Abstract
Members of the polo-like kinases (Plk1, Plk2, Plk3, and Plk4) are involved in the regulation of various stages of the cell cycle and have been implicated in cancer progression. Unlike its other family members the expression of Plk3 remains steady during cell cycle progression, suggesting that its activity may be spatiotemporally regulated. However, the mechanism of regulation of Plk3 activity is not well understood. Here, we show that calcium- and integrin-binding protein 1 (CIB1), a Plk3 interacting protein, is widely expressed in various cancer cell lines. Expression of CIB1 mRNA as well as protein is increased in breast cancer tissue as compared to normal tissue. CIB1 constitutively interacts with Plk3 as determined by both in vitro and in vivo assays. This interaction of CIB1 with Plk3 is independent of intracellular Ca2+. Furthermore, binding of CIB1 results in inhibition of Plk3 kinase activity both in vitro and in vivo. Interestingly, this inhibition of the Plk3 activity by CIB1 is Ca2+-dependent. Taken together, our results suggest that CIB1 is a regulatory subunit of Plk3 and it regulates Plk3 activity in a Ca2+-dependent manner. Furthermore, upregulation of CIB1 in cancer cells could thus inhibit Plk3 activity leading to abnormal cell cycle regulation in breast cancer cells. Thus in addition to Plk3, CIB1 may be a potential biomarker and target for therapeutic intervention of breast cancer.
Keywords: CIB1, calcium- and integrin-binding protein 1, polo-like kinase 3, Plk3, breast cancer
1. Introduction
Altered activity or expression of key regulators of the cell cycle has been shown to be responsible for cancer progression. Polo-like kinases (Plks) a family of serine/threonine kinases have been shown to play pivotal roles in the regulation of cell cycle progression 1–3. The Plk family consists of four members that have been identified in the mammalian cells; Plk1, Plk2/Snk, Plk3/Fnk/Prk (proliferation-related kinase) and Plk4/Sak 4–8. These kinases are characterized by the presence of highly conserved noncatalytic domains termed polo boxes 9. These polo boxes are shown to be involved in protein-protein interactions and are required for subcellular localization of Plks 10. They bind to the phosphorylated serine or threonine residues of the binding partners and regulate subcellular localization of Plks 10–12. In addition, the C-terminus, which contains the polo boxes, is shown to be involved in regulation of kinase activity 13.
Controlled expression or activity of the Plks has been shown to regulate normal progression of cell cycle. Since variations in their activity or expression often leads to oncogenic transformation, these protein kinases are considered to be proto-oncogenes 14. The difference in their function and regulation depends on their spatiotemporal expression, subcellular localization, and substrate specificity 15–17. Expression of Plk1 is strongly correlated with aggressiveness and poor prognosis in many cancers 18–20. Reduction in Plk2 expression has been reported to enhance stress-induced apoptosis 21, whereas, Plk3 expression is shown to increase during G1 phase, but remains mostly unaltered during cell cycle progression 22–24. In contrast to Plk1, Plk3 expression has been negatively correlated with the development of certain cancers 3. It is thus believed that regulation of Plk3 activity or subcellular localization may dictate its function during the cell cycle, but little is known about the mechanism by which this regulation occurs.
Using the yeast two-hybrid system, Plk3 was shown to interact with CIB1 16, which was originally identified as a calcium- and integrin αIIb-binding protein with a sequence similarity to the regulatory molecules calcineurin B and calmodulin 25. CIB1 mRNA and protein expression is widespread, including notable expression in some cancer cell lines 26. We investigated the physiological relevance of interaction between Plk3 and CIB1. Here we show that CIB1 is expressed in several cancer cell lines and its expression is upregulated during breast cancer progression. We also show that CIB1 constitutively binds Plk3 and inhibits its kinase activity in a Ca2+-dependent manner. Taken together, our results suggest that CIB1 acts as a regulatory subunit of Plk3, and thereby contributes to the regulation of the cell cycle in a Ca2+-dependent manner.
2. Materials and methods
2.1. Cell culture and transfection
Human breast carcinoma cell lines T47D, MDA-MB-468, MDA-MB-361, MDA-MB-436, MCF-7, and MCF-10A were all obtained from American Type Culture Collection (ATCC; Manassas, VA) cultured and maintained as per the manufacture’s instruction. T47D cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 100 μg/mL insulin, 100 IU/mL and 100 μg/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA). Human umbilical cord vein endothelial cell (HUVEC) and medium supplements were obtained from Lonza Walkerville, Inc (Conshohocken, PA) and Chinese hamster ovary cells (CHO) were from ATCC. Stable transfection using pcDNA3.1 (Mock) or pcDNA3.1-CIB1 expression vector was performed as described previously 27. Clones stably expressing high levels of CIB1 as determined by Western blot were maintained in the growth medium containing 300 μg/mL G418 (Invitrogen). The design of shRNA specific for Plk3 was performed using OptiRNAi program 28. The top choice of a 23 nucleotide (5′-AAGTCATCCCGCAGAGCCGCGTC-3′) sequence was used to generate a double stranded shRNA with a 9 base pair hairpin loop as described 29. The shRNA construct in pSUPER vector or the empty vector was transiently transfected-using Lipofectamine (Invitrogen) following the manufacture’s instructions. Assays were performed 72 h post transfection. All cells were incubated at 37°C and 5% CO2 with 95% humidity.
2.2. Matched breast tumor/normal expression array
A matched tumor/normal expression array containing cDNA synthesized from 9 human breast tumors and adjacent normal tissue from the same individual was purchased from Clontech (Mountain view, CA, USA). To ensure valid comparisons, the manufactures independently normalized each tumor cDNA to its matched normal sample based on the expression of three housekeeping genes: ubiquitin, 23-kDa highly basic protein, and ribosomal protein S9. Arrays were probed using radiolabeled CIB1 cDNA probe following manufacturer’s protocol. Densitometric quantification of spot intensity was performed using Bio-Rad Gel-Doc scanning software (Richmond, CA, USA).
2.3. Western blot analysis
Cells were lysed using lysis buffer (1% Nonidet P-40 (NP40), 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 10 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mM phenylmethanesulfonyl fluoride (PMSF), and 10 mM NaF) for 30 min on ice, and then centrifuged at 13, 000 rpm for 10 min at 4°C. The proteins (500 μg/mL) were separated by 12% SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to Polyvinylidene fluoride (PVDF) membrane (BioRad). Membranes were blocked with 5% non-fat dry milk in Tris-HCl-buffered saline Tween-20 (TBST) and incubated overnight at 4°C with primary antibodies as indicated. After washing, the membranes were further incubated with corresponding HRP-conjugated secondary antibodies and processed using LumiGLO reagent (Cell Signaling, MA, USA). Band intensity was quantified using Bio-Rad Gel-Doc scanning software. Images were than processed by using Adobe Photoshop Software.
2.4. Immunohistochemistry of breast cancer tissue arrays
A breast cancer tissue array slide containing paraffin sections of 12 tumors specimens and 12 normal breast tissues were purchased from Biomeda (Foster, CA, USA). Another array containing 6 cases of breast tumors of various grades (quadruple core per case) and their corresponding adjacent non-neoplastic tissues (1.5 cm away from tumor) were purchased from Biomax, Rockville, MD, USA. Two separate sets of single core microarray panel of 50 paired breast cancer specimens (various grades and metastasis to lymph nodes) were also purchased from Biomax. Slides were deparaffinized before staining according to the manufacturer’s protocol. Slides were immunofluorescently stained using anti-CIB1 (clone UN7.79) or isotype-specific control IgG (cIgG). Briefly, the slides were treated with 0.2% Triton X-100 in 1X PBS for 5 min, the washed and blocked with 3% BSA in PBS (blocking solution) for 1 h at room temperature (RT). The slides were then incubated with anti-CIB1 (1:100) or anti-Ki67 (Abcam, Cambridge, MA) or cIgG (1:100) at 4°C overnight in a humidified chamber. After incubation, slides were washed three times with blocking solution and incubated with rhodamine X-conjugated Donkey anti-Mouse secondary antibody (1:300) or FITC-conjugated goat anti-rabbit (1:100) for 1 h at RT. Slides were washed three times with blocking solution followed by a final wash of PBS, mounted in Slowfade to minimize fading of the fluorescence intensity. Sections were visualized using LSM Zeiss Laser confocal microscope (Thornwood, NY, USA). Mean fluorescence intensity at the same laser power was measured using Zeiss software. Hematoxylin and Eosin (H&E) stained duplicate slide, which was provided by the manufacturer, was used to identify a tumorigenic phenotype.
2.5. Immunoprecipitation assay
Mock- or CIB1-overexpressing T47D cells were untreated or treated with 50 μM BAPTA-AM (Sigma), a calcium chelator, for 1 h at 37°C, then lysed with ice-cold lysis buffer for 30 min on ice and centrifuged. The lysates were pre-cleared with cIgG and protein G Sepharose beads (Amersham, Piscataway, NJ). Pre-cleared lysates (500 μg/mL) were incubated with anti-CIB1 (7.79) or anti-Plk3 or cIgG for 1 h at RT and incubated further with protein G-Sepharose beads overnight at 4°C. Immunocomplex-captured beads were washed three times with lysis buffer, and boiled in 2X Laemmli sample buffer. The proteins were separated by SDS-PAGE, detected by Western blotting. In a separate set of experiments, where radioactivity was used to determine the kinase activity (as described in the in vitro kinase assay), the membrane was probed with anti-Plk3 (BD Bioscience) to determine the total Plk3 immunoprecipitated. Densitometric analysis of band intensity was quantitated using a Bio-Rad Gel Doc 2000 system.
2.6. Recombinant proteins and in vitro binding assay
Recombinant CIB1 protein was expressed in E. coli and purified as previously described 25. Recombinant Plk3 protein was expressed and purified from insect cells by following the procedure described previously 22. In vitro binding assay was performed as described 25. Briefly, Immulon® 2-HB microtiter wells were coated with 5 μg/mL of recombinant CIB1 protein overnight at 4°C. After blocking with 1% BSA for 1 h at RT, various concentrations of purified recombinant Plk3 was added to the wells and incubated at RT for an additional 1 h. After washing with PBS, bound recombinant Plk3 was detected using anti-Plk3 or isotype specific IgG as control in an ELISA assay and read at 405 nm using a microtiter 96-well plate reader (Dynatech, Chantilly, VA, USA).
2.7. In vitro kinase assay
An in vitro kinase assay was performed as described previously 22. Briefly, purified recombinant Plk3 (500 ng/reaction) along with kinase reaction mixture in the presence or the absence of added recombinant purified CIB1 (1 μg/reaction) was incubated with 20 mg of α-casein along with [γ-32P] ATP (Amersham) for 30 min. In a separate set of experiments, either Plk3 or CIB1 was immunoprecipitated from total cell lysates (500 μg/mL) of mock- and CIB1-transfected T47D cells that were allowed to adhere on collagen and used as a source of Plk3. The 2X sample buffer was added to stop the reaction. Samples were analyzed immediately by SDS-PAGE. Coomassie-stained gels were dried and subjected to autoradiography.
2.8. Statistical analysis
All assays were repeated three times with similar results. Representative data were shown and data analyses were performed using Student’s t test (mean value, s.e.m,). Results were expressed as mean ± s.e.m. P≤ 0.05 were regarded as statistically significant.
3. Results
3.1. CIB1 mRNA expression is upregulated in cancer tissues
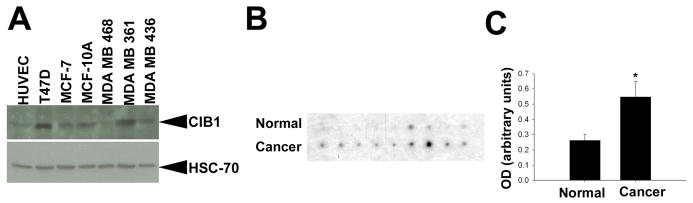
Although CIB1 was originally identified as an integrin αIIb-binding protein 25, it was subsequently shown that CIB1 expression is not limited to platelet-specific lineages. In fact, CIB1 mRNA appears to be widely expressed 26. In order to determine whether CIB1 protein is expressed in breast cancer-derived cell lines, we performed Western blot analysis using CIB1-specific antibody. HUVEC were used as positive control where it was found to express. We found that CIB1 protein is expressed in all breast cancer cell lines tested. Especially, in T47D, MCF-7, MCF-10A, and MDA-MB-361 cell lines, the level of CIB1 expression is substantially more than that of HUVECs (Fig. 1A). Equal loading of protein in each lane was ascertained through HSC-70 probing. We next determined the extent of CIB1 upregulation in breast cancer tissue by probing a cDNA array in which cDNA synthesized from 9 human breast tumors and the adjacent normal tissue from each individual was used. This array provides a sensitive means for the detection and quantitation of differential gene expression relevant to breast cancer. To ensure valid comparison, the manufacturers have independently normalized each tumor cDNA to its matched normal sample using expression of three separate housekeeping genes. We found a detectable level of CIB1 message in the normal tissue. However, all breast tumor tissues showed an increased level of CIB1 mRNA expression compared to their corresponding normal tissue (Fig. 1B). Quantitation of the expression of CIB1 mRNA level in the tumor tissue showed an average of two-fold increase over the normal levels (Fig. 1C). These results suggest that CIB1 mRNA expression is upregulated in breast cancer tissue.
Fig. 1. CIB1 protein is highly expressed in several breast cancer cell lines.
(A) Lysates (25μg/lane) of HUVEC and various breast cancer cell lines as indicated were Western blotted using anti-CIB1 (upper panel) and the same blot was reprobed with anti-HSC-70 to ensure equal loading (lower panel). (B) Autoradiograph of arrays of normalized matched cDNA from breast tissue samples probed with radiolabeled CIB1 cDNA probe. (C) Densitometric quantitation of B. Data are expressed as mean ± S.E. *, P<0.01.
3.2. CIB1 protein expression is upregulated in breast tumor
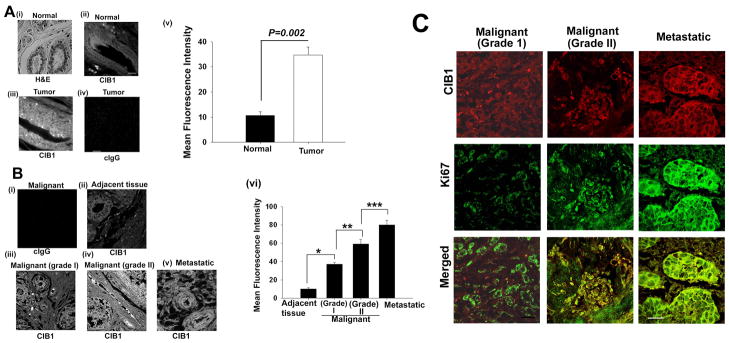
Increased expression of the CIB1 message in breast cancer tissue and a variety of breast carcinoma cell lines, prompted us to determine CIB1 protein expression in normal and tumorigenic breast tissue. Commercially available tumor and normal breast tissue sections were immunohistochemically stained using anti-CIB1 antibody. H&E staining of the normal breast tissue section showed typical glandular morphology and mammary epithelial cell lining (Fig. 2Ai). In normal breast epithelium tissue, CIB1 localized along the cell membrane of glandular epithelial cells as was expected for a myristoylated protein (Fig. 2Aii). Interestingly, in tumor tissue, CIB1 expression appears to be upregulated, and localized throughout the cell, not just restricted to the membrane of the glandular epithelial cells (Fig. 2Aiii). This is not surprising since CIB1 is known to function as a myristoyl switch 30. No staining was observed when isotype-specific control antibody was used, suggesting that the anti-CIB1 staining is highly specific (Fig. 2Aiv). Comparison of pixel intensities of the images captured at identical laser power indicated a significant increase in CIB1 expression in tumors compared to normal breast tissue (Fig. 2Av).
Fig 2. CIB1 protein expression is upregulated in breast tumor tissues.
(A) Breast cancer tissue array from Biomeda (i) Histochemical H&E staining of a representative breast tumor tissue section; Representative image of CIB1 expression in normal (ii) tumor (iii), and (iv) control (isotype-specific control IgG). (v) Quantitation of CIB1 expression in normal and tumor tissue sections. Magnification: × 600. (B) Breast cancer tissue microarray sections from Biomax stained for CIB1 protein, (i) control, (ii) adjacent normal tissue, (iii) malignant grade 1 tumor, (iv) malignant grade 2 tumor, (v) metastatic, and (vi) quantitation of CIB1 expression in tissues shown in ii-v. (C) Confocal images of malignant grade I, grade II, and metastatic tumor stained with anti-CIB1 (red; upper panel), anti-Ki67 (green; middle panel), and merged images showing colocalization (lower panel). Scale bar 10 μm. *P< 0.001; **P< 0.007; ***P<0.01.
In order to assess the expression of CIB1 during breast cancer progression, a tissue array containing samples of various pathological grades assigned by the manufacturer were immunohistochemically stained with anti-CIB1. An isotype specific control IgG had no staining as expected (Fig. 2Bi). Adjacent tissue 1.5 cm away from the tumor showed low staining for CIB1 (Fig. 2Bii). Interestingly, tumor tissue of increasing grade showed increased level of expression of CIB1 (Fig. 2Biii–v). Quantification of mean fluorescence intensity as a measure of CIB1 expression indicated a significant increase in CIB1 expression in various grades of breast tumors compared to adjacent non-cancerous tissue (Fig. 2Bvi). To verify the increase in CIB1 expression is indeed in proliferating tumor cells the sections were double stained for CIB1 and Ki67, a proliferation specific marker. We found that CIB1 and Ki67 colocalized confirming the increased expression of CIB1 in tumor cells (Fig. 2C). Taken together, these results suggest that CIB1 expression is increased during breast cancer progression.
3.3. Plk3 interacts with CIB1 in vivo and in vitro
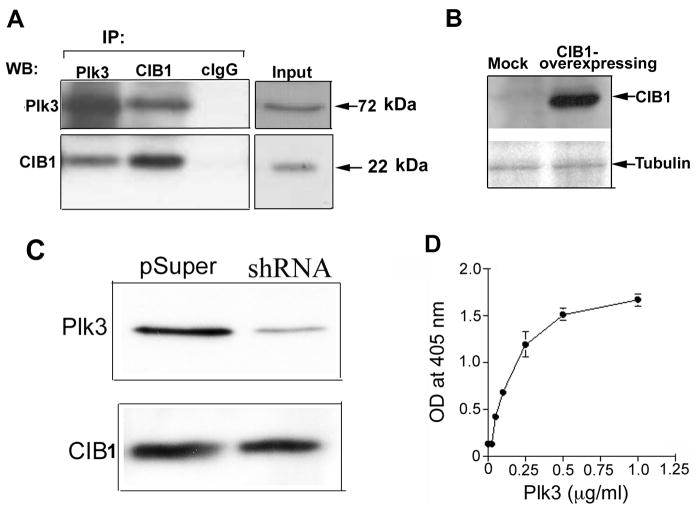
It has been shown previously through the yeast two-hybrid assay that CIB1 and Plk3 interact 16. Due to the established role of Plk3 in cell cycle progression, and the known expression of Plk3 and CIB1 in a number of cancer cell lines, including T47D, it was apparent that the interaction between Plk3 and CIB1 might play a role in cancer progression. To determine their in vivo interaction in a breast cancer cell line, we performed a coimmunoprecipitation assay from T47D cell-lysates using well-characterized, highly-specific antibodies. Plk3 was coimmunoprecipitated with CIB1 by anti-CIB1, but not by an isotype-specific control IgG (cIgG) (Fig. 3A). This was further confirmed in a reciprocal immunoprecipitation experiment where CIB1 was co-immunoprecipitated by anti-Plk3, suggesting that a specific interaction occurs between CIB1 and Plk3 in vivo (Fig. 3A). Although, the antibodies used were shown to be specific to CIB1 25, 27 and Plk3 23, we further confirmed the specificity of CIB1 and Plk3 antibodies by overexpressing human CIB1 protein in CHO cells, and down-regulating Plk3 in T47D cells using Plk3 specific shRNA, respectively. We found that in CHO cells anti-CIB1 does not recognize any band, but in CIB1 overexpressing cells recognizes a 22kDa band corresponding to human CIB1 (Fig. 3B). In T47D cells, anti-Plk3 shows down-regulation of Plk3 by Plk3-specific shRNA (Fig. 3C).
Fig. 3. Endogenous and physiological interactions of CIB1 and Plk3.
(A) Western blot of T47D cell lysate- immunoprecipitates of Plk3 and CIB1 immunoblotted with an anti-Plk3 (upper panel) or anti-CIB1 (lower panel) antibody. Control isotype-specific antibody (cIgG) was used as a control and the whole cell lysate was used as input. (B) Lysates of Mock or CIB1 overexpressing CHO cells blotted with anti-CIB1. Tubulin expression was used for equal loading. (C) Lysates of transiently tranfected T47D cells with pSUPER vector as a control, or with the Plk3-specific shRNA construct were Western blotted using anti-Plk3 (upper panel) and same blot was reprobed with anti-CIB1 (lower panel). (D) Solid-phase in vitro binding assays were performed using immobilized recombinant CIB1 and an increasing concentration of soluble recombinant Plk3 protein, and BSA or IgG were used as a controls to determine non-specific binding. Bound recombinant Plk3 was detected using anti-Plk3 monoclonal antibody in an ELISA assay and read on a plate reader at 405 nm. Data shown in (A) is a representative of three separate experiments.
Coimmunoprecipitation of endogenous CIB1 with Plk3 and vice versa does not determine whether they interact directly or indirectly through other proteins being part of a multi-protein complex. To address this, we generated and purified recombinant Plk3 and CIB1 proteins, and performed an in vitro solid-phase binding assay. We found that Plk3 bound to immobilized CIB1 in a concentration dependent and saturable manner, demonstrating a physical interaction between the two proteins (Fig. 3D). The approximate KD of 2.6 ± 0.5 nM calculated from the above binding data indicate that these proteins bind with a very high affinity. This is consistent with the yeast two-hybrid data reported previously 16. These findings further suggest that the interaction of CIB1 with Plk3 does not require any post-translational modifications such as phosphorylation since the recombinant CIB1 protein used was produced in bacteria.
3.4. Plk3 interacts with CIB1 both in the absence and the presence of Ca2+
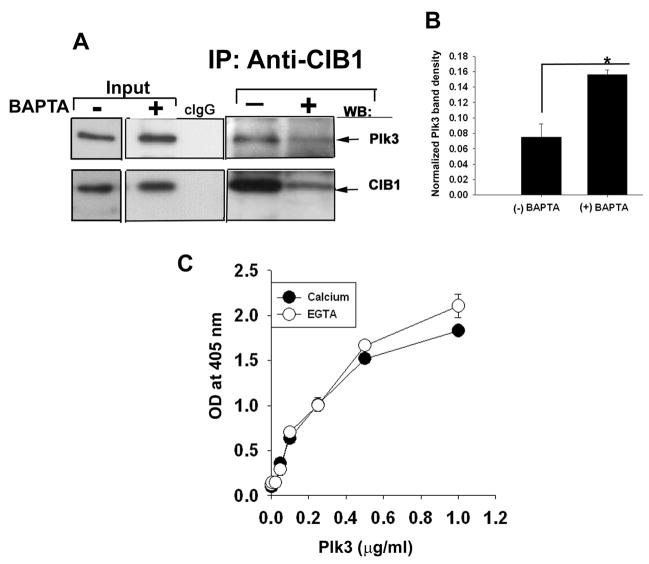
Because CIB1 is known to bind Ca2+ 25, we asked if its interaction with Plk3 is regulated by Ca2+ in vivo. To test this, we repeated the coimmunoprecipitation experiment using lysates of cells pre-treated with or without BAPTA-AM to chelate endogenous Ca2+. Plk3 readily coimmunoprecipitated with CIB1 in the absence of BAPTA-AM treatment (Fig. 4A). The presence of BAPTA-AM greatly affected the amount of CIB1 immunoprecipitated, possibly due to reduced affinity of the antibody for apo-CIB1 (Fig. 4A). The expression of Plk3 was not affected by Ca2+ chelation (Fig. 4A input). It appeared at first that reduced amount of Plk3 in the CIB1 immunoprecipitate of BAPTA-treated cell lysate was due to the reduced interaction with CIB1. However, when normalized to the total immunoprecipitated CIB1, a significantly greater amount of Plk3 was coimmunoprecipitated, indicating that an increased amount of Plk3 bound to CIB1 (Fig. 4B). This suggested a possibility that CIB1 may interact with Plk3 both in the presence and the absence of Ca2+.
Fig. 4. Interaction of CIB1 and Plk3 is independent of intracellular Ca2+.
(A) Lysates of T47D cells pre-treated with or without BAPTA-AM immunoprecipitated and Western blotted for Plk3 (top panel), and CIB1 (lower panel). Inputs represent whole cell lysate (untreated and treated with BAPTA) probed for CIB1. Isotype-specific antibody (cIgG) was used as a control for IP. (B) Quantitation of association of CIB1 and Plk3 in the presence or the absence of BAPTA-AM from (A). Band intensity of the co-immunoprecipitated protein was normalized with band intensity of corresponding total immunoprecipitated protein (P<0.01). Shown in (A) is a representative blot from three separate experiments. (C) Solid-phase in vitro binding assays were performed using immobilized recombinant CIB1 and an increasing concentration of soluble recombinant Plk3 protein in the presence of 2 mM Ca2+ or 2 mM EGTA. BSA was used as a control. Bound recombinant Plk3 was detected using anti-Plk3 monoclonal antibody and quantitated by reading at 405nm using a plate reader. Isotype-specific IgG was used to determine non-specific binding.
To further determine whether the binding affinity between CIB1 and Plk3 is altered in the presence of Ca2+, we performed an in vitro binding assay in the presence of Ca2+ or EGTA. We used purified recombinant Plk3 and CIB1 proteins in this assay. To our surprise, we found that CIB1 bound Plk3 in both the presence and the absence of Ca2+ with slightly better binding in the latter condition (Fig. 4C). These results suggest that CIB1 is constitutively bound to Plk3 and thus may function as a regulatory subunit of Plk3.
3.5. Recombinant Plk3 activity was inhibited by purified recombinant CIB1 in a Ca2+-dependent manner
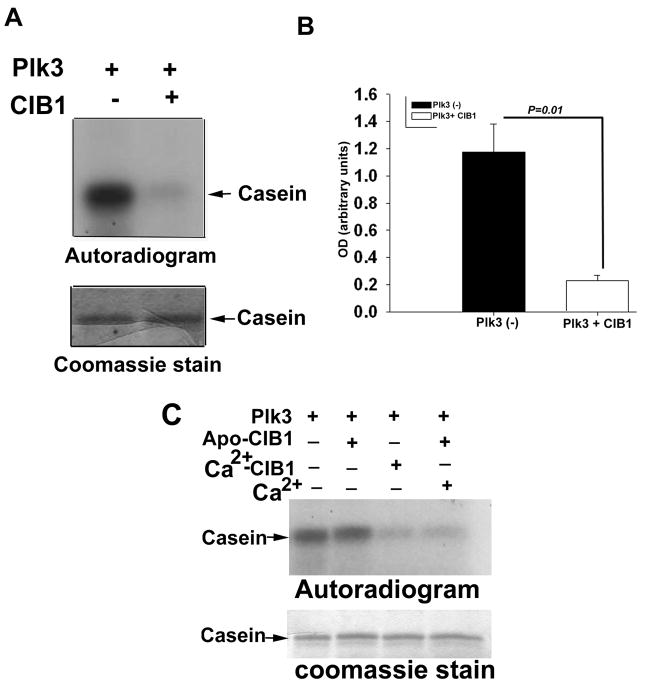
We determined the effect of CIB1 interaction on Plk3 kinase activity in an in vitro kinase assay. We first ascertained that the recombinant Plk3 is pure and active. We also excluded the possibility that some other kinases were present as a contaminant and contributing to the observed activity by showing that recombinant kinase-dead Plk3 prepared similarly does not show any activity in this assay 31. Interestingly, recombinant Plk3 activity was inhibited upon addition of purified recombinant CIB1 (Fig. 5A). Densitometric quantitation of the band intensity from at least three separate experiments indicated a five-fold decrease in kinase activity that was statistically significant (Fig. 5B). This inhibition of Plk3 activity was found to be dependent on the concentration of CIB1 added (data not shown).
Fig 5. CIB1 inhibits Plk3 kinase activity in a calcium-dependent manner.
Autoradiogram analysis of in vitro kinase assay. (A) Phosphorylation of α-casein by Plk3 in the presence or the absence of CIB1. (B) Quantitation of the band intensity of A from more than three separate experiments (P=0.01). (C) Phosphorylation of α-casein by Plk3 (top panel) in the presence of Ca2+-stripped (apo-CIB1) or Ca2+-bound recombinant CIB1 or apo-CIB1 with Ca2+. Coomassie stained gel (bottom panel) is shown to indicate equal loading. Data shown is a representative autoradiograph of at least three separate experiments.
Because CIB1 binds Plk3 in both the presence and the absence of Ca2+, we next investigated the role of Ca2+ in this inhibition. We found that Ca2+-bound CIB1 inhibited Plk3 activity, whereas apo-CIB1 (obtained by extensive dialysis of recombinant CIB1 in the presence of EGTA) had no effect (Fig. 5C). The inability of apo-CIB1 to block kinase activity is not due to misfolding caused by the removal of Ca2+, since the circular dichroism spectrum of apo- and Ca2+-bound CIB1 were identical (data not shown). This was further confirmed by that fact that addition of Ca2+ to apo-CIB1 reestablished its ability to inhibit Plk3 activity (Fig. 5C), indicating that CIB1 regulates Plk3 activity in a Ca2+-dependent manner.
3.6. Ectopic overexpression of CIB1 in T47D cells attenuates Plk3 activity
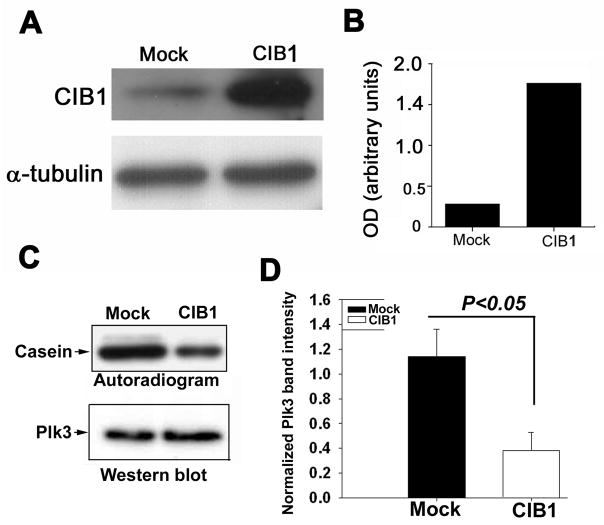
To delineate if CIB1 inhibits Plk3 activity in vivo, we first investigated the endogenous level of CIB1 expression in T47D cells. Western blot analysis showed that a detectable amount of CIB1 expression is endogenous to this cell line (Fig. 6A), as had been reported previously 26. In order to overexpress CIB1, we transfected these cells with a CIB1-pcDNA construct. The cells transfected with CIB1 showed a several-fold higher level of CIB1 expression, as compared to mock cells transfected with an empty vector as a control (Fig. 6A and B). To determine that CIB1 inhibited Plk3 activity in vivo, we immunoprecipitated Plk3 from mock- and CIB1-transfected T47D cell lysates and performed an immunoprecipitate kinase assay. In agreement with our in vitro data, under similar amounts of immunoprecipitation, Plk3 from mock-transfected cells had greater activity than Plk3 from CIB1-transfected cells (Fig. 6C). Quantification of data from several experiments suggested a significant decrease in Plk3 activity in the immunoprecipitates of CIB1-overexpressing cell lysates (Fig. 6D). Taken together, these results suggest that CIB1 is a regulatory subunit of Plk3 and it regulates Plk3 activity in a Ca2+-dependent manner.
Fig 6. Ectopically CIB1-overexpressing cells showed reduced Plk3 activity.
(A) Western blot analysis of cell lysates from mock- and CIB1-overexpressing T47D cells using anti-CIB1 (top panel). Blot was reprobed using anti-a-tubulin antibody to ensure equal loading (bottom panel). (B) Densitometric quantitation of A. (C) Immunoprecipitate kinase assay of Plk3 from mock- and CIB1-overexpressing T47D cell lysates (top panel). The blot was reprobed for Plk3 to ensure equal loading (bottom panel). Shown is a representative blot of three independent experiments.
Discussion
In the present study, we sought to determine the physiological relevance of the interaction between Plk3 and CIB1 in breast cancer progression. We found that CIB1 mRNA as well as protein is upregulated in various cancer cell lines, and in particular breast cancer tissues. CIB1 expression appears to be increased during breast cancer progression. Our results further demonstrate that CIB1 constitutively binds to Plk3 and inhibits Plk3 activity in a Ca2+-dependent manner. Taken together, these results provide evidence that CIB1 is a regulatory subunit of Plk3 and may be involved in the spatiotemporal regulation of this important tumor suppressor.
CIB1 has been shown to interact with Plk2 and Plk3, two of the members of polo-like kinase family 16. Here we show that CIB1 interacts with Plk3 in the presence and the absence of Ca2+, but inhibits Plk3 activity only in the presence of Ca2+. Consistent with our results, it has recently been shown that CIB1 also inhibits Plk2 activity in Cos-7 cells 17. It is possible that CIB1, a calcium-binding protein, is activated upon a rise in intracellular Ca2+ and inhibits these kinases. Polo-like kinase family members are characterized by the presence of a highly conserved polo box, a motif involved in protein-protein interactions, which has been shown to be important for the physiological function of these kinases 32. It has been shown that in addition to its kinase activity, the C-terminus of Plk3 is also important for its function, because overexpression of Plk3 or its kinase-dead mutant induces chromatin condensation and apoptosis 33. This phenomenon seems to be dependent on Plk3′s C-terminal half, where the strictly-conserved polo box domains are situated, because overexpression of a C-terminal deletion mutant of Plk3 failed to induce cell death 33. Recently, it has been shown that the polo box functions as a specific phosphoserine or phosphothreonine binding domain and is involved in the localization of the kinase to the centrosome 32. It is also known that the polo box is important for the subcellular localization of polo-like kinase family members 10, and it is interesting to note that CIB1 has been shown to bind to the polo box of Plk3 possibly in a phosphorylation independent manner 16. It is therefore possible that binding of CIB1 to Plk3 not only inhibits its activity, but may also regulate its subcellular localization.
Plk3 expression is consistently downregulated in several carcinomas 7, 34. It is thus hypothesized that Plk3 plays the role of a safeguard gene in controlling normal cell division, and that its downregulation in carcinomas leads to compromised cell division. Plk3 has also been shown to phosphorylate the tumor suppressor protein, p53 on serine-20 resulting in its activation 35. Thus inhibition or downregulation of Plk3 may lead to tumor progression. Consistent with this notion it has been recently shown that genetic ablation of Plk3 results in spontaneous development of tumors in mice underscoring its function as a tumor suppressor 36.
Expression of Plk3 is rapidly induced upon exposure of serum-starved cells to growth factors, implicating Plk3 in the regulation of cell proliferation 7. Unlike other members of the polo-like kinase family, Plk3 expression levels remain rather constant during the normal cell cycle 37 except that it is slightly upregulated during the G1 phase 24. However, in head, neck, and lung carcinomas, it has been shown that Plk3 levels are significantly downregulated, contrasting that of another family member, Plk1 7, 34, 38. It is thus possible that Plk3 activity is required for normal cell division in such precursor cells, and that its downregulation may aid to cancer development. How Plk3 activity is regulated during the cell cycle in normal cells where Plk3 is substantially expressed is still not clear. However, our finding that CIB1 inhibits Plk3 kinase activity in a Ca2+-dependent manner may provide such an explanation. Further experimentation will be necessary to address this possibility.
Taken together, our results suggest that CIB1 is a regulatory subunit of Plk3, and it affects the activity of Plk3 in a Ca2+-dependent manner. Thus, during the normal cell cycle, Plk3 activity may be regulated by the level of CIB1 and an unwanted change in the expression of either may be a key determinant in the transformation from the normal to the malignant phenotype, making Plk3 and CIB1 potential targets for therapeutic intervention.
Acknowledgments
The authors would like to thank C. Blamey for recombinant CIB1 protein and X. Huang for his help with the in vitro binding assay. This work was supported by the National Institutes of Health Grant HL57630, National Center for Research Resources Grant 1P20RR155801 (to U.P.N.).
Footnotes
Importance: CIB1 expression is upregulated in breast cancer cells. CIB1 constitutively binds to Plk3 and inhibits its activity in a Ca2+-dependent manner.
References
- 1.Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776–83. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- 2.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–76. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 3.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–91. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 4.Clay FJ, McEwen SJ, Bertoncello I, Wilks AF, Dunn AR. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci U S A. 1993;90:4882–6. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–7. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 6.Fode C, Motro B, Yousefi S, Heffernan M, Dennis JW. Sak, a murine protein-serine/threonine kinase that is related to the Drosophila polo kinase and involved in cell proliferation. Proc Natl Acad Sci U S A. 1994;91:6388–92. doi: 10.1073/pnas.91.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, Lu L, Dai W. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402–8. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 8.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–9. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover DM, Hagan IM, Tavares AA. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777–87. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci U S A. 1998;95:9301–6. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roshak AK, Capper EA, Imburgia C, Fornwald J, Scott G, Marshall LA. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–11. doi: 10.1016/s0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 12.Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–8. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci U S A. 2002;99:1984–9. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2:672–6. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EF, Stewart KD, Woods KW, Giranda VL, Luo Y. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry. 2007;46:9551–63. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- 16.Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, Staubli U, Bereiter-Hahn J, Strebhardt K, Kuhl D. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. Embo J. 1999;18:5528–39. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, Liu MA, Yuan YL, Erikson RL. The Serum-Inducible Protein Kinase Snk Is a G(1) Phase Polo-Like Kinase That Is Inhibited by the Calcium- and Integrin-Binding Protein CIB. Mol Cancer Res. 2003;1:376–84. [PubMed] [Google Scholar]
- 18.Ito Y, Yoshida H, Matsuzuka F, Matsuura N, Nakamura Y, Nakamine H, Kakudo K, Kuma K, Miyauchi A. Polo-like kinase 1 (PLK1) expression is associated with cell proliferative activity and cdc2 expression in malignant lymphoma of the thyroid. Anticancer Res. 2004;24:259–63. [PubMed] [Google Scholar]
- 19.Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002;29:354–8. doi: 10.1034/j.1600-0560.2002.290605.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, Ferris DK. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397–405. doi: 10.1006/bbrc.1997.6633. [DOI] [PubMed] [Google Scholar]
- 21.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–71. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang B, Pan H, Lu L, Li J, Stambrook P, Li B, Dai W. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J Biol Chem. 1997;272:28646–51. doi: 10.1074/jbc.272.45.28646. [DOI] [PubMed] [Google Scholar]
- 23.Xie S, Wu H, Wang Q, Cogswell JP, Husain I, Conn C, Stambrook P, Jhanwar-Uniyal M, Dai W. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J Biol Chem. 2001;276:43305–12. doi: 10.1074/jbc.M106050200. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci U S A. 2007;104:1847–52. doi: 10.1073/pnas.0610856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem. 1997;272:4651–4. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- 26.Shock DD, Naik UP, Brittain JE, Alahari SK, Sondek J, Parise LV. Calcium-dependent properties of CIB binding to the integrin alphaIIb cytoplasmic domain and translocation to the platelet cytoskeleton. Biochem J. 1999;342(Pt 3):729–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Naik UP, Naik MU. Association of CIB with GPIIb/IIIa during outside-in signaling is required for platelet spreading on fibrinogen. Blood. 2003;102:1355–62. doi: 10.1182/blood-2003-02-0591. [DOI] [PubMed] [Google Scholar]
- 28.Cui W, Ning J, Naik UP, Duncan MK. OptiRNAi, an RNAi design tool. Computer Methods and Programs in Biomedicine. 2004 doi: 10.1016/j.cmpb.2003.09.002. In Press. [DOI] [PubMed] [Google Scholar]
- 29.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 30.Jarman KE, Moretti PA, Zebol JR, Pitson SM. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. J Biol Chem. 285:483–92. doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, Darzynkiewicz Z, Jhanwar-Uniyal M, Dai W. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22:3450–9. doi: 10.1128/MCB.22.10.3450-3459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of plks by the polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 33.Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res. 2000;60:6826–31. [PubMed] [Google Scholar]
- 34.Dai W, Liu T, Wang Q, Rao CV, Reddy BS. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol. 2002;20:121–6. [PubMed] [Google Scholar]
- 35.Xie S, Wang Q, Wu H, Cogswell J, Lu L, Jhanwar-Uniyal M, Dai W. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–9. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, Winkles JA, Dai W. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–85. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahassi el M, Conn CW, Myer DL, Hennigan RF, McGowan CH, Sanchez Y, Stambrook PJ. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene. 2002;21:6633–40. doi: 10.1038/sj.onc.1205850. [DOI] [PubMed] [Google Scholar]
- 38.Holtrich U, Wolf G, Yuan J, Bereiter-Hahn J, Karn T, Weiler M, Kauselmann G, Rehli M, Andreesen R, Kaufmann M, Kuhl D, Strebhardt K. Adhesion induced expression of the serine/threonine kinase Fnk in human macrophages. Oncogene. 2000;19:4832–9. doi: 10.1038/sj.onc.1203845. [DOI] [PubMed] [Google Scholar]