Abstract
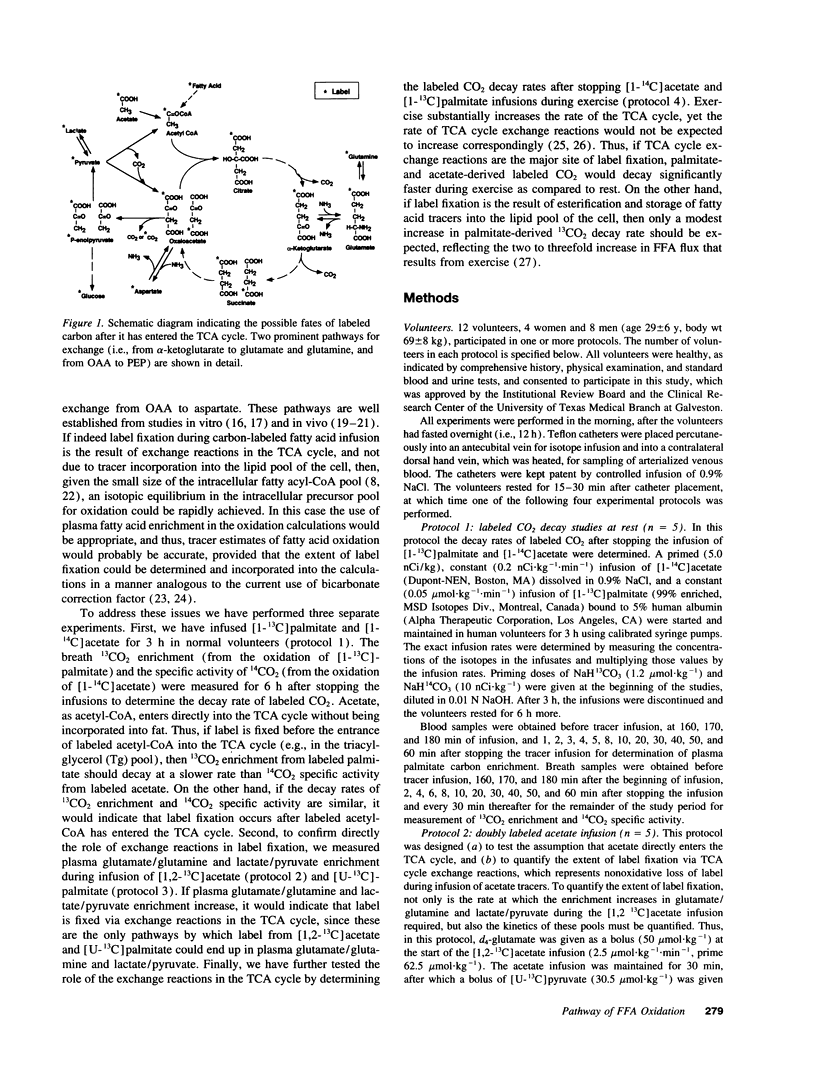
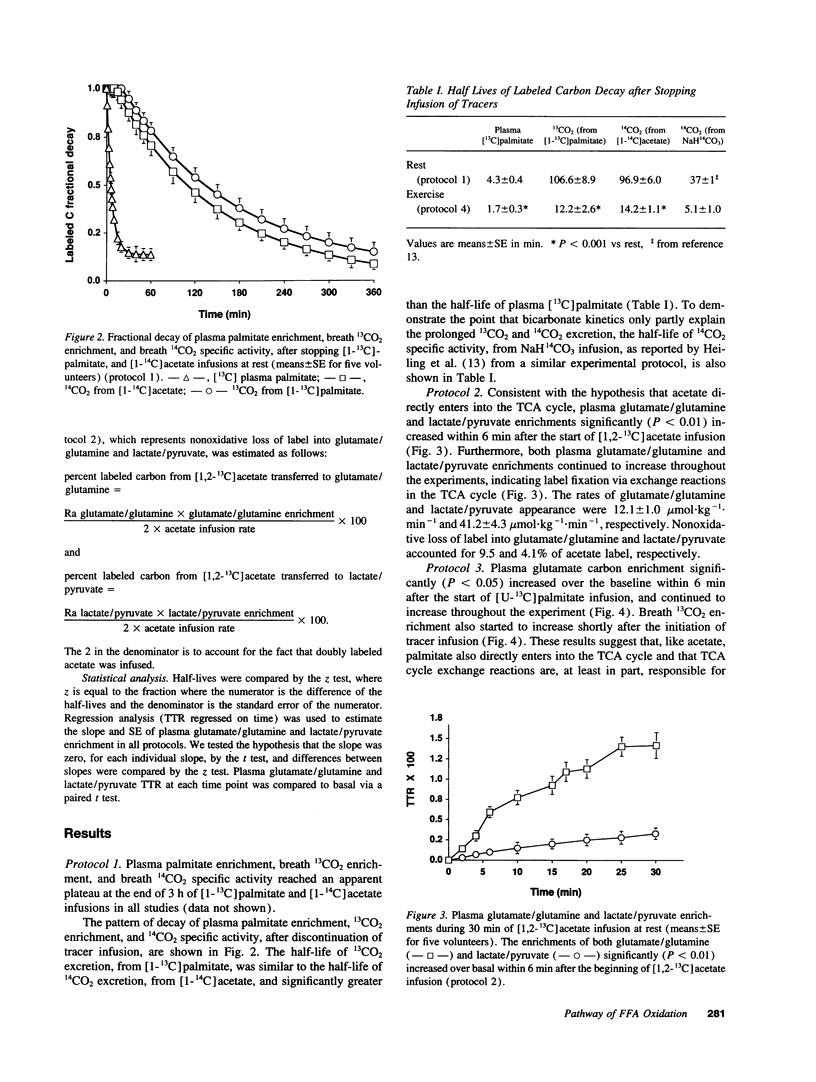
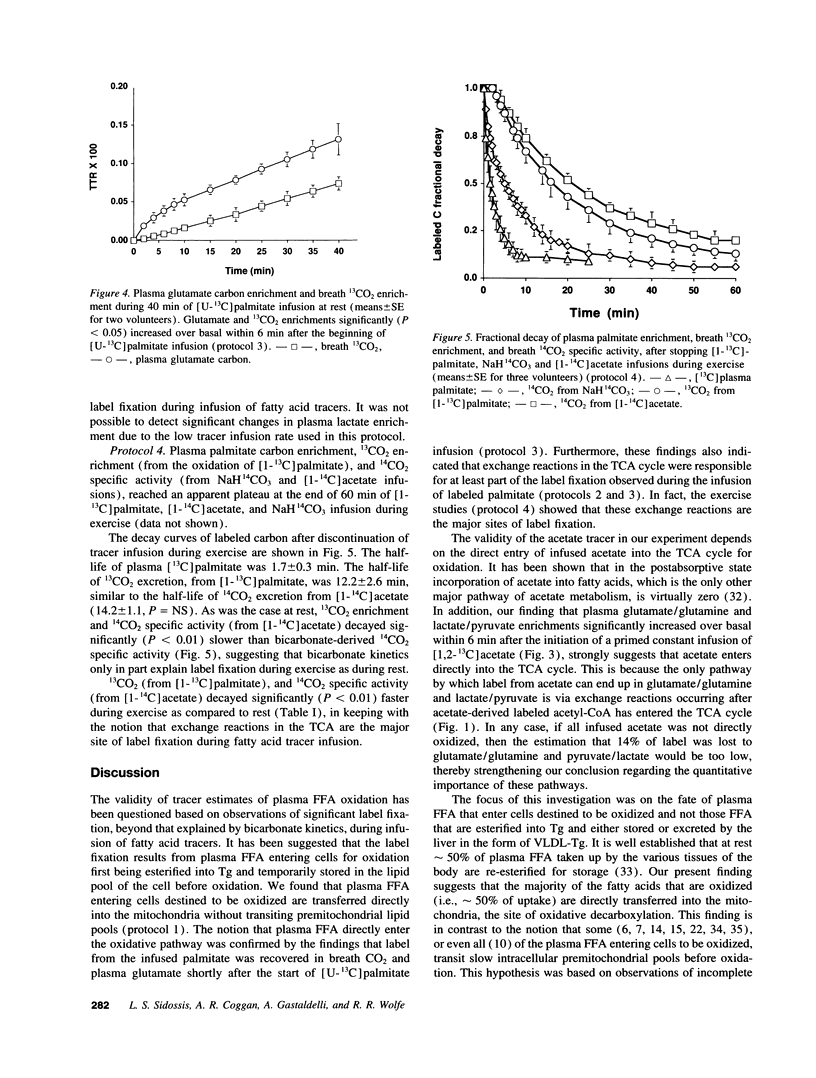
To determine the pathway of plasma FFA oxidation and the site(s) of label fixation observed during infusion of FFA tracers, [1-13C]palmitate and [1-14C]acetate were infused intravenously for 3 h in five volunteers. Breath 13CO2 enrichment and 14CO2 specific activity were followed for 6 h to determine the labeled CO2 decay rates. Acetate enters directly into the TCA cycle; hence, if palmitate transits a large lipid pool before oxidation, 13CO2 enrichment (from palmitate) should decay slower than 14CO2 specific activity (from acetate). Breath 13CO2 enrichment and 14CO2 specific activity decayed at a similar rate after stopping the tracer infusions (half-lives of 13CO2 and 14CO2 decay: mean [+/- SE] 106.6 +/- 8.9 min, and 96.9 +/- 6.0 min, respectively, P = NS), which suggests that palmitate enters the TCA cycle directly and that label fixation occurs after citrate synthesis. Significant label fixation was shown in plasma glutamate/glutamine and lactate/pyruvate during infusion of either [1,2-13C]acetate or [U-13C]palmitate, suggesting that TCA cycle exchange reactions are at least partly responsible for label fixation. This was consistent with our finding that the half-lives of 13CO2 enrichment and 14CO2 specific activity decreased significantly during exercise to 14.4 +/- 3 min and 16.8 +/- 1 min, respectively, since exercise significantly increases the rate of the TCA cycle in relation to that of the TCA cycle exchange reactions. We conclude that plasma FFA entering cells destined to be oxidized are directly oxidized and that tracer estimates of plasma FFA oxidation will underestimate the true value unless account is taken of the extent of label fixation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna R. C., Groop L. C., Zych K., Shank M., DeFronzo R. A. Dose-dependent effect of insulin on plasma free fatty acid turnover and oxidation in humans. Am J Physiol. 1990 Nov;259(5 Pt 1):E736–E750. doi: 10.1152/ajpendo.1990.259.5.E736. [DOI] [PubMed] [Google Scholar]
- Chinkes D. L., Zhang X. J., Romijn J. A., Sakurai Y., Wolfe R. R. Measurement of pyruvate and lactate kinetics across the hindlimb and gut of anesthetized dogs. Am J Physiol. 1994 Jul;267(1 Pt 1):E174–E182. doi: 10.1152/ajpendo.1994.267.1.E174. [DOI] [PubMed] [Google Scholar]
- Coggan A. R. Plasma glucose metabolism during exercise in humans. Sports Med. 1991 Feb;11(2):102–124. doi: 10.2165/00007256-199111020-00003. [DOI] [PubMed] [Google Scholar]
- Consoli A., Kennedy F., Miles J., Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987 Nov;80(5):1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais G. R., Tancredi R. G., Zierler K. L. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976 Aug;58(2):421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOERANSSON G., OLIVECRONA T. THE METABOLISM OF FATTY ACIDS IN THE RAT. I. PALMITIC ACID. Acta Physiol Scand. 1964 Nov;62:224–239. doi: 10.1111/j.1748-1716.1964.tb03970.x. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., Shank M., Petrides A. S., DeFronzo R. A. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest. 1991 Jan;87(1):83–89. doi: 10.1172/JCI115005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., Simonson D. C., Petrides A. S., Shank M., DeFronzo R. A. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. Am J Physiol. 1992 Jul;263(1 Pt 1):E79–E84. doi: 10.1152/ajpendo.1992.263.1.E79. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., NAIMARK A., BORCHGREVINK C. F. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest. 1968;21(3):263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. VII. FFA uptake and oxidation at different work intensities. Scand J Clin Lab Invest. 1972 Dec;30(4):429–436. doi: 10.3109/00365517209080281. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Ekelund L. G., Holmgren A. Kinetic analysis of the oxidation of palmitate-1-14C in man during prolonged heavy muscular exercise. J Lipid Res. 1967 Jul;8(4):366–373. [PubMed] [Google Scholar]
- Havel R. J., Pernow B., Jones N. L. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967 Jul;23(1):90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Heiling V. J., Miles J. M., Jensen M. D. How valid are isotopic measurements of fatty acid oxidation? Am J Physiol. 1991 Nov;261(5 Pt 1):E572–E577. doi: 10.1152/ajpendo.1991.261.5.E572. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Christiansen M., Kaempfer S., Kletke C., Wu K., Reid J. S., Mulligan K., Hellerstein N. S., Shackleton C. H. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J Clin Invest. 1991 May;87(5):1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I., Bortz W. M. Oxidation of plasma FFA in lean and obese humans. Metabolism. 1968 Jan;17(1):62–73. doi: 10.1016/s0026-0495(68)80008-3. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Schumann W. C., Bartsch G. E., Chandramouli V., Kumaran K., Wahren J., Landau B. R. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem. 1991 Apr 15;266(11):6975–6984. [PubMed] [Google Scholar]
- Malmendier C. L., Delcroix C., Berman M. Interrelations in the oxidative metabolism of free fatty acids, glucose, and glycerol in normal and hyperlipemic patients. A compartmental model. J Clin Invest. 1974 Aug;54(2):461–476. doi: 10.1172/JCI107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn J. A., Coyle E. F., Sidossis L. S., Gastaldelli A., Horowitz J. F., Endert E., Wolfe R. R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993 Sep;265(3 Pt 1):E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- STRISOWER E. H., KOHLER G. D., CHAIKOFF I. L. Incorporation of acetate carbon into glucose by liver slices from normal and alloxan-diabetic rats. J Biol Chem. 1952 Sep;198(1):115–126. [PubMed] [Google Scholar]
- Schumann W. C., Magnusson I., Chandramouli V., Kumaran K., Wahren J., Landau B. R. Metabolism of [2-14C]acetate and its use in assessing hepatic Krebs cycle activity and gluconeogenesis. J Biol Chem. 1991 Apr 15;266(11):6985–6990. [PubMed] [Google Scholar]
- Wisneski J. A., Gertz E. W., Neese R. A., Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987 Feb;79(2):359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R., Durkot M. J., Wolfe M. H. Investigation of kinetics of integrated metabolic response to adrenergic blockade in conscious dogs. Am J Physiol. 1981 Nov;241(5):E385–E395. doi: 10.1152/ajpendo.1981.241.5.E385. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Evans J. E., Mullany C. J., Burke J. F. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980 Apr;7(4):168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Jahoor F., Miyoshi H. Evaluation of the isotopic equilibration between lactate and pyruvate. Am J Physiol. 1988 Apr;254(4 Pt 1):E532–E535. doi: 10.1152/ajpendo.1988.254.4.E532. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Jahoor F. Recovery of labeled CO2 during the infusion of C-1- vs C-2-labeled acetate: implications for tracer studies of substrate oxidation. Am J Clin Nutr. 1990 Feb;51(2):248–252. doi: 10.1093/ajcn/51.2.248. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Klein S., Carraro F., Weber J. M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990 Feb;258(2 Pt 1):E382–E389. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Puhakainen I., Saloranta C., Groop L., Taskinen M. R. Demonstration of a novel feedback mechanism between FFA oxidation from intracellular and intravascular sources. Am J Physiol. 1991 May;260(5 Pt 1):E680–E689. doi: 10.1152/ajpendo.1991.260.5.E680. [DOI] [PubMed] [Google Scholar]



