Abstract
High mobility group box chromosomal protein 1 (HMGB1) is a DNA binding protein that exhibits pro-inflammatory properties when present in the extracellular compartment. Putative receptors for HMGB1 include Toll-like receptor (TLR) 4, TLR2 and the receptor for advanced glycation end products (RAGE). We tested the hypothesis that extracellular HMGB1 can induce tolerance to the bacterial product, lipoteichoic acid (LTA). Pretreatment of human monocyte-like THP-1 cells with 1 μg/ml HMGB1 18 h before exposure to LTA (10 μg/ml) decreased secretion of TNF, NF-κB DNA-binding, and degradation of IκBα. Denaturation of HMGB1 with boiling water or co-incubation with anti-HMGB1 antibody abrogated the induction of tolerance to LTA. In contrast, co-incubation with polymyxin B failed to diminish HMGB1-induced tolerance to LTA. These findings support the view that the induction of LTA tolerance by HMGB1 was not due to LPS contamination. Bone marrow-derived macrophages obtained from C57Bl/6 wild-type and RAGE-deficient mice became LTA-tolerant following HMGB1 exposure ex vivo. We were unable to demonstrate LTA tolerance in TLR2 and TLR4-deficient macrophages, as they are hyporesponsive to LTA. These findings suggest that extracellular HMGB1 induces LTA tolerance, and RAGE receptor is not required for this induction.
Keywords: High mobility group box chromosomal protein 1 (HMGB1), Lipoteichoic acid, tolerance, signal transduction, Toll-like receptor -2
Introduction
HMGB1 is a non-histone nuclear protein that is instrumental for nucleosomal structure and stability, and is important for the binding of transcription factors to their cognate DNA sequences [1]. A decade ago, in a landmark study, HMGB1 was demonstrated to be a late mediator of lipopolysaccharide (LPS; endotoxin) induced-mortality in mice [2-4]. HMGB1 is secreted by immunostimulated monocytes and macrophages [2], pituicytes [5], dendritic cells [6], NK cell [7], endothelial cells [8] and enterocytes [9]. HMGB1 is also released into the extracellular environment by necrotic and damaged cells [10, 11]. Extracellular HMGB1 is thought to signal via several cell-surface receptors, including the receptor for advanced glycation end products (RAGE) and the pathogen associated molecular pattern (PAMP) receptors i.e. Toll-like receptor (TLR)2 and TLR4 [12, 13]. HMGB1 is a prototypical “danger associated molecular pattern” molecule or alarmin that serves to alert and activate the innate immune system in response to tissue injury due to trauma, ischemia/reperfusion or infection [14]. Increased circulating concentrations of HMGB1 have been documented in patients with sepsis [15, 16], pneumonia [17] and trauma [18]. Previously, we had demonstrated that pretreatment of differentiated THP-1 cells with HMGB1 attenuates the inflammatory response to a subsequent exposure to LPS or simply stated, extracellular HMGB1 induces LPS tolerance [19].
It has been established that pre-treating mice [20] with one or more small doses of LPS markedly attenuates the deleterious inflammatory response to subsequent administration of a larger dose of endotoxin. This phenomenon, which is termed “endotoxin tolerance” likewise, has been documented in human volunteers [21]. Similarly, “endotoxin tolerance” can be demonstrated in vitro by pre-incubating cultured macrophages or monocytes with a relatively low concentration of LPS prior to exposing the cells to a larger dose of the same substance [22]. Endotoxin tolerance, or some variant of this process, is thought to be responsible for the observation that peripheral blood mononuclear cells isolated from patients with sepsis are hyporesponsive to LPS as assessed by cytokine production ex vivo [23].
Lipoteichoic acid (LTA) is a pro-inflammatory glycolipid produced by Gram-positive bacteria [24]. Via a TLR2-dependent pathway, highly purified LTA derived from Staphylococcus aureus stimulates monocytes and macrophages to produce TNF, IL-1β and IL-6 [25]. Furthermore, pretreatment of human monocyte-like THP-1 cells with LTA induces tolerance to the glycolipid. Compared to naïve cells, THP-1 cells pre-treated with LTA secrete less IL-1β and TNF following subsequent challenge with a higher concentration of LTA [26].
Herein, we tested the hypothesis that prior exposure to a low concentration of HMGB1 (TLR2 ligand) would blunt the response of monocytes or macrophages to a subsequent challenge with another TLR2 ligand, LTA.
Materials and Methods
Cell culture
The human acute monocytic leukemia cell line, THP-1, was purchased from American Type Culture Collection (ATCC# TIB-202, Manassas, VA). Unless noted otherwise, all reagents were from Sigma-Aldrich (St. Louis MO). For all experiments, cells first underwent a differentiation step by treatment with IFN-γ (100 U/ml; Pierce Biotechnology, Rockford, IL) for 18 h. THP-1 cells were cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin/streptomycin, 0.35 % β-mercaptoethanol, and 10 mM Hepes(pH 7.35)
Studies were performed in 12-well plates (Becton Dickinson, Mountain View, CA) at a density of 1 × 106 cells/ml. Where indicated, stimulation was performed with 10 μg/ml Staphylococcus aureus LTA or 10 μg/ml (S)-(2,3-bis(palmitoyloxy)-(2RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser(S)-Lys4-OH, trihydrochloride (Pam3Cys) (Enzo Life Sciences, Plymouth Meeting, PA). Since LPS is a potential contaminant of recombinant proteins and LPS pretreatment has been demonstrated to induce cross tolerance to LTA in THP-1 cells [27], we tested the recombinant HMGB1 for the presence of LPS. We sought to detect contaminating levels LPS using a chromogenic Limulus amoebocyte lysate assay (Associates of Cape Cod Cape Cod, MA). The concentration of LPS present in the recombinant HMGB1 used for the present studies was less than the lower detection limit for this assay (0.06 ng/ml).
Bone marrow-derived macrophage cultures
Isolation of murine bone marrow-derived macrophages has been described previously [28]. Briefly, bone marrow cells were obtained from C57Bl/6, TLR2−/−, TLR 4−/− (Charles River Laboratories, Wilmington, MA) and RAGE−/− mice were centrifuged in lymphocyte separation medium (LSM) (Mediatech Inc, Herndon, VA). Cells harvested from the interface were cultured in 75 cm2 flasks in Eagle’s minimal essential medium (EMEM) (Mediatech Inc, Hernadon, VA) supplemented with 10% FBS, 2mM glutamine, 15 mM HEPES, 0.02% sodium bicarbonate, 1% Penicillin/Streptomycin and 10 ng/ml granulocyte-macrophage colony stimulating factor (GM-CSF) (Thermo Scientific, Rockford, IL). After a 24-h adherence step (day 2), which allowed for the removal of mature monocytes and fibroblasts from the bone marrow, non-adherent cells from each flask were transferred to a second flask and were fed with medium containing recombinant GM-CSF (10 ng/ml). The cells were again incubated with GM-CSF (10 ng/ml) on day 4. After a total of 7 days in culture, the macrophages were removed enzymatically with the neutral protease, dispase (Worthington Biochemical Corporation, Lakewood, NJ) and collected by gentle scraping. The cells were resuspended in complete EMEM and exposed to experimental conditions.
Model of LTA tolerance with HMGB1
THP-1 cells cultured in RPMI media containing 10% heat-inactivated FBS were incubated with IFN- γ for 18 h to allow for differentiation. The following day, cells were treated with 1 μg/ml of HMGB1 in complete RPMI media, and incubated at 37 °C for 18 h. The cells were then stimulated with 10 μg/ml of LTA for the indicated time intervals. Based on our previous study, we used 1 μg/ml HMGB1 to induce LTA tolerance in THP-1 cells and bone marrow derived macrophages [19]. In preliminary experiments, 1 μg/ml HMGB1 did not induce the NF-κB-dependent luciferase expression in transfected THP-1 cells.
Transient transfections and luciferase assays
An NF-κB-luciferase reporter plasmid was used to measure LTA-dependent activation of NF-κB. The plasmid (3x-NF-κB-Luc) contains the luciferase reporter gene under the control of three tandem NF-κB binding motifs and a minimal interferon-β promoter. THP-1 cells were transfected using DEAE-dextran. THP-1 cells (1 × 106 per ml) were seeded into 12-well plates on the day before transfection. The next day, the cell suspension was washed with STBS (25 mM Tris·Cl, pH 7.4, 137 mM NaCl, 5 mM KCl, 0.6 mM Na2HPO4, 0.7 mM CaCl2, and 0.5 mM MgCl2) and resuspended in STBS (1 × 106 per ml). DEAE-dextran was added to the cell suspension (100 μg/ml), followed by NF-κB-luciferase reporter plasmid (1 μg/ml). The cells were incubated at 37 °C for 15 min, washed twice with STBS, resuspended, and cultured in complete RPMI medium. The transfected cell lines were cultivated for 24 h and harvested. In the initial experiments, HMGB1 (1 μg/ml) was added 24 h after transfection, followed by addition of LTA (10 μg/ml) for 4 h. Further experiments were conducted by exposing differentiated THP-1 cells transfected with the 3x NF-κB luciferase reporter plasmid to increasing log concentrations of recombinant HMGB1 (0.01-1 μg/ml) for 18 h followed by addition of LTA (10 μg/ml) or Pam3Cys (10 μg/ml) for 4 h. After treatment, cellular proteins were extracted and analyzed for luciferase activity according to the manufacturer’s instructions (Enhanced Luciferase Assay Kit, BD Biosciences Pharmingen, San Diego, CA) using a BioOrbit 1250 Luminometer. Luciferase activity is reported as fold induction over control cells (transfected and treated with basal growth media) and corrected for total cellular protein.
Additional tolerance experiments to rule out LPS contamination
As described above, differentiated THP-1 cells were preconditioned with HMGB1 (1 μg/ml) for 18 h and then treated with LTA (10 μg/ml) for 4 h. Further experiments were designed to demonstrate that induction of tolerance to LTA is secondary to HMGB1 and not due to LPS contamination of HMGB1. For these studies, THP-1 cells were pretreated with denatured HMGB1 (1 μg/ml). HMGB1 was denatured by immersion in boiling water for 60 min. For comparison, other THP-1 cells were pretreated with the same concentration of HMGB1, which was not subjected to prolonged heating. The cells were incubated with denatured or intact HMGB1 for 18 h and then stimulated with 10 μg/ml LTA for 4 h. Cells were then harvested for protein extraction and assayed for luciferase activity as described above. To further test the contribution of HMGB1 in inducing tolerance, we pretreated THP-1 cells with HMGB1 and simultaneously added anti-HMGB1 neutralizing antibody (1 μg/ml) for 18 h. Cell supernatant was analyzed for TNF levels.
Enzyme-linked immunosorbent assay (ELISA)
TNF concentrations were measured in culture supernatants from treated cells using a commercially available sandwich ELISA (Biosource, Camarillo, CA). All procedures were performed as recommended by the manufacturer.
Nuclear protein extraction
All nuclear protein extraction procedures were performed on ice with ice-cold reagents. Cells were treated HMGB1 for 18 h before incubation with LTA for 60 min. Cells were washed twice with PBS and harvested by scraping. Cells were pelleted in 1 ml of PBS at 14,000 rpm for 1 min. The pellet was washed twice with PBS and resuspended in lysis buffer (10 mM Tris-HCl, pH 7.8, 10 mM KCl, 1 mM EGTA, 5 mM MgCl2, 1mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). The suspension was incubated on ice for 15 min and Nonidet P-40 was added followed by centrifugation at 4 °C at 2,000 rpm for 5 min. The supernatant was discarded and the cell pellet was dissolved in extraction buffer (20 mM Tris-HCl ,pH 7.8, 32 mM KCl, 0.2 mM EGTA, 5 mM MgCl2, 1 mM DTT, 0.5 mM PMSF 0.1 mM Na2VO4 and 25% v/v glycerol) and incubated on ice for 15 min. Nuclear proteins were isolated by centrifugation at 14,000 rpm at 4 °C for 10 min. Protein concentrations of the resultant supernatants were determined using the Bradford assay. Nuclear proteins were stored at −80 °C until used for electromobility gel shift assays (EMSA).
EMSA
EMSA were performed as previously described [29]. A double stranded oligonucleotide probe corresponding to the NF-κB oligonucleotide probe (5′-GTGGAATTTCCTCTGA-3′) was labeled with γ-[32P] adenosine triphosphate using T4 polynucleotide kinase (Promega, Madison, WI) and purified in Bio-Spin chromatography columns (GE Healthcare, Buckinghamshire, UK). For each sample 4 μg of nuclear proteins were incubated with Bandshift buffer (10 mM Tris-HCl, 40 mM KCl, 1 mM EDTA, 1 mM DTT, 50 ng/ml poly [d(I-C], 10% glycerol) at room temperature with subsequent addition of the radiolabeled oligonucleotide probe for 30 min. Protein-nucleic acid complexes were resolved using a nondenaturing polyacrylamide gel consisting of 5% acrylamide (29:1 ratio of acrylamide: bisacrylamide) and run in 0.25 X TBE (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA) for 1 h at constant current (100 mA). Gels were transferred to Whatman 3MM paper, dried under a vacuum at 80 °C for 1 h, and used to expose to X-ray film at −80 °C with an intensifying screen.
Western blot analysis
Western blot analyses were performed as previously described [29]. Briefly, whole cell lysates containing 30 μg of protein were boiled in equal volumes of loading buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, and 10% β-mercaptoethanol). Proteins were separated electrophoretically on 8–16% gels and subsequently transferred to polyvinylidene difluoride (PVDF; GE Healthcare, Buckinghamshire, UK). For immunoblotting, membranes were blocked with 5% non-fat dried milk in phosphate - buffered saline (PBS) for 1 h. Primary antibody against IκBα (Santa Cruz Biotechnology, Santa Cruz, CA) was applied at 1:250 dilution. After washing thrice with PBS containing 0.5% Tween 20 (PBST), secondary antibody (horse radish peroxidase-conjugated goat anti- rabbit immunoglobulin G, Stressgen, Victoria, British Columbia) was applied at 1:4,000 dilution for 1 h. Blots were washed in PBST thrice for 5 min, incubated in Enhanced Chemiluminescence Reagent (GE Healthcare), and used to expose X-ray film (GE Healthcare).
Statistical Analyses
Differences among groups were evaluated by one-way analysis of variance and Student-Newman-Keuls test. P ≤ 0.05 was considered statistically significant.
Results
HMGB1 pretreatment induces LTA tolerance in differentiated THP-1 cells
Differentiated THP-1 cells were transiently transfected with the 3xNF-κB luciferase reporter plasmid. When these cells were exposed to LTA, they expressed 20.3 ± 1.8 fold more luciferase activity than resting cells (Figure 1a). Preconditioning with 1 μg/ml HMGB1 for 18 h before LTA stimulation significantly attenuated NF-κB-dependent promoter activity (15.5 ± 2.2 fold induction, p <0.05 compared to LTA alone, Figure 1a). Furthermore, HMGB1 induces LTA tolerance in a dose dependent manner (Figure 1b).
Figure 1.

a Effect of HMGB1 preconditioning on LTA-mediated NF-κB promoter activity as assessed by luciferase assay. Differentiated THP-1 cells were transfected with a 3xNF-κB promoter luciferase reporter plasmid and preconditioned with HMGB1 (1 μg/ml) for 18 h, as indicated. Cells were then exposed to LTA (10 μg/ml) for 4 h. Figure 1b Differentiated THP-1 cells were transfected with a 3xNF-κB promoter luciferase reporter plasmid and preconditioned with HMGB1 (0.01, 0.1 or 1 μg/ml) for 18 h, as indicated. Cells were then exposed to LTA (10 μg/ml) for 4 h. Data represent mean ± SEM of three separate experiments with each condition performed in triplicate (*, P<0.05, vs. LTA alone).
Figure 1c Effect of HMGB1 preconditioning on LTA and Pam3Cys mediated NF-κB promoter activity as assessed by luciferase assay. Differentiated THP-1 cells were transfected with a 3xNF-κB promoter luciferase reporter plasmid and preconditioned with HMGB1 (1 μg/ml) for 18 h, as indicated. Cells were then exposed to LTA or Pam3Cys (10 μg/ml) for 4 h (*P<0.05, vs. LTA alone, #P<0.05, vs. Pam3Cys alone). Figure 1d Differentiated THP-1 cells were transfected with a 3xNF-κB promoter luciferase reporter plasmid and preconditioned with HMGB1 (0.01, 0.1 or 1 μg/ml) for 18 h, as indicated. Cells were then exposed to Pam3Cys (10 μg/ml) for 4 h. Data represent mean ± SEM of three separate experiments with each condition performed in triplicate (*P<0.05, vs. Pam3Cys alone).
Figure 1e ELISA results demonstrating the effects of HMGB1 pretreatment on TNF levels in the supernatants of differentiated THP-1 cells. Control cells were maintained in a basal growth medium. Cells were preconditioned with HMGB1 (1 μg/ml) for 18 h before the addition of LTA (10 μg/ml) for 4 h. Data represent the mean ± SEM of three separate experiments with each condition performed in triplicate (*, P<0.05, vs. LTA alone).
Similar to LTA, Pam3Cys is a highly specific synthetic TLR2 agonist that activates the signal transduction cascade leading to downstream TNF expression. In order to test that tolerance induced by HMGB1 pretreatment is not limited to LTA but extends to other TLR2 agonists, we repeated the experiment with Pam3Cys. Pretreatment with 1 μg/ml HMGB1 prior to stimulation with Pam3Cys significantly attenuated NF-κB-dependent promoter activity (43.78 ± 2.62 vs. 60.84 ± 1.09 fold induction, p <0.05 compared to Pam3Cys alone) (Figure 1c). Similar to our observation with LTA, induction of Pam3Cys tolerance by HMGB1 is also dose dependent (Figure 1d).
Tolerance to LTA is associated with marked inhibition of TNF secretion [27]. To determine whether HMGB1 pretreatment inhibits LTA-mediated TNF secretion, we measured the concentration of this cytokine in supernatants from THP-1 cells. As expected, when naive cells were incubated with LTA, they secreted TNF into the medium (Figure 1b). However, if the cells were pre-treated with HMGB1, LTA-induced TNF secretion was significantly down-regulated (713±42 pg/ml vs. 1041±45, P<0.05; Figure 1b). These data suggest that pretreatment with HMGB1 induced tolerance to subsequent LTA-mediated NF-κB activation.
Preconditioning with boiled HMGB1 fails to induce LTA tolerance
A potential confounding factor with the use of E.coli-derived recombinant proteins is the presence of LPS as a contaminant. We conducted additional experiments to test the hypothesis that induction of LTA tolerance is a property of HMGB1 and not due to contamination with LPS. As a first step, we compared the effectiveness of heat-denatured HMGB1 with nonboiled HMGB1 for the induction of tolerance to LTA. Note that boiling for 60 min is sufficient to denature the protein, whereas this treatment has no effect on the pro-inflammatory properties of LPS. Differentiated THP-1 cells were pretreated with nonboiled or boiled HMGB1 (1μg/ml) for 18 h, followed by exposure to 10 μg/ml LTA for 4 h. We observed that NF-κB promoter activity was comparable in THP-1 cells preconditioned with boiled HMGB1 and cells treated with LTA alone (19.3±0.5 vs. 20.3±1.8 fold induction; Figure 2). In contrast, when cells were treated with nonboiled HMGB1, we observed attenuation of the NF-κB-dependent reporter activity compared with cells treated with LTA alone (15.5 ± 2.2 vs. 20.3±1.8 fold induction; Figure 2). As a next step, we compared the induction of LTA tolerance by HMGB1 when incubations were carried out in the absence or presence of the LPS neutralizing agent, polymyxin B. Similar to pretreatment with HMGB1 alone, pretreatment with HMGB1 in the presence of polymyxin B successfully induced LTA tolerance (15.5±2.2 vs. 14.6±1.1 fold induction of luciferase activity; Figure 2). These data support the view that induction of tolerance to LTA is a property of HMGB1 and not endotoxin contamination of recombinant HMGB1.
Figure 2.

Effect of preconditioning with nonboiled HMGB1, boiled HMGB1and nonboiled HMGB1 in the presence of endotoxin neutralizer polymyxin, on LTA-mediated NF-κB promoter reporter luciferase activity. Differentiated THP-1 cells were transfected with 3xNF-κB promoter luciferase reporter plasmid and preconditioned with HMGB1 (1 μg/ml), or boiled HMGB1 (1 μg/ml) and nonboiled HMGB1 in the presence of endotoxin neutralizer polymyxin, for 18 h. Cells were then exposed to LTA (10 μg/ml) for 4 h. Data represent mean ± SEM of three separate experiments with each condition performed in triplicate. (*, P<0.05, vs. LTA alone and #, P<0.05, vs. LTA + HMGB1).
HMGB1 antibody blocks induction of LTA tolerance by HMGB1
To further ascertain that induction of LTA tolerance is a property of HMGB1, we sought to determine if simultaneous addition of HMGB1 and anti-HMGB1 neutralizing antibody (1 μg/ml) would block induction of LTA tolerance. As expected, TNF levels in culture supernatants were lower when THP-1 cells were preconditioned by exposure to HMGB1 prior to being challenged with LTA (1041±45 pg/ml for cells treated with LTA alone vs. 713±42 pg/ml for cells treated with HMGB1 then LTA, P<0.05; Figure 3). Simultaneous addition of HMGB1and anti-HMGB1 antibody during preconditioning blocked induction of LTA tolerance, as compared with cells preconditioned with HMGB1 alone (1154±56 vs. 713±42 pg/ml, P<0.05; Figure 3). As an additional control, we showed that addition of an irrelevant antibody (anti-β-actin antibody failed to block the induction of tolerance by HMGB1 (698±45 vs. 1041±45 pg/ml, P<0.05; Figure 3). Collectively, these data reinforce the notion that induction of LTA tolerance is a property of HMGB1. Furthermore, contamination of the recombinant HMGB1 preparation with LPS does not appear to be the basis for our observations.
Figure 3.
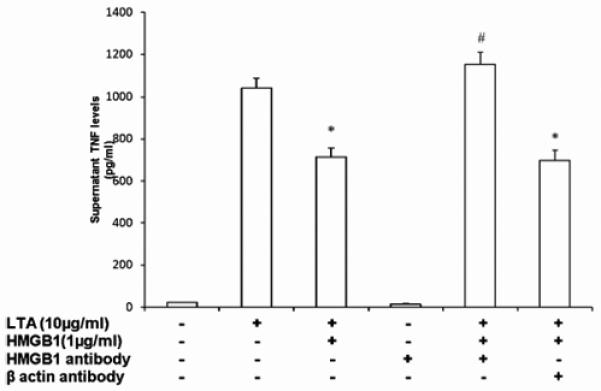
ELISA results demonstrating the effects of HMGB1 pretreatment, HMGB1+ HMGB1 antibody, HMGB1+ βactin antibody on TNF levels in the supernatants of differentiated THP-1 cells. Control cells were maintained in a basal growth medium. Cells were preconditioned with HMGB1 (1 μg/ml), HMGB1+ HMGB1 antibody(1 μg/ml), HMGB1+ βactin antibody(1 μg/ml) for 18 h before the addition of LTA 10 μg/ml for 4 h. Data represent the mean ± SEM of three separate experiments with each condition performed in triplicate (*, P<0.05, vs. LTA alone and #, P<0.05, vs. LTA + HMGB1).
HMGB1 preconditioning inhibits LTA-mediated DNA binding of NF-κB
NF-κB is transcription factor, which has been implicated in the up-regulation of proinflammatory gene expression following exposure of responsive cells to LTA [30]. We sought to determine the effect of preconditioning with HMGB1 on LTA mediated NF-κB DNA binding in nuclear extracts prepared from THP-1 cells. Cells treated with LTA alone demonstrated increased DNA binding of NF-κB as compared with control cells (Figure 4). In contrast, nuclear NF-κB DNA-binding activity was markedly inhibited in HMGB1 preconditioned cells as compared with cells treated with LTA alone (Figure 4). According to Ziegler-Heitbrock et al., one of the mechanisms responsible for the development of tolerance to proinflammatory pathogen-associated molecules, such as LTA, is a switch in the composition of NF-κB from the transcriptionally active p65/p50 heterodimeric form to the transcriptionally inactive p50/p50 homodimeric form. Accordingly, we examined the composition of the NF-κB dimers by carrying out supershift analyses of the protein complexes bound to DNA. Nuclear proteins purified from THP-1 cells were incubated with antibodies specific for p65 and p50 prior to assembling the DNA-binding reaction while performing EMSA. HMGB1 preconditioning did not alter the NF-κB subunit composition in response to LTA (Figure 4). In summary, preconditioning with HMGB1 decreases LTA mediated NF-κB DNA binding but does not alter the NF-κB subunit composition.
Figure 4.

DNA binding of NF-κB in THP-1 cells stimulated with LTA. Representative autoradiograph of EMSA for NF-κB is representative of three similar, separate experiments. Control cells were maintained in basal growth medium. LTA-treated cells were treated with LTA (10 μg/ml) for 1 h. HMGB1-treated cells were preconditioned with HMGB1 (1 μg/ml) for 18 h before the addition of LTA. To analyze the composition of the DNA-binding complex, supershift of the DNA-binding complex was performed with p65 and p50 antibody. LTA-treated cells were treated with LTA (1 μg/ml) for 1 h. LTA-treated cells were preconditioned with HMGB1 (1 μg/ml) for 18 h before the addition of LTA. Nuclear protein purified from THP-1 cells was incubated with antibodies for p65 and p50 for 30 min before addition of the radiolabeled oligonucleotide.
HMGB1 pretreatment inhibits LTA-mediated degradation of IκB
IκBα belongs to the IκB family of proteins, which bind to NF-κB dimers and sterically block the function of their nuclear localizing sequences, thereby promoting cytoplasmic retention. Degradation of IκB is required for translocation of NF-κB from the cytoplasm into the nucleus. Hence, we sought to determine whether HMGB1 preconditioning affected LTA-induced degradation of IκBα. After exposure to LTA, degradation of IκBα was apparent as early as 5 min, and significant degradation was apparent by 15 min after LTA addition (Figure 5). IκBα degradation was markedly reduced when cells were pretreated with HMGB1 prior to LTA addition (Figure 5).
Figure 5.

(a) Representative autoradiograph of Western blot analysis for IκBα, demonstrating the effect of HMGB1 preconditioning on IκBα degradation following LTA stimulation. Control cells were maintained in basal growth medium. THP-1 cells were preconditioned with HMGB1 (1 μg/ml) for 18 h before the addition of LTA (10 μg/ml) for 5 and 15 min. The gel is representative of three experiments with similar results. Figure 5 (b) Image analysis of IκB content determined by densitometry. Decrease calculated as percentage of THP-1 cells treated with media alone (*, P<0.05, vs. control; #, P <0.05, vs. LTA).
RAGE receptor is not necessary for induction of LTA tolerance
We next sought to identify the receptor(s), which are important for the induction of LTA tolerance by HMGB1 pretreatment. Three receptors, namely TLR2, TLR4 and RAGE, have been implicated as being involved in transducing the proinflammatory effects of extracellular HMGB1. We were able to investigate the roles of TLR2, TLR4 and RAGE in the pathway responsible for HMGB1-induced tolerance to LTA by carrying out studies, using bone marrow-derived macrophages harvested from C57Bl/6 wild-type mice, TLR2−/−, TLR4−/− mice, and RAGE−/− mice. The recognition of LTA by mammalian cells is dependent upon the interaction of the ligand with a receptor complex, which includes TLR2. Hence, murine macrophages that lacked TLR2 demonstrated minimal TNF secretion following LTA stimulation as compared to control cells (3 ± 2 vs. 3 ± 2 pg/ml). TLR2−/− macrophages that were pretreated with HMGB1 demonstrated similar TNF levels to untreated TLR2−/− macrophages (3.2 ± 2 vs. 3 ± 2 pg/ml). Previously, it has been reported that TLR4-deficient macrophages are hyporesponsive to LTA [31, 32]. Our results agreed with these observations as TLR4−/− deficient macrophages demonstrated minimal TNF secretion following LTA stimulation in comparison to control cells (5.0 ± 2.7 vs.0 pg/ml). TNF levels in supernatants from TLR4−/− macrophages pretreated with HMGB1 were similar to levels in supernatants from untreated TLR4−/− macrophages (5.0 ± 2.7 vs. 3.89 ± 2.7 pg/ml). HMGB1 pretreated wild type demonstrated attenuated supernatant TNF levels compared to cells that were not preconditioned (1092 ± 54 vs. 710 ± 17 pg/ml). This observation was consistent with our data obtained from differentiated THP-1 cells. Similar to the results we obtained with wild-type macrophages, TNF levels in supernatants from RAGE−/− macrophages pretreated with HMGB1 were decreased compared to supernatant TNF levels in untreated TLR4−/− cells (1359 ± 77 vs. 1001 ± 41 pg/ml; Figure 6). These results suggest that RAGE is not required for the induction of LTA tolerance by HMGB1.
Figure 6.

TNF ELISA demonstrating the effect of HMGB1 pretreatment ex vivo in bone marrow-derived macrophages from C57Bl/6, TLR2−/− TLR4−/− and RAGE−/− mice. Cells were preconditioned with HMGB1 (1 μg/ml) for 18 h before the addition of LTA 10 μg/ml for 4 h. Data represent the mean ± SEM of four separate experiments with each condition performed in triplicate (*, P<0.05, vs. LTA alone).
Discussion
HMGB1 is actively secreted by immunostimulated macrophages and monocytes [2], endothelial cells [8],dendritic cells [6], and enterocytes [9]. Additionally, when cells undergo necrosis, HMGB1 is released into the surrounding extracellular milieu. In contrast, during apoptosis, HMGB1 is bound firmly to chromatin because of generalized histone underacetylation and minimal quantities of HMGB1 are released [10, 33-35]. These observations support the notion that HMGB1 is an endogenous danger signal, alerting the immune system to the presence of inflammation and necrosis [2, 15, 36].
The presence of HMGB1 in the extracellular compartment serves many other functions. In addition to its ability to mediate inflammation [2, 37] [38], extracellular HMGB1 regulates the migration of monocytes [39]and induces the migration and proliferation of smooth muscle cells and mesoangioblasts [40] [41]. Furthermore, HMGB1 contributes to dendritic cell maturation and induction of immune responses [11] [6, 42, 43].
In a fashion, which is analogous to the induction of LPS tolerance, macrophages and monocytes demonstrate reduced responses to subsequent LTA challenges if they have previously been exposed to a lower (tolerizing) concentration of LTA; this phenomenon is referred to as LTA tolerance [44]. Pretreatment with LTA has also been shown to confer myocardial protection in a rat model of coronary artery ligation [45]. In this study, we demonstrate that acquired tolerance to LTA also can be induced by HMGB1.
Contamination of recombinant proteins with LPS can confound the interpretation of results in studies such as the ones reported here carried out using recombinant HMGB1. However, in the HMGB1 preparation used for our experiments, the concentration of LPS was less than the lower limit of detection for the standard Limulus lysate assay we employed. This finding reduces the likelihood that LPS contamination was responsible for the apparent induction of LTA tolerance by HMGB1. Nevertheless, the absence of LPS as detected by the Limulus lysate assay does not complete exclude the possibility that very low levels of LPS contamination were responsible for our key observations. In this regard, it is important that we obtained additional evidence that HMGB1 per se and not a contaminant, such as LPS, was responsible for the induction of tolerance to LTA. Specifically, we showed that denaturation of HMGB1 by immersion in boiling water abrogated the ability of the protein to induce tolerance to LTA. Furthermore, we showed that a specific antibody against HMGB1 also neutralized the protein’s tolerance-inducing activity.
The foregoing notwithstanding, it seems possible (or even likely) that trace levels of LPS or LTA in combination with HMGB1 confer tolerogenic activity to the protein. This view is supported by the results of several recent studies, which document that very highly purified HMGB1 has minimal cytokine-like activity in vitro [46-48], whereas E. coli-derived recombinant HMGB1 is a potent pro-inflammatory agent. Hreggvidsdottir et al. have proposed that HMGB1 forms a complex with other TLR ligands or inflammation-promoting molecules to initiate or augment cytokine production. It is possible that presence of such complexes can induce tolerance to various microbial products; for example, complexes of HMGB1 with LPS can induce tolerance to LPS or complexes of HMGB1 with LTA can induce tolerance to LTA.
There continues to be considerable debate, regarding the clinical relevance of tolerance. Data from animal models suggests that there is decreased severity of infections and less tissue damage in these models. However, tolerance is responsible for diminished responsiveness to repeated bacterial challenge, leading to increased sensitivity to nosocomial infections [49]. A phase I trial of endotoxin tolerance in humans was recently completed, but the results have not yet been published (NCT00246714).
Co-immunoprecipitation studies done by Park et al. suggest that there is no interaction between HMGB1 and RAGE [50]. These authors suggest that similar to LPS, HMGB1 activates inflammatory processes due to its early interaction with TLR4 and TLR2. In contrast, Kokkola et al. demonstrated that HMGB1 stimulation of RAGE-deficient macrophages leads to less production of TNF, IL-1β, and IL-6 than occurs when RAGE-sufficient macrophages are incubated with HMGB1[51]. In this context, it is important to point out that in our study we found that HMGB1 induced LTA tolerance to a similar degree in RAGE−/− and wild-type bone marrow-derived macrophages. This finding was quite unexpected, since in previous work from our laboratory, we showed that induction of tolerance to LPS by prior exposure to HMGB1 was demonstrable in wild-type but not RAGE−/− murine bone marrow-derived macrophages. Collectively, these findings support the view that distinct mechanisms are responsible for HMGB1-induced tolerance to LPS and LTA.
In our study, HMGB1 pretreatment decreased LTA-mediated NF-κB activation and preserved the NF-κB inhibitory protein IκB-α as compared to cells treated with LTA alone. Enhanced expression of transcriptionally inert p50 NF-κB homodimer and reduction in p50/p65 NF-κB heterodimer expression has been suggested as a possible mechanism for LPS tolerance induction in an in vitro LPS-preconditioning model. Siedlar and colleagues demonstrated tolerance to (S)-(2,3-bis(palmitoyloxy)-(2RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser(S)-Lys4-OH, trihydrochloride (Pam3Cys), a TLR2 agonist, by pretreating monocytes with small doses of Pam3Cys [52]. Similar to our study, they reported no change in the p50/p65 heterodimer composition.
In conclusion we have demonstrated HMGB1 pretreatment can induce LTA tolerance. Potential mechanisms involved in HMGB1-mediated LTA tolerance include cytosolic preservation of IκBα and decreased NF-κB activation. As compared to LPS tolerance, where RAGE receptor played a key role, we were able to induce LTA tolerance in RAGE −/− macrophages. Our data also suggests that LPS contamination of the HMGB1 preparation does not account for our observations.
Acknowledgements
We thank Dr. Tim Oury of the University of Pittsburgh and Dr. Angelika Bierhaus of the University of Heidelberg (Heidelberg, Germany) for providing bone marrow derived macrophages from RAGE knockout mice.
Footnotes
Supported by NIH grants KO8 GM076344 (R.A.)
S.M.R. and H.S. contributed equally to this work
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bustin M. Regulation of DNA-Dependent Activities by the Functional Motifs of the High-Mobility-Group Chromosomal Proteins. Mol Cell Biol. 1999;19(8):5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. Journal of internal medicine. 2004;255(3):320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126(2):389–392. [PubMed] [Google Scholar]
- 6.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174(12):7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 7.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106(2):609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 8.Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, Rauvala H, Palmblad J, Andersson J, et al. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scandinavian journal of immunology. 2004;60(6):566–573. doi: 10.1111/j.0300-9475.2004.01518.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290(4):C990–999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Muller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5(8):825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of biological chemistry. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock (Augusta, Ga. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 15.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Critical care medicine. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 17.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Critical care medicine. 2007;35(4):1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 18.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock (Augusta, Ga. 2009;32(1):17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aneja RK, Tsung A, Sjodin H, Gefter JV, Delude RL, Billiar TR, Fink MP. Preconditioning with high mobility group box 1 (HMGB1) induces lipopolysaccharide (LPS) tolerance. J Leukoc Biol. 2008;84(5):1326–1334. doi: 10.1189/jlb.0108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greisman SE, Hornick RB. The nature of endotoxin tolerance. Transactions of the American Clinical and Climatological Association. 1975;86:43–50. [PMC free article] [PubMed] [Google Scholar]
- 21.Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Critical care medicine. 2009;37(4):1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 22.West MA, Heagy W. Endotoxin tolerance: a review. Critical care medicine. 2002;30(1 Suppl):S64–73. [PubMed] [Google Scholar]
- 23.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. The Journal of clinical investigation. 1991;88(5):1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer W. Physiology of lipoteichoic acids in bacteria. Advances in microbial physiology. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 25.Ellingsen E, Morath S, Flo T, Schromm A, Hartung T, Thiemermann C, Espevik T, Golenbock D, Foster D, Solberg R, et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med Sci Monit. 2002;8(5):BR149–156. [PubMed] [Google Scholar]
- 26.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J Immunol. 2002;168(12):6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- 27.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and Lipoteichoic Acid-Induced Tolerance and Cross-Tolerance: Distinct Alterations in IL-1 Receptor-Associated Kinase. J Immunol. 2002;168(12):6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- 28.Falk LA, Vogel SN. Comparison of bone marrow progenitors responsive to granulocyte-macrophage colony stimulating factor and macrophage colony stimulating factor-1. J Leukoc Biol. 1988;43(2):148–157. doi: 10.1002/jlb.43.2.148. [DOI] [PubMed] [Google Scholar]
- 29.Chen PC, Wheeler DS, Malhotra V, Odoms K, Denenberg AG, Wong HR. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 2002;26(5):233–241. doi: 10.1023/A:1019718718977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao SJ, Lei HC, Kuo CT, Chang MS, Chen BC, Chang YC, Chiu WT, Lin CH. Lipoteichoic acid induces nuclear factor-kappaB activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, Akt, and p38 MAPK in RAW 264.7 macrophages. Immunology. 2005;115(3):366–374. doi: 10.1111/j.1365-2567.2005.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164(2):554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 33.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291(6):C1318–1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol. 2007;178(10):6495–6503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- 35.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203(7):1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 37.Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72(6):1084–1091. [PubMed] [Google Scholar]
- 38.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 39.Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepantalo M, Carpen O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104(4):1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 40.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152(6):1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164(3):441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35(7):2184–2190. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- 43.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 44.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of Cross-Tolerance by Lipopolysaccharide and Highly Purified Lipoteichoic Acid Via Different Toll-Like Receptors Independent of Paracrine Mediators. J Immunol. 2001;166(8):5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 45.Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart: A comparison with endotoxin. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(6):1521–1528. doi: 10.1161/01.atv.20.6.1521. [DOI] [PubMed] [Google Scholar]
- 46.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81(1):49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann K, Volkel D, Pable S, Lindner T, Kramberger F, Bahrami S, Scheiflinger F. Native versus recombinant high-mobility group B1 proteins: functional activity in vitro. Inflammation. 2004;28(4):221–229. doi: 10.1023/b:ifla.0000049047.61014.e3. [DOI] [PubMed] [Google Scholar]
- 48.Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009 doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 49.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9(2):101–107. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- 50.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 51.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scandinavian journal of immunology. 2005;61(1):1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 52.Siedlar M, Frankenberger M, Benkhart E, Espevik T, Quirling M, Brand K, Zembala M, Ziegler-Heitbrock L. Tolerance induced by the lipopeptide Pam3Cys is due to ablation of IL-1R-associated kinase-1. J Immunol. 2004;173(4):2736–2745. doi: 10.4049/jimmunol.173.4.2736. [DOI] [PubMed] [Google Scholar]


