Abstract
p38 MAPK is activated potently during cardiac ischaemia, although the precise mechanism by which it is activated is unclear. We used the isolated perfused rat heart to investigate the signalling pathways activated upstream of p38 during global cardiac ischaemia. Ischaemia strongly activated p38α but not the JNK pathway. The MAPKKs, MKK3, MKK4 and MKK6 have previously been identified as potential upstream activators of p38; however, in the ischaemic perfused heart, we saw activation of MKK3 and MKK6 but not MKK4. MKK3 and MKK6 showed different temporal patterns of activity, indicating distinct modes of activation and physiological function. Consistent with a lack of JNK activation, we saw no activation of MKK4 or MKK7 at any time point during ischaemia. A lack of MKK4 activation indicates, at least in the ischaemic heart, that MKK4 is not a physiologically relevant activator of p38. The MAPKKK, ASK1, was strongly activated late during ischaemia, with a similar time course to that of MKK6 and in ischaemic neonatal cardiac myocytes ASK1 expression preferentially activated MKK6 rather than MKK3. These observations suggest that during ischaemia ASK1 is coupled to p38 activation primarily via MKK6. Potent activation of ASK1 during ischaemia without JNK activation shows that during cardiac ischaemia, ASK1 preferentially activates the p38 pathway. These results demonstrate a specificity of responses seldom seen in previous studies and illustrate the benefits of using direct assays in intact tissues responding to physiologically relevant stimuli to unravel the complexities of MAPK signalling.
Abbreviations: ASK1, apoptosis-sensitive kinase; MAPK, mitogen-activated protein kinase; MAPKK/MKK, MAPK kinase; MAPKKK, MAPKK kinase; JNK, c-Jun N-terminal kinase; ERK, extracellularly regulated kinase; SAPK, stress-activated protein kinase; MEKK, MAPK/ERK kinase; MLK, mixed lineage kinase; TAK, TGF β-activated kinase; GCK, germinal centre kinase; Tpl-2, tumour progression locus-2; PAK, p21-activated protein kinase; TAO Kinase, thousand and one kinase; DTT, dithiothreitol; BSA, bovine serum albumin; GST, glutathione S-transferase
Keywords: Ischaemia, Heart, ASK1, JNK, p38, MAPK
1. Introduction
In mammalian cells, mitogen-activated protein kinase (MAPK) cascades are differentially activated by a wide variety of extracellular stimuli and contribute to the regulation of many cellular responses [1,2]. The extracellularly regulated kinases or ERK family of MAPKs regulate predominantly the growth and proliferation of cells in response to factors acting on tyrosine kinase and G-protein-coupled receptors, and in most cell types, are only weakly activated by stressful stimuli. This is in contrast to the stress-activated protein kinase or SAPK family of MAPKs, which includes the c-Jun N-terminal Kinase (JNK) and p38 MAPK. JNK and p38 are activated strongly in response to cellular stresses such as heat and osmotic shock, genotoxic agents, UV irradiation, trophic factor withdrawal and also by pro-inflammatory cytokines. JNK and p38 are also potently activated by ischaemia/reperfusion injury in the heart where they are thought to contribute to the apoptotic and hypertrophic responses of cardiac myocytes [3,4]. In the isolated perfused rat heart, JNK and p38 are differentially activated by ischaemia and reperfusion [5–7]. p38 is rapidly activated at the onset of ischaemia and remains active during reperfusion. JNK on the other hand is not activated by ischaemia alone but is potently activated upon reperfusion, probably in response to reactive oxygen species. Although the kinetics of activation of JNK and p38 are well documented in the perfused heart, the mechanism of their activation is poorly characterised. A number of upstream activators of JNK and p38 have been identified by cloning and transfection experiments using cells in culture and mouse gene knockout experiments. The proposed immediate upstream regulators of JNK are the MAP kinase kinases MKK4 and MKK7 [8–11], while those directly activating p38 are MKK3, MKK6 and possibly MKK4 [8,12–15]. Numerous MAP kinase kinase kinases that activate MKKs have been proposed, and these include the MAPK/ERK kinases (MEKKs), mixed lineage kinases (MLKs), TGFβ-activated kinase (TAK), germinal centre kinase (GCK), tumour progression locus-2 (Tpl-2), p21-activated protein kinases (PAKs), apoptosis-sensitive kinase (ASK1) and thousand and one (TAO) kinase [2,16,17]. However, the precise arrangement of these pathways is unclear and since candidate regulators have been proposed mainly by transfection studies in cultured cell lines, the physiological relevance of many of them remains to be established. We have attempted to address this issue using a physiologically relevant experimental system. In this study, we have used the isolated perfused rat heart to investigate the pathways that lead to the activation of p38 during cardiac ischaemia.
2. Materials and methods
2.1. Langendorff perfusion
Terminal anaesthesia was administered to adult male (250–300 g) Wistar rats by interperitoneal injection of sodium pentobarbitone (250 mg/kg). After induction of anaesthesia, intravenous heparin (150 IU) was administered and the heart surgically removed. Hearts were perfused retrogradely at a pressure of 10 kPa (70 mm Hg) with Krebs–Henseleit bicarbonate-buffered saline (25 mM NaHCO3, 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2 , 1.2 mM KH2 PO4, pH 7.6) at 37 °C supplemented with 10 mM glucose and equilibrated with 95% O2/5% CO2 . The temperature of the hearts and perfusates were maintained at 37 °C by the use of a water-jacketed apparatus. Hearts were pre-equilibrated for 15 min prior to induction of ischaemia, during which time the coronary flow was approximately 10 ml/min. Simple global ischaemia was induced by interruption of flow for the times indicated by clamping the aortic perfusion line. Hearts ceased beating within 1–3 min of ischaemia. As a positive control for kinase activation, some hearts were perfused with buffer containing 0.5 M sorbitol for 20 min. Control hearts were perfused for up to 60 min after the pre-equilibration period without interruption to the perfusate flow. At the end of all perfusions, hearts were freeze–clamped between aluminium tongs cooled in liquid N2 and the frozen tissue stored at −80 °C until required.
2.2. Preparation of neonatal cardiac myocytes
Neonatal cardiac myocytes were isolated from 1- to 3-day-old Wistar/Sprague–Dawley rat hearts as described previously [18,19]. Briefly, ventricular myocytes were dissociated by digestion at 37 °C with 0.8 mg/ml collagenase type II (Gibco, #17101-015) and 1.2 mg/ml pancreatin (Sigma, #P3292) in sterile ADS buffer (116 mM NaCl, 20 mM HEPES, 0.8 mM Na2HPO4, 5.6 mM glucose, 5.4 mM KCl, 0.8 mM MgSO4, pH 7.35). The cells were resuspended in maintenance medium; Dulbecco's modified Eagle's medium/medium 199 (4:1 (v/v)), containing 100 U/ml of both penicillin and streptomycin, supplemented with 10% horse serum and 5% foetal calf serum (Gibco), prior to preplating on uncoated culture dishes for 60 min at 37 °C to deplete fibroblasts. The non-adherent myocytes were plated at a final density of 1 × 106 cells/dish in 4 ml maintenance medium on 60-mm dishes coated with gelatin. The culture medium was exchanged for fresh maintenance medium after overnight incubation.
2.3. Cell treatments and preparation of lysates
Neonatal cardiac myocytes, plated onto 60-mm collagen-coated dishes and incubated overnight in maintenance medium containing 2% foetal calf serum, were washed once with PBS at 37 °C prior to incubation for the indicated times with control buffer (4 mM HEPES, pH 7.5, 137 mM NaCl, 3.58 mM KCl, 0.49 mM MgCl2, 0.9 mM MgCl2, 5.59 mM d-glucose, 2% FCS) or ischaemia buffer (4 mM HEPES, pH 6.5, 137 mM NaCl, 16 mM KCl, 0.49 mM MgCl2, 0.9 mM MgCl2, 5.59 mM d-glucose, 2% FCS, 10 mM 2-deoxyglucose, 20 mM sodium lactate, 1 mM sodium dithionite). As a positive control and to allow the measurement of maximal kinase activation, some cells were osmotically shocked by incubation with control buffer containing 0.5 M sorbitol. After the treatments indicated, cells were washed once with ice-cold PBS and lysed by scraping into 0.5 ml of ice-cold Triton lysis buffer (20 mM HEPES, pH 7.5, 137 mM NaCl, 25 mM β-glycerol phosphate, 2 mM sodium pyrophosphate, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 2.5 μg/ml each of pepstatin, antipain and leupeptin, 2 mM benzamidine, 0.5 mM DTT, 1 mM Na3VO4). Lysates were vortexed and clarified by centrifugation at 20,000×g for 15 min at 4 °C. Heart extracts were prepared from frozen perfused hearts which were pulverised under liquid N2 in a pre-cooled pestle and mortar. Approximately 200 μg of the powdered heart tissue was extracted into 900 μl of ice-cold lysis buffer (20 mM HEPES, pH 7.5, 137 mM NaCl, 25 mM β-glycerol phosphate, 2 mM sodium pyrophosphate, 2 mM EDTA, 10% glycerol, 1 mM PMSF, 2.5 μg/ml each of pepstatin, antipain and leupeptin, 2 mM benzamidine, 0.5 mM DTT, 1 mM Na3VO4) using a PowerGen 35 tissue homogeniser set at full power (Fisher Scientific). Lysates were clarified by centrifugation at 20,000×g for 15 min at 4 °C.
2.4. RT-PCR analysis
Total RNA was extracted from approximately 100 mg rat heart tissue or neonatal cardiac myocytes using RNAwiz reagent (Ambion). cDNA was generated from 1 μg of total RNA in a reverse transcription reaction containing 10 mM Tris–HCl, pH 9.0, 5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100, 1 mM dNTPs, 1 U/μl RNasin RNase inhibitor, 0.5 μg oligo (dT)15 primers and 16 U AMV reverse transcriptase (Promega). Reverse transcription was carried out for 1 hour at 42 °C. cDNA generated by reverse transcription was used as template in polymerase chain reactions to amplify p38 isoforms using specific primers. Their sequences were: p38α forward 5′-AAG AAA ATC TCC TCA GAG TCT-3′, reverse 5′-AAT GAC TTC ATC GTA GGT CAG-3′; p38β forward 5′-TCC TCG GAG CAT GCC CGG ACA-3′, reverse 5′-CAG GGA GCT GTG AGG GTT CCA-3′; p38γ forward 5′TGC TTC TGT CCT GAC CAA TGC-3′, reverse 5′-ACT CTG GCT CCT AGC TGC CTG-3′; p38δ forward 5′-CAC ACA GCT TTT CCC ACG CGC-3′, reverse 5′-CCT CGA GTC CTT CCG GGC TAT-3′. PCR products were separated by electrophoresis on a 1% agarose gels and their identity confirmed by DNA sequencing (Applied Biosystems).
2.5. Immunoprecipitation and kinase assays
Clarified lysates from neonatal cardiac myocytes or perfused hearts (500–700 μg total protein) were incubated with 5 mg Protein-A Sepharose and 5 μl of antiserum to either Flag, JNK, p38, MKK3, MKK6, MKK4, MKK7 or ASK1 in lysis buffer, adjusted to a final volume of 500 μl and mixed by tumbling at 4 °C. After 3 hours, immune complexes were isolated by a brief low-speed spin at 4 °C and precipitates washed three times with 1 ml of ice-cold lysis buffer. Precipitates were then either resuspended in SDS–PAGE sample buffer prior to western blotting analysis or washed once more with 1 ml of kinase assay buffer (25 mM HEPES, pH 7.4, 25 mM β-glycerol phosphate, 25 mM MgCl2, 0.5 mM Na3VO4, 0.5 mM DTT) prior to kinase assay. For kinase assays, immunoprecipitates were resuspended in kinase assay buffer to a final volume of 50 μl containing 50 μM [γ-32P] ATP (2000 cpm/pmol) and the relevant protein substrates required for each kinase, which were; 5 μg of GST-ATF2 (1-109) for p38, 5 μg of GST-c-Jun (1-79) for JNK, 1 μg GST-p38 and 4 μg GST-ATF2 for MKK3 and MKK6, 1 μg GST-JNK and 4 μg of GST-c-Jun (1-79) for MKK4 and MKK7 or 5 μg of myelin basic protein for ASK1. All kinase assays were terminated, after incubation for 30 min at 30 °C, by the addition of SDS–PAGE sample buffer. The samples were then subjected to electrophoresis on 10% SDS–PAGE gels. 32P-incorporation into protein substrates was determined by PhosphorImager analysis of the dried gels (Molecular Dynamics).
2.6. Cell culture
HEK-293 human embryonic kidney cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% (v/v) foetal bovine serum, 2 mM-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
2.7. Transfection of neonatal cardiac myocytes
Rat neonatal cardiac myocytes, plated at 1 × 106 cells/dish on 60-mm collagen-coated dishes, were transfected 48 hours after isolation. Plasmid cDNA constructs (1 μg) were transfected using Fugene-6 according to the manufacturer's instructions. Myocytes were cultured for a further 48 hours, replacing medium after 24 hours, before treatment and lysis. For adenoviral transfection, neonatal cardiac myocytes were infected with adenovirus pAdEasy1-WT-ASK1 (expressing GFP and WT-ASK1 under separate CMV promoters) at an MOI of 50 using a viral stock of titre 109–1010 viral particles/ml. GFP fluorescence indicated ∼ 50% efficiency of infection after 24 hours. Cells were used for experiments 48 h post-infection.
2.8. Western blotting
Immunoprecipitates or cell lysates (200 μg total protein) were subjected to SDS–PAGE on 8% or 10% polyacrylamide gels [20]. Proteins were transferred to Immobilon-P membranes (Millipore) using semi-dry electrophoretic transfer (Biorad) and the membranes blocked for 1 hour in 3% (w/v) bovine serum albumin (BSA) in TBST (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.02% (v/v) Tween-20). Blots were incubated for 1 hour with primary antibody diluted in TBST containing 3% (w/v) BSA. The blots were then washed three times for 15 min in TBST prior to incubation in the appropriate horseradish peroxidase-coupled secondary antibody (GE Healthcare) at 1:10,000 dilution in TBST with 3% (w/v) BSA. Blots were again washed three times in TBST before being developed using enhanced chemiluminescence (GE Healthcare) and exposure to X-ray film (Fuji).
2.9. GST-fusion proteins
GST fusions of JNK, p38, c-Jun (1-79) and ATF2 (1-109) were expressed in E. coli using pGEX vectors and purified by glutathione agarose affinity chromatography as described previously [21].
2.10. Antibodies
Anti-JNK antibody (Cat. no. 554 285) was from BD Biosciences. A rabbit polyclonal antibody to p38 was generated against a GST fusion of full-length p38 [21]. The phosphorylation site-specific antibodies anti-phospho-p38 (Cat. no. 9211), anti-phospho-JNK (Cat. no. 9251) and anti-phospho-Thr 845 ASK1 (Cat. no. 3765) were from Cell Signalling Technology. Antibodies to MAPK kinases were from Santa Cruz Biotechnology: MKK3 (Cat. no. sc-960), MKK6 (Cat. no. sc-1991), MKK4 (Cat. no. sc-964) and MKK7 (Cat. no. sc-7103). Anti-GFP (Cat. no. G6539), anti-Flag M2 (Cat. no. F-3165) and anti-γ-tubulin (Cat. no. T6557) were from Sigma. Anti-ASK1 rabbit polyclonal antiserum was the kind gift of Prof. Hidenori Ichijo, University of Tokyo. Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were from GE Healthcare, UK.
2.11. Constructs
Vectors expressing Flag-tagged MKK3, MKK6, MKK4 and MKK7 have been described previously [22] as have pGEX vectors for expressing GST fusions of JNK, p38, c-Jun and ATF2 [21]. Constructs expressing Flag-tagged p38 isoforms (p38α, β, γ and δ) were obtained from Dr. Raj Patel, University of Leicester. pcDNA3-HA-ASK1 was the kind gift of Prof. Hidenori Ichijo, University of Tokyo. Adenoviral vectors expressing full-length, wild-type, HA-tagged ASK1 were constructed by subcloning the ASK1 cDNA into the pAdEasy transfer vector prior to homologous recombination in E. coli into the pAdEasy-1 viral backbone (Stratagene). pAdEasy-1-WT-ASK1 vector was purified from E. coli, linearised and used to infect HEK-293A cells to produce packaged recombinant adenovirus. High-titre adenoviral stocks were generated by sequential passage in HEK-293A cells according to the manufacturer's instructions (Stratagene).
3. Results
3.1. SAPK activation during cardiac ischaemia
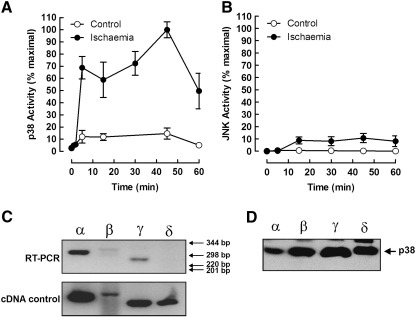
To confirm which SAPKs are activated during cardiac ischaemia, perfused rat hearts were subjected to global ischaemia and extracts assayed for p38 and JNK activity by immune-complex kinase assay. As expected, we saw a large and rapid increase in p38 kinase activity in response to ischaemia, which appeared to be bi-phasic, with little or no JNK activation (Fig. 1A and B). To determine which p38 isoforms are expressed and might be activated by ischaemia in the heart, we performed RT-PCR analysis. We were able to detect a strong signal for p38α, with weaker signals for the β and γ isoforms (Fig. 1C, upper panel). We were unable to detect the p38δ transcript in heart extracts even though a control p38δ cDNA gave a strong RT-PCR signal (Fig. 1C, lower panel). To determine which p38 isoforms our polyclonal p38 antiserum was capable of precipitating, we immunoprecipitated Flag-tagged p38 from HEK cells transfected with the various p38 isoforms. Western blotting with anti-Flag showed that the p38 antibody was able to precipitate each of the p38 isoforms equally well (Fig. 1D). These results suggest that in the perfused heart p38α is the major contributor to increased p38 activity during ischaemia.
Fig. 1.
Time course of SAPK activation during cardiac ischaemia. Freshly isolated perfused rat hearts were subjected to global ischaemia for the times indicated, extracted and assayed for p38 (A) or JNK (B) activity by immune-complex kinase assay. The range of p38 isoforms expressed in the normal adult rat heart was determined by RT-PCR analysis with isoform-specific primers using tissue from unstimulated hearts (C; upper panel). cDNAs for p38α, β, γ and δ were used as positive controls (lower panel). The isoform specificity of the p38 antibody used in immune-complex kinase assays was determined by immunoprecipitation from cell lysates containing epitope-tagged forms of p38α, β, γ and δ, followed by SDS–PAGE and western blotting with anti-Flag antibody (D).
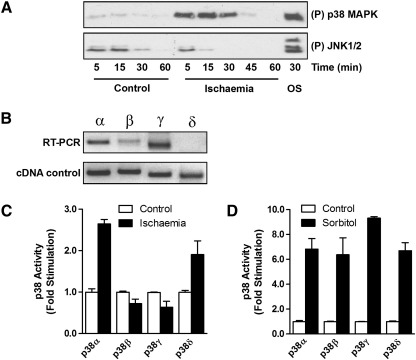
To further investigate the contribution of the various p38 isoforms to SAPK activity during ischaemia, we investigated p38 activation in neonatal cardiac myocytes subjected to simulated ischaemia. Similar to the experiments in the perfused heart, we saw robust activation of p38 during ischaemia with little or no activation of JNK (Fig. 1A). p38 isoform expression in neonatal cardiac myocytes was similar to that in the adult heart, RT-PCR analysis revealing the expression of p38α, β and γ but not δ, although the expression of p38γ seemed to be higher in the neonatal myocytes (Fig. 2B). To determine which p38 isoforms could be activated by ischaemia, we transfected neonatal myocytes with Flag-tagged p38 isoforms and subjected them to ischaemia for 20 min prior to immunoprecipitation with anti-Flag antibody and immune-complex kinase assay. Both p38α and p38δ were strongly activated by ischaemia while p38β and p38γ remained unaffected (Fig. 2C). In a control experiment, osmotic shock strongly activated all of the transfected p38 isoforms showing that all were efficiently expressed and capable of activation (Fig. 2D). Taken together, these experiments suggest that p38α is the SAPK most significantly activated during cardiac ischaemia.
Fig. 2.
Differential activation of SAPK isoforms during simulated ischaemia. Primary neonatal cardiac myocytes were subjected to simulated ischaemia for the times indicated and cell lysates examined by SDS–PAGE and western blotting with anti-phospho p38 and anti-phospho JNK antibodies. As a positive control, cells were osmotically shocked (OS) by treatment with 0.5 M sorbitol for 20 min (A). The range of p38 isoforms expressed in unstimulated neonatal cardiac myocytes was determined by RT-PCR analysis using isoform-specific primers (B; top panel). cDNAs for p38α, β, γ and δ were used as positive controls (lower panel). Primary neonatal cardiac myocytes were transfected with Flag-tagged p38 isoforms α, β, γ and δ. The cells were subjected to simulated ischaemia for 20 min (C) or treated with 0.5 M sorbitol for 20 min (D). Transfected p38 was immunoprecipitated from the cell extracts using anti-Flag antibody and assayed in an immune-complex kinase assay.
3.2. MAPKK activation during cardiac ischaemia
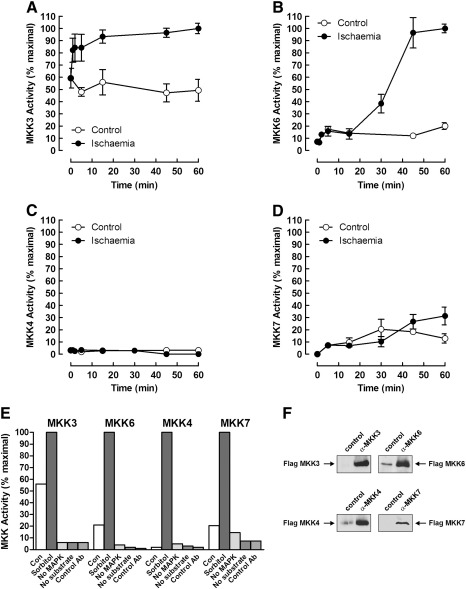
To investigate further the pathway of SAPK activation during ischaemia, we assayed the kinases thought to lie upstream of p38 and JNK. Perfused hearts were subjected to ischaemia and extracts assayed for MKK activity using immune-complex kinase assays. Both of the known p38 activators MKK3 and MKK6 were activated by ischaemia but with different time courses (Fig. 3A and B). MKK3 appeared to have a relatively high basal activity but was activated further by ischaemia and remained active throughout the time course (Fig. 3A). MKK6 was also activated significantly by ischaemia but not until later, with the first detectable increase at 30 min and remaining active until the end of the time course (Fig. 3B). In contrast, neither of the JNK activators MKK4 or MKK7 showed a significant increase in activity during ischaemia in the perfused heart (Fig. 3C and D). In control experiments in which hearts were perfused with sorbitol, we saw robust activation of all MKK isoforms assayed showing that all of the MKKs were present and could be activated by osmotic shock (Fig. 3E). No MKK activity was detected in immunoprecipitates using control antibody, or in assays lacking GST-MAPK or substrate (Fig. 3E). Antibody specificity was demonstrated by specific immunoprecipitation of Flag-tagged MKKs (Fig. 3F).
Fig. 3.
Differential activation of SAPK activators during cardiac ischaemia in perfused hearts. Freshly isolated perfused rat hearts were subjected to global ischaemia for the times indicated, extracted and assayed for MKK3 (A), MKK6 (B), MKK4 (C) and MKK7 (D) activity by coupled immune-complex kinase assay. Extracts prepared from hearts perfused without (Con) or with 0.5 M sorbitol were used in control MKK assays lacking either GST-p38/JNK (no MAPK), GST-ATF2/Jun (no substrate) or immunoprecipitated with control antiserum (E). Extracts of HEK 293 cells, transfected with Flag-tagged MKKs, were immunoprecipitated with either control serum or antisera specific for the various MKKs assayed. Immunoprecipitated MKKs were detected by western blotting with anti-Flag antibody (F).
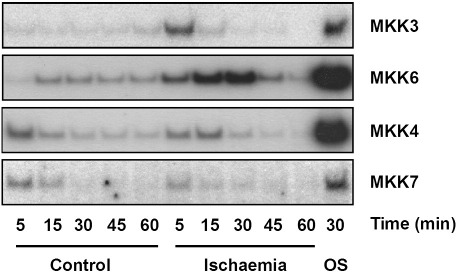
These data are entirely consistent with activation of p38 but not JNK and rule out a role for MKK4 in the activation of p38 during cardiac ischaemia. We obtained similar results in neonatal cardiac myocytes subjected to simulated ischaemia with activation of MKK3 and MKK6 and poor activation of MKK4 and MKK7 (Fig. 4A).
Fig. 4.
Differential activation of SAPK activators by simulated ischaemia in neonatal cardiac myocytes. Primary neonatal cardiac myocytes were subjected to simulated ischaemia or maintained in control buffer for the times indicated and cell extracts assayed for MKK activity by coupled immune-complex kinase assay. As a positive control, extracts from cells treated with 0.5 M sorbitol for 30 min (OS) were also assayed for each MKK.
3.3. ASK1 activation during cardiac ischaemia
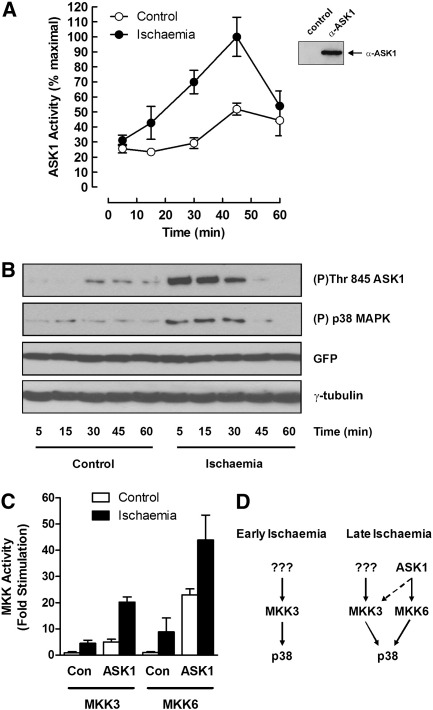
To test if the MAPKKK ASK1 were activated by ischaemia, we immunoprecipitated ASK1 from ischaemic and control hearts and assayed its activity in an immune-complex kinase assay. There was a gradual but strong activation of ASK1 during ischaemia peaking at 45 min (Fig. 5A) similar to that seen for MKK6 (Fig. 3B). At later time points ASK1 activity declined, similarly to that seen for p38 (Fig. 1A).
Fig. 5.
Activation ASK1 and downstream MKKs during cardiac ischaemia. Perfused rat hearts were subjected to global ischaemia for the times indicated, extracted and assayed for ASK1 activity by immune-complex kinase assay (A). Heart extracts were immunoprecipitated with control serum or anti-ASK1 antibody and precipitated ASK1 detected by western blotting with anti-ASK1 antibody (inset). Primary neonatal cardiac myocytes, infected with an adenoviral vector expressing wild-type ASK1, were subjected to simulated ischaemia or maintained in control buffer for the times indicated. Cell extracts were prepared, separated by SDS–PAGE and blotted with anti-phospho-ASK1 and anti-phospho-p38 antibody. Equal infection rates and gel loading were confirmed by blotting with anti-GFP and anti-γ-tubulin, respectively (B). Primary neonatal cardiac myocytes, infected with an adenoviral vector expressing wild-type ASK1 (ASK1) or empty vector (Con), were subjected to simulated ischaemia (black bars) or maintained in normal buffer (open bars) for 15 min. Lysates were prepared and MKK3 and MKK6 activity measured using coupled immune-complex kinase assays. Kinase activity is expressed relative to the empty vector control in unstimulated cells (C). Proposed routes for p38 activation by MKKs during ischaemia (D).
To investigate the coupling of ASK1 to downstream pathways, we subjected neonatal cardiac myocytes expressing ASK1 to simulated ischaemia and measured the activity of the kinases thought to lie downstream. Cells were transfected with ASK1 to ensure robust activation of downstream pathways and to compare the coupling in both stimulated and unstimulated cells.
Similarly to the perfused heart experiments, we saw a strong activation of ASK1 during ischaemia which correlated with increased p38 activity (Fig. 5B). To determine which downstream kinases ASK1 was likely to couple to, we compared MKK3 and MKK6 activation by ischaemia in cells transfected with or without ASK1. In untransfected cells, we saw activation of both MKK3 and MKK6 by ischaemia as expected. However, in cells transfected with ASK1, we saw enhanced activation of both MKK3 and MKK6 with the activation of MKK6 being most affected, particularly in unstimulated cells (Fig. 5C). Taken together with the co-incident activation of MKK6 and ASK1 during ischaemia, these data suggest that activation of ASK1 leads to preferential activation of MKK6 and consequent activation of p38.
4. Discussion
Genetic and biochemical studies have allowed the rapid delineation of the multiple MAPK signalling pathways found in all eukaryotes and the involvement of MAPKs themselves in many important biological processes is clear [1,2]. However, the precise arrangement of many of the upstream components in these pathways is uncertain since relatively few of them have been assayed directly after physiologically relevant stimulation in intact tissues. SAPKs are strongly activated by ischaemia/reperfusion in the heart and play an important role in cardiac function, although the exact mechanism of activation is poorly understood [3,4]. To address this, we set out to assay directly the upstream activators of SAPKs during ischaemia in the perfused rat heart. This an ideal experimental system with which to investigate the activators upstream of p38 independently of JNK, since most investigators report p38 but not JNK activation during ischaemia, with both activated on reperfusion [5–7]. This is in contrast with many other stimuli which often activate both p38 and JNK together [1,2].
4.1. p38 activation during ischaemia
In agreement with earlier reports, we found good activation of p38 during ischaemia without significant JNK activation in the perfused rat heart (Fig. 1A and B). This was confirmed in neonatal cardiac myocytes exposed to the metabolic stresses associated with ischaemia (Fig. 1E).
A lack of good, precipitating, isoform-specific p38 antibodies makes it difficult to determine exactly which isoforms are activated by ischaemia in the perfused heart by direct assay. By RT-PCR analysis, we found that the p38α transcript was readily detected in both adult heart and neonatal cardiac myocytes (Figs. 1C and 2C). This is in agreement with studies in other species and the consensus seems to be that the predominant p38 isoform expressed in the heart is α [23–26]. However, we also found evidence for a low level of expression of p38β, as have others [24,26], although some earlier studies concluded that p38β was absent [23,25,27]. Together these observations suggest that p38β is clearly less abundant in the heart than the α isoform. In agreement with previous studies in the rat, mouse and in humans [28], we also detected the p38γ transcript in the adult rat tissue, although at low levels. The p38γ transcript was detected at a higher level in neonatal myocytes, possibly reflecting a role in cardiac myocyte differentiation [29]. In contrast to studies in humans and mouse [24,25], we were unable to detect the p38δ transcript in either adult heart or neonatal rat cardiomyocytes. This would seem to rule out a role for p38δ in cardiac ischaemia/reperfusion injury, at least in the rat.
In neonatal cardiac myocytes transfected with Flag-tagged p38 isoforms, we saw significant activation of p38α and p38δ but not β or γ, even though they were all strongly activated by osmotic shock (Fig. 2B). Since the antibody used in our p38 immune-complex kinase assays recognises all of the four p38 isoforms equally (Fig. 1D) and given the levels of expression of the p38 isoforms in the heart and their differential activation by the stresses associated with ischaemia, our observations suggest that it is the α isoform contributing most to the p38 activity we can measure in heart extracts and therefore the p38 isoform most relevant to cardiac ischaemia in vivo.
4.2. Activation of MKK isoforms
It is clear from genetic and biochemical studies that the immediate upstream activators of JNK are MKK4 [8,9] and MKK7 [10,11] while p38 seems to be activated by MKK3 [8] and MKK6 [12,13], although some studies have also suggested a role for MKK4 [8,14,15]. However, as far as we are aware, the direct upstream activators of JNK and p38 have not been assayed directly in the perfused heart. Consistent with robust p38 activation during ischaemia, we saw strong activation of both MKK3 and MKK6. There was no significant activation of either MKK4 or MKK7 during ischaemia, which is consistent with the lack of JNK activation. The absence of MKK4 activation is significant since previous reports have shown that MKK4 may activate p38 [8,14], although the exact route to p38 activation may be stimulus dependent [15]. In our study, the lack of MKK4 activation under conditions where p38 is strongly activated clearly rules out a role for MKK4 in the activation of p38 during cardiac ischaemia.
Interestingly, we observed different kinetics of activation for MKK3 and MKK6 during ischaemia, with MKK3 being activated rapidly and remaining active, while MKK6 was not activated significantly until later in the time course. This clearly suggests that there are different pathways operating upstream of MKK3 and MKK6 which are active at different times during ischaemia. It also suggests different physiological functions for MKK3 and MKK6, which is in accordance with previous studies showing differences apparent in T-cell apoptosis [30], cardiac stress responses [31] and remodelling [23]. These effects may be linked to the ability of MKK3 and MKK6 to activate different p38 isoforms [32–34].
4.3. Activation of ASK1 by ischaemia
MAPKKs are themselves activated by a diverse array of MAPKKKs that link extracellular signals to downstream MAPK signalling pathways [16,17]. We found the assay of MAPKKKs in heart extracts to be rather problematic, probably due to a combination of relatively low abundance of the kinases themselves and/or the poor quality of some the MAPKKK antisera available to us. However, we had good reagents for ASK1, a redox-sensitive MAPKKK that has been shown to activate the p38 and JNK pathways and which is thought to be involved in the response to many cellular stresses [35,36]. ASK1 assays were straightforward and we found ASK1 to be strongly activated by ischaemia in the perfused rat heart, with a gradual increase in activity during ischaemia, reaching maximal activation at 45 min (Fig. 5A). This is in agreement with other studies showing activation of ASK1 by ischaemia in the brain, spinal cord, kidney, heart and in a mouse model of ischaemia-induced angiogenesis [37–42].
4.4. Coupling of ASK1 to downstream pathways
Since both p38 and MKK3 were activated strongly at the onset of ischaemia, before significant ASK1 activation, it is unlikely that ASK1 plays a role in activation of p38 via MKK3 at early time points, implying that a different MAPKKK is responsible (Fig. 5D). Further work will be required to determine which other MAPKKKs are active early in ischaemia [16,17]. The time course of ASK1 activation more closely matched that of MKK6 than MKK3, suggesting that ASK1 might be coupled to MKK6 during ischaemia. This idea is further reinforced by the observation that expression of ASK1 alone in unstimulated neonatal cardiac myocytes activated both MKK3 and MKK6 but with a much larger fold effect on MKK6. MKK6 activation in ischaemic cells was also increased by expression of ASK1 but the fold effect appeared lower than that for MKK3 due to the higher basal MKK6 activity (Fig. 5C). ASK1 activation occurring late during ischaemia also correlates well with the phenotype of ASK1(−/−) MEF cells, where only the late, sustained activation of p38 required for apoptosis by TNFα is abolished by the knockout of ASK1 [43].
These observations show that there are different pathways active during early vs. late ischaemia. Activation of p38 in early ischaemia probably occurs via an unidentified MAPKKK and MKK3 without involvement of ASK1 or MKK6. Later, ASK1 contributes to p38 activation, most likely via MKK6, although MKK3 may also be involved (Fig.D).
4.5. MKK-independent pathways of p38 activation
Recent work has suggested that there may be MKK-independent pathways to p38 activation during cardiac ischaemia that involve TAB1-mediated autophosphorylation of p38, perhaps in conjunction with the AMP-activated protein kinase (AMPK) [44–47]. Although we see activation of both MKK3 and MKK6 in the perfused rat heart, which should be sufficient for full activation of p38, we cannot rule out the possibility that an MKK-independent component, such as TAB1 or AMPK, may contribute to p38 activation during ischaemia. However, full activation of p38 requires dual phosphorylation of the TGY motif in the kinase domain, and this requires an MKK since p38 poorly autophosphorylates the tyrosine in this motif. In addition, MKK3/MKK6 compound knockouts seem to abolish p38 activation completely in MEFs, at least for TNFα [15]. Recent work also suggests that although AMPK is activated by cardiac ischaemia, it may not be involved in p38 activation [48]. Clearly further work will be required to clarify the role of MKK-independent modes of p38 activation during ischaemia in vivo.
4.6. ASK1 coupling to the JNK pathway
It is clear that ASK1 can contribute to activation of the JNK pathway by cell stresses and cytokines [43]. However, during cardiac ischaemia, we observed strong stimulation of ASK1 activity in the absence of JNK activation, suggesting that, at least in this context, ASK1 does not appear to be an activator of the JNK pathway. This is consistent with the results of a study of ASK1(−/−) mice which showed a clear attenuation of p38 activation during cardiac ischaemia in the knockout [42]. However, there was also no JNK activation in the wild-type during ischaemia, when ASK1 was clearly active, and only a small reduction of JNK activity upon reperfusion in the ASK1 knockout. Taken together, these results suggest that during cardiac ischaemia ASK1 couples preferentially to p38 rather than JNK.
4.7. Role of the ASK1/p38 pathway in cardiac responses to ischaemia
The involvement of ASK1 and p38 pathways in coordinating tissue responses to cellular stresses including ischaemia/reperfusion injury is clear and has been documented extensively [3,4,36,42,49,50].
The general consensus appears to be that p38 activation during ischaemia is likely to be detrimental and contributes to tissue injury since inhibition of p38 with the pyridinyl imidazole SB203580, and related compounds, seems to be protective [50]. The interpretation of this is complicated by the fact that these compounds inhibit p38α and β but not γ or δ. However, our data and those of others suggest that in the perfused heart p38γ and δ may not play a major role in ischaemic injury.
The different p38 isoforms appear to have different functions, with p38α having been linked to apoptosis and p38β promoting survival and hypertrophy [31,50–53]. p38α(−/−) mice do not survive [54] but those expressing a dominant-negative p38α appear to be protected from cardiac ischaemia/reperfusion injury, thus reinforcing the notion that p38α activation is detrimental [55].
The regulation of the p38 pathway in the ischaemic heart is clearly complex. A fuller understanding of the role of p38 in cardiac ischaemia will require further work with better isoform-specific p38 inhibitors combined with a careful analysis of cardiac-specific knockouts of the various p38 isoforms.
Acknowledgements
We would like to thank Dr. Raj Patel (University of Leicester) and Prof. Hidenori Ichijo (University of Tokyo) for the generous provision of reagents. This work was supported by a grant from the British Heart Foundation (PG 98111).
References
- 1.Johnson G.L., Lapadat R. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 2.Kyriakis J.M., Avruch J. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y. Circulation. 2007;116:1413–1423. doi: 10.1161/CIRCULATIONAHA.106.679589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausenloy D.J., Yellon D.M. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Bogoyevitch M.A., Gillespie-Brown J., Ketterman A.J., Fuller S.J., Ben-Levy R., Ashworth A., Marshall C.J., Sugden P.H. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 6.Yin T., Sandhu G., Wolfgang C.D., Burrier A., Webb R.L., Rigel D.F., Hai T., Whelan J. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 7.Knight R.J., Buxton D.B. Biochem Biophys Res Commun. 1996;218:83–88. doi: 10.1006/bbrc.1996.0016. [DOI] [PubMed] [Google Scholar]
- 8.Derijard B., Raingeaud J., Barrett T., Wu I.H., Han J., Ulevitch R.J., Davis R.J. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez I., Hughes R.T., Mayer B.J., Yee K., Woodgett J.R., Avruch J., Kyriakis J.M., Zon L.I. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 10.Moriguchi T., Toyoshima F., Masuyama N., Hanafusa H., Gotoh Y., Nishida E. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tournier C., Whitmarsh A.J., Cavanagh J., Barrett T., Davis R.J. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raingeaud J., Whitmarsh A.J., Barrett T., Derijard B., Davis R.J. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriguchi T., Kuroyanagi N., Yamaguchi K., Gotoh Y., Irie K., Kano T., Shirakabe K., Muro Y., Shibuya H., Matsumoto K., Nishida E., Hagiwara M. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 14.Lin A., Minden A., Martinetto H., Claret F.X., Lange-Carter C., Mercurio F., Johnson G.L., Karin M. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 15.Brancho D., Tanaka N., Jaeschke A., Ventura J.J., Kelkar N., Tanaka Y., Kyuuma M., Takeshita T., Flavell R.A., Davis R.J. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter-Vann A.M., Johnson G.L. J Cell Biochem. 2007;102:848–858. doi: 10.1002/jcb.21522. [DOI] [PubMed] [Google Scholar]
- 17.Cuevas B.D., Abell A.N., Johnson G.L. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 18.Finn S.G., Dickens M., Fuller S.J. Biochem J. 2001;358:489–495. doi: 10.1042/0264-6021:3580489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwaki K., Sukhatme V.P., Shubeita H.E., Chien K.R. J Biol Chem. 1990;265:13809–13817. [PubMed] [Google Scholar]
- 20.Laemmli U.K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Raingeaud J., Gupta S., Rogers J.S., Dickens M., Han J., Ulevitch R.J., Davis R.J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 22.Kelkar N., Gupta S., Dickens M., Davis R.J. Mol Cell Biol. 2000;20:1030–1043. doi: 10.1128/mcb.20.3.1030-1043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R.P., Kass D.A., Wang Y. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beardmore V.A., Hinton H.J., Eftychi C., Apostolaki M., Armaka M., Darragh J., McIlrath J., Carr J.M., Armit L.J., Clacher C., Malone L., Kollias G., Arthur J.S. Mol Cell Biol. 2005;25:10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemke L.E., Bloem L.J., Fouts R., Esterman M., Sandusky G., Vlahos C.J. J Mol Cell Cardiol. 2001;33:1527–1540. doi: 10.1006/jmcc.2001.1415. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.K., Pedram A., Razandi M., Levin E.R. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 27.Rakhit R.D., Kabir A.N., Mockridge J.W., Saurin A., Marber M.S. Biochem Biophys Res Commun. 2001;286:995–1002. doi: 10.1006/bbrc.2001.5477. [DOI] [PubMed] [Google Scholar]
- 28.Court N.W., dos Remedios C.G., Cordell J., Bogoyevitch M.A. J Mol Cell Cardiol. 2002;34:413–426. doi: 10.1006/jmcc.2001.1523. [DOI] [PubMed] [Google Scholar]
- 29.Seta K., Sadoshima J. J Mol Cell Cardiol. 2002;34:597–600. doi: 10.1006/jmcc.2002.2000. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka N., Kamanaka M., Enslen H., Dong C., Wysk M., Davis R.J., Flavell R.A. EMBO Rep. 2002;3:785–791. doi: 10.1093/embo-reports/kvf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Huang S., Sah V.P., Ross J., Jr., Brown J.H., Han J., Chien K.R. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 32.Enslen H., Brancho D.M., Davis R.J. EMBO J. 2000;19:1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enslen H., Raingeaud J., Davis R.J. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 34.Remy G., Risco A.M., Inesta-Vaquera F.A., Gonzalez-Teran B., Sabio G., Davis R.J., Cuenda A. Cell Signal. 2010;22:660–667. doi: 10.1016/j.cellsig.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 36.Hattori K., Naguro I., Runchel C., Ichijo H. Cell Commun Signal. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q., Zhang G. Neurosci Lett. 2002;329:232–236. doi: 10.1016/s0304-3940(02)00650-x. [DOI] [PubMed] [Google Scholar]
- 38.Stetler R.A., Cao G., Gao Y., Zhang F., Wang S., Weng Z., Vosler P., Zhang L., Signore A., Graham S.H., Chen J. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P., Cao X., Nagel D.J., Yin G. Neurosci Lett. 2007;415:248–252. doi: 10.1016/j.neulet.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 40.Terada Y., Inoshita S., Kuwana H., Kobayashi T., Okado T., Ichijo H., Sasaki S. Biochem Biophys Res Commun. 2007;364:1043–1049. doi: 10.1016/j.bbrc.2007.10.122. [DOI] [PubMed] [Google Scholar]
- 41.Izumi Y., Kim-Mitsuyama S., Yoshiyama M., Omura T., Shiota M., Matsuzawa A., Yukimura T., Murohara T., Takeya M., Ichijo H., Yoshikawa J., Iwao H. Arterioscler Thromb Vasc Biol. 2005;25:1877–1883. doi: 10.1161/01.ATV.0000174801.76234.bd. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T., Otsu K., Takeda T., Yamaguchi O., Hikoso S., Kashiwase K., Higuchi Y., Taniike M., Nakai A., Matsumura Y., Nishida K., Ichijo H., Hori M. Biochem Biophys Res Commun. 2005;333:562–567. doi: 10.1016/j.bbrc.2005.05.151. [DOI] [PubMed] [Google Scholar]
- 43.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanno M., Bassi R., Gorog D.A., Saurin A.T., Jiang J., Heads R.J., Martin J.L., Davis R.J., Flavell R.A., Marber M.S. Circ Res. 2003;93:254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- 45.Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R.J., Luo Y., Han J. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 46.Ge B., Xiong X., Jing Q., Mosley J.L., Filose A., Bian D., Huang S., Han J. J Biol Chem. 2003;278:2286–2293. doi: 10.1074/jbc.M210918200. [DOI] [PubMed] [Google Scholar]
- 47.Li J., Miller E.J., Ninomiya-Tsuji J., Russell R.R., III, Young L.H. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 48.Jacquet S., Zarrinpashneh E., Chavey A., Ginion A., Leclerc I., Viollet B., Rutter G.A., Bertrand L., Marber M.S. Cardiovasc Res. 2007;76:465–472. doi: 10.1016/j.cardiores.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Taniike M., Yamaguchi O., Tsujimoto I., Hikoso S., Takeda T., Nakai A., Omiya S., Mizote I., Nakano Y., Higuchi Y., Matsumura Y., Nishida K., Ichijo H., Hori M., Otsu K. Circulation. 2008;117:545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 50.Bassi R., Heads R., Marber M.S., Clark J.E. Curr Opin Pharmacol. 2008;8:141–146. doi: 10.1016/j.coph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Saurin A.T., Martin J.L., Heads R.J., Foley C., Mockridge J.W., Wright M.J., Wang Y., Marber M.S. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 52.Nemoto S., Xiang J., Huang S., Lin A. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 53.Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondoh G., Uno Y., Kashiwase K., Taniike M., Nakai A., Matsumura Y., Miyazaki J., Sudo T., Hongo K., Kusakari Y., Kurihara S., Chien K.R., Takeda J., Hori M., Otsu K. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams R.H., Porras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A.R. Mol Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- 55.Kaiser R.A., Bueno O.F., Lips D.J., Doevendans P.A., Jones F., Kimball T.F., Molkentin J.D. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]