, Sheffield D, Xiang Y, Starrenburg E. Stress influences the level of negative affectivity after forehead cold pressor pain. J Pain. 2003;4:520–9. doi: 10.1016/j.jpain.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Long RR. Sensitivity of cutaneous cold fibers to noxious heat: paradoxical cold discharge. J Neurophysiol. 1977;40:489–502. doi: 10.1152/jn.1977.40.3.489. [DOI] [PubMed] [Google Scholar]
- 34.Lovatsis D, Jose JB, Tufman A, Drutz HP, Murphy K. Assessment of patient satisfaction with postoperative pain management after ambulatory gynaecologic laparoscopy. J Obstet Gynaecol Can. 2007;29:664–7. doi: 10.1016/s1701-2163(16)32552-x. [DOI] [PubMed] [Google Scholar]
- 35.MacGregor AJ, Griffiths GO, Baker J, Spector TD. Determinants of pressure pain threshold in adult twins: evidence that shared environmental influences predominate. Pain. 1997;73:253–7. doi: 10.1016/S0304-3959(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 36.Macintyre PE, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1996;64:357–64. doi: 10.1016/0304-3959(95)00128-X. [DOI] [PubMed] [Google Scholar]
- 37.Maggirias J, Locker D. Psychological factors and perceptions of pain associated with dental treatment. Community Dent Oral Epidemiol. 2002;30:151–9. doi: 10.1034/j.1600-0528.2002.300209.x. [DOI] [PubMed] [Google Scholar]
- 38.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen CS, Price DD, Vassend O, Stubhaug A, Harris JR. Characterizing individual differences in heat-pain sensitivity. Pain. 2005;119:65–74. doi: 10.1016/j.pain.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136:21–9. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130:3041–9. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- 42.Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, Washburn SA, Harris L, Eisenach JC. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–25. doi: 10.1097/00000542-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen JL, Kehlet H. Hyperalgesia in a human model of acute inflammatory pain: a methodological study. Pain. 1998;74:139–51. doi: 10.1016/s0304-3959(97)00160-7. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain. 1998;76:377–84. doi: 10.1016/S0304-3959(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 45.Petersen-Felix S, Arendt-Nielsen L, Bak P, Bjerring P, Breivik H, Svensson P, Zbinden AM. Ondansetron does not inhibit the analgesic effect of alfentanil. Br J Anaesth. 1994;73:326–30. doi: 10.1093/bja/73.3.326. [DOI] [PubMed] [Google Scholar]
- 46.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–9. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- 48.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 49.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–90. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 50.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. App Psychol Meas. 1977;1:385–401. [Google Scholar]
- 51.Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 52.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–16. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 54.Schanberg LE, Sandstrom MJ, Starr K, Gil KM, Lefebvre JC, Keefe FJ, Affleck G, Tennen H. The relationship of daily mood and stressful events to symptoms in juvenile rheumatic disease. Arthritis Care Res. 2000;13:33–41. doi: 10.1002/1529-0131(200002)13:1<33::aid-art6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 55.Schuler M, Njoo N, Hestermann M, Oster P, Hauer K. Acute and chronic pain in geriatrics: clinical characteristics of pain and the influence of cognition. Pain Med. 2004;5:253–62. doi: 10.1111/j.1526-4637.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 56.Sindrup SH, Poulsen L, Brosen K, Arendt-Nielsen L, Gram LF. Are poor metabolisers of sparteine/debrisoquine less pain tolerant than extensive metabolisers? Pain. 1993;53:335–9. doi: 10.1016/0304-3959(93)90229-I. [DOI] [PubMed] [Google Scholar]
- 57.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29:2684–94. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 59.Staud R, Price DD, Robinson ME, Vierck CJ., Jr Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–43. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70:678–90. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 62.Watson D, Clark L, editors. X manual for the positive and negative schedule — Expanded form. University of Iowa; Iowa City: 1991. The Panas. [Google Scholar]
- 63.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100:115–9. doi: 10.1097/00000542-200401000-00020. discussion 5A. [DOI] [PubMed] [Google Scholar]
- 64.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–13. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Wise EA, Price DD, Myers CD, Heft MW, Robinson ME. Gender role expectations of pain: relationship to experimental pain perception. Pain. 2002;96:335–42. doi: 10.1016/s0304-3959(01)00473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. 2008;134:69–79. doi: 10.1016/j.pain.2007.03.032. [DOI] [PubMed] [Google Scholar]