Abstract
Background. About 30% of dilated cardiomyopathy (DCM) cases are familial. Mutations are mostly found in the genes encoding lamin A/C, beta-myosin heavy chain and the sarcomeric protein cardiac troponin-T (TNNT2). Mutations in TNNT2 are reported in approximately 3% of DCM patients. The overall phenotype caused by TNNT2 mutations is thought to be a fully penetrant, severe disease. This also seems to be true for a recurrent deletion in the TNNT2 gene; p.K217del (also known as p.K210del).
Methods. We compared the phenotype of all Dutch patients identified as carrying the TNNT2 p.K217del mutation with those described in the literature. All index patients underwent cardiological evaluation. Family screening was done in all described families.
Results. Six DCM patients carrying the TNNT2 p.K217del mutation were identified from four Dutch families. Mean age of disease manifestation was 33 years. Heart transplantation was required in three of them at ages 12, 18 and 19 years. These outcomes are comparable with those described in the literature.
Conclusion. Carriers of the TNNT2 p.K217del mutation in our Dutch families, as well as in families described in the literature before, generally show a severe, early-onset form of DCM. (Neth Heart J 2010;18:478–85.)
Keywords: Cardiomyopathy, Dilated, Genetics , Troponin T
According to the European Society of Cardiology (ESC), cardiomyopathy is defined as a myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of an underlying cause.1 One of the subtypes, dilated cardiomyopathy (DCM), is characterised by abnormal ventricular enlargement together with impaired systolic function, eventually leading to heart failure. It is the most common indication for cardiac transplantation.2 DCM can be isolated, but also multiple family members can be affected. About 30% of DCM cases are reported to be familial.3-7
Familial DCM is clinically and genetically highly heterogeneous, with more than 20 genes being identified as underlying the disease.8 These genes involved in DCM mainly encode cytoskeletal, nuclear envelope, and sarcomeric proteins.9 Mutations in these genes are found in up to 25% of cases.4,10,11 Causative mutations in DCM are mostly found in the lamin A/C (LMNA), beta myosin heavy chain (MYH7), and troponin T (TNNT2) genes. These mutations have been found in familial DCM in about 7%, 7% and 3% of index patients, respectively.4-6,9,12-17 Screening for mutations in genes coding for sarcomeric proteins is therefore considered useful in cases of both familial and non-familial DCM, when other causes for DCM are excluded.
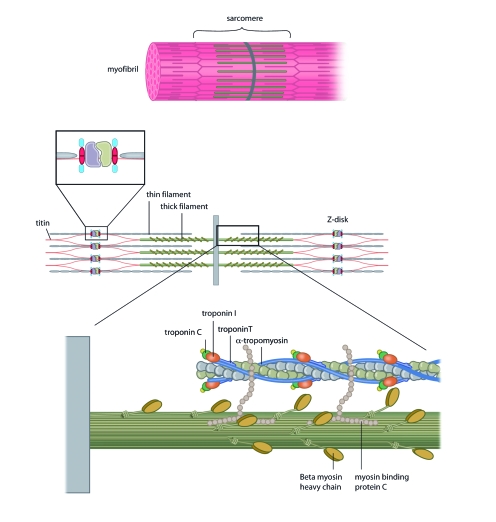
The specific mechanisms by which mutations in sarcomeric protein genes lead to different forms of cardiomyopathy still remain largely unclear. Some authors state that depending on the way in which the protein function of cardiac troponin T is altered, mutations will either lead towards a hypertrophic cardiomyopathy (HCM) or a DCM phenotype.7,18,19 Troponin T, a sarcomeric protein, forms a cytoskeletal complex with troponin C (TnC) and troponin I (TnI), thereby playing a central role in calcium regulation of (cardiac) muscle contraction, which makes it essential for maintaining normal cardiac contractile function (figure 1).20
Figure 1.
The myocytic troponin complex forms part of the sarcomere. Muscle contraction is achieved by the ATP-dependant interaction of the troponin subunits with actin and tropomyosin. Adapted from: Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. J Clin Invest. 2005;115:518-26.
Thus far, 11 different mutations in TNNT2 have been reported to cause DCM.12 An overall genotype-phenotype correlation observed for DCM caused by TNNT2 mutations suggests a fully penetrant, aggressive disease, manifesting at a relatively young age.12 This also seems to be true for patients with DCM caused by the p.K217del mutation, which is believed to be a recurrent mutation in DCM.3,5,7,9,12
Our study aimed to describe all the Dutch patients identified as carrying the recurrent p.K217del mutation (also known as p.K210del) in TNNT2, and to compare the patient characteristics with those of patients described in the literature.
Materials and Methods
Clinical evaluation
All index patients included in this study underwent cardiological evaluation consisting of at least electrocardiography, echocardiography and a family history. A diagnosis of DCM was made, based on the ESC criteria.1
Genetic evaluation
Total genomic DNA of index patients was extracted from EDTA (peripheral) blood according to standard procedures. PCR amplification and mutation analysis for TNNT2 was performed using primers located in flanking intronic sequences (available upon request) using DHPLC (denaturing high-performance liquid chromatography) on the WAVE system (Transgenomics, Santa Clara, California, USA) or sequence analysis. Nomenclature for the description of the mutation is according to http://www.hgvs.org/mutnomen/ using NM_000364.2 as the reference sequence.
Results
Genetic data
Six patients from four Dutch families were identified as carrying the TNNT2 p.K217del mutation causing DCM. This mutation causes an in frame deletion of three base pairs, which leads to the deletion of the amino-acid lysine in the troponin T protein. This leads to the forming of a functionally altered protein.19,21
Clinical data
In family A, the TNNT2 p.K217del mutation was found in a 10-year-old girl (table 1) who complained of progressive shortness of breath on exertion and lying flat. She was diagnosed with severe DCM (left ventricular shortening fraction 12 to 13%, left ventricular dilatation). At age 12 her cardiac function had deteriorated (left ventricular end-diastolic diameter (LVEDD) 72 mm, LV shortening fraction 7%) and she required heart transplantation. The TNNT2 p.K217del mutation was not found in her parents and paternity was confirmed, so this mutation had occurred de novo. No additional pathogenic mutation was found in the LMNA, MYH7 and desmin (DES) genes.
Table 1.
Characteristics and outcomes of Dutch DCM families carrying the TNNT2 p.K217del mutation.
| Family | Age at diagnosis | Sex | Presentation | Family characteristics | Mutation confirmed | Outcome (age) |
|---|---|---|---|---|---|---|
| A | 10 | ♀ | General complaints | De novo mutation | + | Progression, waiting for HtX (12) |
| B; III-1 | 70 | ♀ | Tiredness, dyspnoea | Sister (13): died of unknown heart disease, father (46) and 2 brothers of father (55 and 58): SCD | + | Current age 72 |
| B; IV-1 | 10 | ♀ | Progressive tiredness | NA | Died (10) DCM | |
| B; IV-2 | 13 | ♂ | Progressive tiredness | NA | Died (13) DCM | |
| C | 18 | ♂ | Progressive exercise intolerance, general weakness | Mother (40) died of CM, grandfather (57) SCD | + | HtX (19) |
| D; III-2 | 18 | ♂ | Cardiogenic shock | + | HtX (18) | |
| D; II-1 | 54 | ♀ | ↓ Exercise tolerance | + | ICD, current age 56 | |
| D; III-1 | 29 | ♂ | Family screening | + | Palpitations (31) |
♀ female, ♂ male, + TNNT2 p.K217del mutation identified, NA=clinical phenotype of DCM but formal mutation analysis not available, CM=cardiomyopathy, HtX=heart transplantation, ICD=implantable cardioverter defibrillator, SCD=sudden cardiac death.
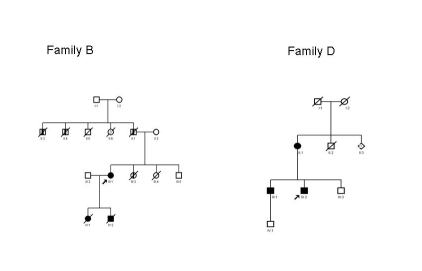
The 70-year old female index patient of family B (III-1, figure 2; table 1) was evaluated by a cardiologist since she had complained of tiredness and dyspnoea on exertion for about six months. She was found to have DCM (LVEDD 66 mm, left ventricular ejection fraction 18%). The family history was remarkable: her two children had died from DCM at ages 10 and 13 years, respectively. Her daughter (IV-1, figure 2) had suffered from progressive tiredness and was admitted to hospital at the age of 9. During admission her condition worsened and she died (age 10 years). Autopsy revealed a dilated heart with fibro-elastosis. The woman’s son (IV-2, figure 2) was also admitted to hospital, at age 13 years, because of progressive tiredness. On echocardiography a dilated heart with minimal contractile activity was seen. He died one month after the initial evaluation. Autopsy confirmed the DCM. Moreover, the woman’s sister (III-3, figure 2) had died at age 13 because of an unknown heart disease and the woman’s father (II-1, figure 2) had died from sudden cardiac death (SCD) at age 46. Two brothers of her father (II-3 and II-4, figure 2) had died suddenly and without evident reason, at ages 55 and 58. There were no DNA samples available for these deceased family members.
Figure 2.
Pedigrees of family B and D. □ male, ○ female, ⋄ sex not determined. Closed symbols depict the patients showing dilated cardiomyopathy. Half closed symbols depict the family members with possible dilated cardiomyopathy. The arrow indicates the index patient carrying the TNNT2 mutation p.K217del.
In family C, the TNNT2 p.K217del mutation was found in a male who was admitted to hospital at age 18 with a short history of progressive exercise intolerance, and general weakness (table 1). Echocardiography showed a severely dilated left ventricle with a severely depressed systolic left ventricular function. The right ventricular function was moderately diminished. A diagnosis of DCM was made. At age 19 he underwent cardiac transplantation. The family history revealed that his mother had died at age 40 due to a cardiomyopathy and that his mother’s father died at age 57 from sudden cardiac death.
The male index patient of family D underwent urgent cardiac transplantation at age 18 (III-2; table 1, figure 2), shortly after presenting with severe and rapidly progressive heart failure caused by DCM. A few years after the cardiac transplant, his mother (II-1; table 1, figure 2) was also diagnosed with DCM. Both carry the TNNT2 p.K217del mutation. Echocardiography of his elder brother (III-1, table 1, figure 2) demonstrated non-compaction cardiomyopathy. He was confirmed to be a carrier of the TNNT2 p.K217del mutation. His younger brother (III-3, figure 2) has so far refused DNA testing, but his cardiac evaluation was normal. Their mother (II-1) has twelve siblings. Nine were tested for the TNNT2 p.K217del mutation and were all negative. One of her brothers had died at age 18 months (II-2, figure 2), possibly because of a cardiac disease. Her parents both lived into their eighties, and they were both under cardiological surveillance. Her father was known with atrial fibrillation and a narrowing of his mitral valve. Her mother had a myocardial infarction.
Discussion
The yield of TNNT2 analysis in familial DCM patients from different published studies thus far ranges from 0 to 9.5% (average 2.7%), in a total of 654 index patients.4,6,7,9,12,14,22-25 In these patients 17 TNNT2 mutations have been found, of which six (35%) were the specific TNNT2 p.K217del mutation. Two studies only analysed exon 13 of the TNNT2 gene.3,5 These revealed two more families with the TNNT2 p.K217del mutation. Finally, two more DCM families in which a TNNT2 mutation was found have been described separately.26,27 The observation that the TNNT2 p.K217del mutation is present in different populations over the world suggests that this mutation is a recurrent mutation although formally a founder mutation can not be excluded. Our observation of a proven de novo case (patient A), however, proves that this mutation is indeed a recurrent TNNT2 mutation. This means that this particular mutation occurs at a higher frequency due to susceptibility of this part (hotspot) of the gene.
Mutation p.K217del in the TNNT2 gene has thus been described in the literature in eight (unrelated) families in total. These families comprised 33 affected persons and the mutation was confirmed in 20 of them. These patients show a phenotype of severe DCM, with clinical onset in infancy or in early adulthood in the majority of affected persons (table 2).3,5,7,9,12
Table 2.
Characteristics and outcomes of DCM families previously described in the literature, carrying the TNNT2 delta K.217 mutation.
| Family | Age at diagnosis | Sex | Presentation | Family characteristics | Mutation confirmed | Outcome (age) |
|---|---|---|---|---|---|---|
| Martins et al.3 | ||||||
| Patient 1 (index) | 25 | ♀ | + | HtX (25) | ||
| Patient 2 (father) | 45 | ♂ | 1 sister + mother died due to HF (both ± 40), 1 sister (53) + 1 brother (27) SCD | NA | Died (53) DCM | |
| Hanson et al.5 | ||||||
| Patient 1 (index) | 59 | ♀ | Maternal grandfather (38) SCD | + | Current age 60 | |
| Patient 2 (mother) | 74 | ♀ | NA | Died (74) due to HF | ||
| Patient 3 (sib) | 16 | ♂ | NA | Died (16) due to HF | ||
| Patient 4 (son) | 20 | ♂ | NA | Died (20) due to HF | ||
| Patient 5 (cousin) | 12 | ♀ | Brother: death (13 months) due to HF | NA | Died (12) | |
| Patient 6 (cousin) | ? | ♂ | + | Asymptomatic, current age 50 | ||
| Kamisago et al. family 17 | ||||||
| Patient 1 (index) | 53 | ♀ | 2 sibs died (1/8 months) CM, 2 sibs of mother (26/27) SCD | + | Current age 59 | |
| Patient 2 (daughter) | 23 | ♀ | + | Current age 29 | ||
| Patient 3 (sib) | 48 | ♂ | + | Current age 51 | ||
| Kamisago et al. family 27 | ||||||
| Patient 1 (index) | 49 | ♀ | 1 daughter died (17) HF, 1 daughter postpartum HF, SCD (19), post-mortem LV and RV dilatation | + | Died (68) CVA | |
| Patient 2 (daughter) | 23 | ♀ | + | Current age 44 | ||
| Patient 3 (grandson) | 14 | ♂ | + | Died (15) due to HF | ||
| Mogensen et al.9 | ||||||
| Patient 1 | 25 | ♂ | Heart failure | + | Died (26) | |
| Patient 2 | 21 | ♂ | + | HtX (22) | ||
| Patient 3 | 35 | ♂ | + | Current age 36 | ||
| Hershberger et al. family 112 | ||||||
| Patient 1 (index) | 31 | ♂ | + | HtX (31) | ||
| Patient 2 (daughter) | 5 | ♀ | NA | Died (5) | ||
| Patient 3 (father) | ? | ♂ | NA | Died (52) | ||
| Hershberger et al. family 212 | ||||||
| Patient 1 (father) | 50 | ♂ | NA | DCM at autopsy (50) | ||
| Patient 2 (sib) | 34 | ♀ | NA | Died (34) after HtX | ||
| Patient 3 (sib) | 37 | ♂ | + | Asymptomatic (50) | ||
| Patient 4 (index) | 26 | ♂ | + | HF | ||
| Hershberger et al. family 312 | ||||||
| Patient 1 | 74 | ♀ | + (oc) | Died | ||
| Patient 2 | ? | ♀ | + (oc) | Died (34) of cancer | ||
| Patient 3 | 59 | ♀ | + | HF | ||
| Patient 4 | 16 | ♂ | NA | Died (16) of HF, DCM at autopsy | ||
| Patient 5 | 52 | ♂ | + | HF | ||
| Patient 6 | 47 | ♂ | + | Asymptomatic | ||
| Patient 7 | 1 | ♂ | NA | Died (1) DCM | ||
| Patient 8 | 12 | ♀ | NA | Died (12) DCM | ||
| Patient 9 | 21 | ♂ | NA | Died (21) DCM |
♀ female, ♂ male, + TNNT2 p.K217del mutation identified, NA=clinical phenotype of DCM but formal mutation analysis not available, oc=obligate carrier, CM=cardiomyopathy, CVA=cerebrovascular accident, HtX=heart transplantation, LV=left ventricle, RV=right ventricle, SCD=sudden cardiac death.
We describe here another six patients from four families with the TNNT2 p.K217del mutation, of which five patients had a DCM phenotype and one showed a non-compaction cardiomyopathy (table 1). Another two family members, for whom no DNA was available, were also diagnosed with DCM. The median age of diagnosis of our six proven TNNT2 p.K217del mutation carriers was 23.5 years (10 to 70 years). They are all still alive, with three patients requiring heart transplantation at ages 12, 18 and 19 years, respectively. Overall, in these families, two DCM patients (2/8) had died at ages 10 and 13 years, respectively. These data do not differ significantly from the patients described in the literature.
The median age of diagnosis in the patients described in the literature with a confirmed or obligate TNNT2 p.K217del mutation (20 patients) was 37 years (14 to 74 years).3,5,7,9,12 Eight of these patients and obligate carriers had died, had been transplanted or were on a waiting list for cardiac transplantation. Excluding one patient who died of cancer, the median age for these events was 26 years (15 to 74 years). Another three persons (3/20) were still asymptomatic at a median age of 50 years (47 to 50 years).3,5,7,9,12 Including the affected family members without mutation analysis results, but who are highly likely to be carriers of the identical mutation, there are 16 patients (16/31) who died from DCM at a median age of 20.5 years (1 to 74 years).3,5,7,9,12 In some of these families there is a marked intrafamilial variability in age of disease onset and severity. This may be due to environmental or additional genetic influences, or both.4
However, both in our patients as well as those described in the literature, the general phenotype associated with the TNNT2 p.K217del mutation is that of early onset, severe cardiac disease, with a relatively high incidence of sudden cardiac death.
Thus far, eleven different TNNT2 mutations are known to cause DCM, with an overall severe phenotype in most affected patients. Mutations in TNNT2 can also cause HCM as well as restrictive cardiomyopathy (RCM) and non-compaction cardiomyopathy (NCCM).28-30 Non-compaction cardiomyopathy caused by a TNNT2 mutation was only reported once in the literature and was also identified in person III-1 from family D (figure 2).30 Until recently, NCCM was believed to be a rare form of cardiomyopathy.31 However, increasing numbers of patients with this diagnosis are being described. This might be attributed to increased awareness of this phenotype by cardiologists and the use of modern ultrasound technology with increased detection of NCCM features, for example in patients that were previously diagnosed with DCM.32
Functional studies revealed that the TNNT2 p.K217del mutation has a desensitising effect on force generation in cardiac muscle cells and on ATP-ase activity.19,21 A decrease in Ca2+ sensitivity is thought to cause a significant reduction in the force generation by the sarcomere of cardiac muscle cells, leading to impaired systolic function. Subsequently, ventricular dilatation will develop as a compensatory mechanism for the decreased stroke volume.18,19,21
These functional data provide evidence that the decrease in Ca2+ sensitivity might be a primary mechanism for the pathogenesis of DCM in patients with this specific TNNT2 mutation. Functional consequences of the mutations in TNNT2 that are associated with DCM seem to be opposite to the consequences of the HCM-causing mutations in TNNT2, which appear to cause increased Ca2+ sensitivity.21
Conclusion
Mutations in genes encoding sarcomeric proteins are seen to cause DCM in a substantial part of familial and non-familial DCM. Consequently, screening for mutations in these genes is relevant. In the literature, the screening of familial DCM patients revealed a TNNT2 mutation in 0 to 9.5% of index patients (average 2.7%), with the p.K217del mutation accounting for one-third (6 of 17) of all the mutations found in TNNT2. Carriers of this mutation generally show a severe form of DCM with early disease manifestation. We were able to evaluate six patients from four Dutch families carrying this identical mutation. We can confirm the severe phenotype associated with the TNNT2 p.K217del mutation since we observed an early age of disease manifestation, with a median age of 23 years (10 to 70) and three of six carriers requiring cardiac transplantation at ages younger than 20 years.
Acknowledgement
We would like to thank Jackie Senior for editing the manuscript.
References
- 1.Elliot P, Andersson B, Arbustini E, Bininska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270-6. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the international society for heart and lung transplantation: Twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009;28:1007-22. [DOI] [PubMed] [Google Scholar]
- 3.Martins E, Silva-Cardoso J, Alves C, Pereira H, Soares B, Damasceno A, et al. Familial dilated cardiomyopathy with troponin T K210del mutation. Rev Port Cardiol. 2006;25:295-300. [PubMed] [Google Scholar]
- 4.Møller DV, Andersen PS, Hedley P, Ersbøll MK, Bundgaard H, Moolman-Smook J, et al. The role of sarcomere gene mutations in patients with idiopathic dilated cardiomyopathy. Eur J Hum Genet. 2009;17:1241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson EL, Jakobs PM, Keegan H, Coates K, Bousman S, Dienel NH, et al. Cardiac troponin T lysine 210 deletion in a family with dilated cardiomyopathy. J Card Fail. 2002;8:28-32. [DOI] [PubMed] [Google Scholar]
- 6.Villard E, Duboscq-Bidot L, Charron P, Benaiche A, Conraads V, Sylvius N, et al. Mutation screening in dilated cardiomyopathy: prominent role of the beta myosin heavy chain gene. Eur Heart J. 2005;26:751-4. [DOI] [PubMed] [Google Scholar]
- 7.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688-96. [DOI] [PubMed] [Google Scholar]
- 8.Van Spaendonck-Zwarts KY, van den Berg MP, van Tintelen JP DNA analysis in inherited cardiomyopathies: current status and clinical relevance. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S46-9. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, et al. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2033-40. [DOI] [PubMed] [Google Scholar]
- 10.Osterziel KJ, Haßfeld S, Geier C, Perrot A. Familiare dilatative Kardiomyopathie. Herz. 2005;30:529-34. [DOI] [PubMed] [Google Scholar]
- 11.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557-67. [DOI] [PubMed] [Google Scholar]
- 12.Hershberger RE, Pinto J, Parks SB, Kushner JD, Li D, Ludwigsen S, et al. Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2009; 2:306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MRG, Towbin JA. Genetic evaluation of cardiomyopathy – a heart failure society of America practice guideline. J Card Fail. 2009;15:83-97. [DOI] [PubMed] [Google Scholar]
- 14.Daehmlow S, Erdmann J, Knueppel T, Gille C, Froemmel C, Hummel M, et al. Novel mutations in sarcomeric protein genes in dilated cardiomyopathy. Biochem Biophys Res Commun. 2002;298:116-20. [DOI] [PubMed] [Google Scholar]
- 15.Parks SB, Kushner JD, Naumann D, Burgess D, Ludwigsen S, Peterson A, et al. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am Heart J. 2008;156:161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor MRG, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, et al. Natural history of dilated cardiomyopathy due to Lamin A/C gene mutations. J Am Coll Cardiol. 2003;41:771-80. [DOI] [PubMed] [Google Scholar]
- 17.van Tintelen JP, Hofstra RMW, Katerberg H, Rossenbacker T, Wiesfeld ACP, du Marchie Sarvaas GJ, et al. High yield of LMNA mutations in patients with dilated cardiomyopathy and/or conduction disease referred to cardiogenetics outpatient clinics. Am Heart J. 2007;154:1130-9. [DOI] [PubMed] [Google Scholar]
- 18.Venkatraman G, Harada K, Gomes AV, Kerrick WG, Potter JD. Different functional properties of troponin T mutants that cause dilated cardiomyopathy. J Biol Chem. 2003;278:41670-6. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, et al. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada K, Morimoto S. Inherited cardiomyopathies as a troponin disease. Jpn J Physiol. 2004;54:307-18. [DOI] [PubMed] [Google Scholar]
- 21.Robinson P, Mirza M, Knott A, Abdulrazzak H, Willott R, Marston S, et al. Alterations in thin filament regulation induced by a human cardiac troponin T mutant that causes dilated cardiomyopathy are distinct from those induced by troponin T mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2002;277:40710-6. [DOI] [PubMed] [Google Scholar]
- 22.Kärkkäinen S, Heliö T, Jääskeläinen P, Miettinen R, Tuomainen P, Ylitalo K, et al. Two novel mutations in the β-myosin heavy chain gene associated with dilated cardiomyopathy. Eur J Heart Fail. 2004;6:861-8. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Arimura T, Itoh-Satoh M, Ueda K, Hohda S, Inagaki N, et al. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2192-201. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu M, Ino H, Yasuda T, Fujino N, Uchiyama K, Mabuchi T, et al. Gene mutations in adult Japanese patients with dilated cardiomyopathy. Circ J. 2005;69:150-3. [DOI] [PubMed] [Google Scholar]
- 25.Zeller R, Ivandic BT, Ehlermann P, Mücke O, Zugck C, Remppis A, et al. Large-scale mutation screening in patients with dilated or hypertrophic cardiomyopathy: a pilot study using DGGE. J Mol Med. 2006;84:682-91. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Czernuszewicz GZ, Gonzalez O, Tapscott T, Karibe A, Durand JB, et al. Novel cardiac troponin T mutation as a cause of familial dilated cardiomyopathy. Circulation. 2001;104:2188-93. [DOI] [PubMed] [Google Scholar]
- 27.Stefanelli CB, Rosenthal A, Borisov AB, Ensing GJ, Russell MW. Novel troponin T mutation in familial dilated cardiomyopathy with gender-dependant severity. Mol Genet Metab. 2004;83:188-96. [DOI] [PubMed] [Google Scholar]
- 28.Menon SC, Michels VV, Pellikka PA, Ballew JD, Karst ML, Herron KJ, et al. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin Genet. 2008;74:445-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaski JP, Syrris P, Burch M, Tomé-Esteban M-T, Fenton M, Christiansen M, et al. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart. 2008; 94:1478-84. [DOI] [PubMed] [Google Scholar]
- 30.Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117:2893-901. [DOI] [PubMed] [Google Scholar]
- 31.Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26-31. [DOI] [PubMed] [Google Scholar]
- 32.Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, et al. Clinical features of isolated nancompaction of the ventricular myocardium: Long term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol. 1999;34:233-40. [DOI] [PubMed] [Google Scholar]