Abstract
Frequent monomorphic ventricular premature beats (VPBs) may lead to left ventricular dysfunction. We describe two patients with frequent monomorphic VPBs and dilated cardiomyopathy in whom left ventricular function normalised after elimination of the VPBs by radiofrequency catheter ablation. The recent literature on this topic is summarised and potential candidates for catheter ablation are discussed. (Neth Heart J 2010;18:493–8.)
Keywords: Cardiomyopathy, Ablation, Premature Ventricular Complex
Ventricular premature beats (VPBs) are commonly observed in clinical practice.1-2In patients with no underlying heart disease, VPBs are considered benign and besides reassurance, no specific treatment is generally indicated.3 In recent case reports and small case series, however, it has been demonstrated that frequent monomorphic VPBs may cause dilated cardiomyopathy.4-13 A causal relation between VPBs and left ventricular dysfunction was demonstrated by the fact that successful elimination of the VPBs, either by radiofrequency catheter ablation or antiarrhythmic drugs, improved or even normalised left ventricular systolic function.4-13
In this article we describe two patients with cardiomyopathy and symptomatic frequent monomorphic ventricular beats in whom left ventricular function markedly improved after elimination of the VPBs by radiofrequency catheter ablation. The recent literature on this topic is summarised and patient characteristics of potential candidates for catheter ablation are discussed.
Case reports
Case 1
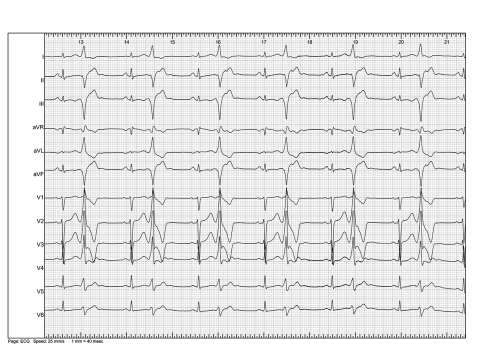
A 34-year-old female was referred for evaluation of frequent palpitations, dizziness and mild fatigue. The symptoms had been present for one year. There was no history of familial cardiac disease. Physical examination was unremarkable. The 12-lead electrocardiogram showed frequent monomorphic VPBs with a right bundle branch block morphology and superior axis (figure 1). Holter recording demonstrated an average heart rate of 74 beats/min with 40,300 monomorphic VPBs during a 24-hour period (43% of all QRS complexes). No (non)-sustained ventricular tachycardias were observed. The echocardiogram showed a reduced ejection fraction of 40% (biplane Simpson method) and mild mitral regurgitation. Of note, echocardiographic measurements were hampered by the frequent VPBs. Additional investigations included an exercise test and a cardiac MRI with gadolinium. Her exercise tolerance was good. No anginal symptoms or ECG changes were observed. Of note, the number of VPBs decreased during exercise and reappeared in the recovery phase. The MRI showed no abnormalities except an enlarged left ventricle and reduced systolic function. She was treated with valsartan 80 mg and nebivolol 2.5 mg, both once daily. During one year of follow-up she continued to have symptoms of palpitations. Repeated Holter recordings demonstrated frequent monomorphic VPBs and the mild systolic left ventricular dysfunction persisted.
Figure 1.
Twelve-lead electrocardiogram showing frequent monomorphic ventricular premature beats in a bigeminal pattern.
To eliminate the frequent symptomatic VPBs she was scheduled for an electrophysiological study and radiofrequency catheter ablation. In addition we hypothesised that elimination of the VPBs could improve left ventricular function. Baseline electrophysiological measurements were normal. Based on the VPB morphology on the 12-lead ECG, a left-sided origin of the VPBs was suspected. Using a retrograde approach from the femoral artery, a roving ablation catheter was used for mapping, pacing and ablation of the left ventricle. To identify the origin of the VPBs, the morphology of QRS complexes during pacing was subsequently compared with QRS morphology during spontaneous VPBs (pace mapping). Multiple ventricular sites were used for pacing. A good match between paced complexes and spontaneous VPBs was observed while pacing in the mid-septal region (figures 2 and 3). Activation mapping at this site during the spontaneous VPBs showed the local activation 35 ms before onset of the QRS (figure 4). Radiofrequency energy was delivered in this region using a cooled 4 mm-tip ablation catheter. After several applications, VPBs had disappeared and also after isoprenaline VPBs were no longer observed.
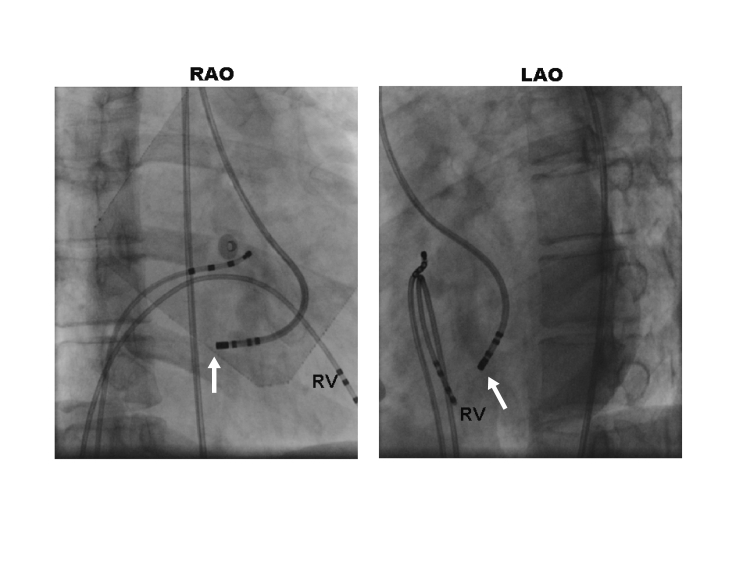
Figure 2.
Fluoroscopic images showing a mid-septal position of the ablation catheter (arrows). Also shown are catheters in the right ventricle (RV) and in the His-bundle position.
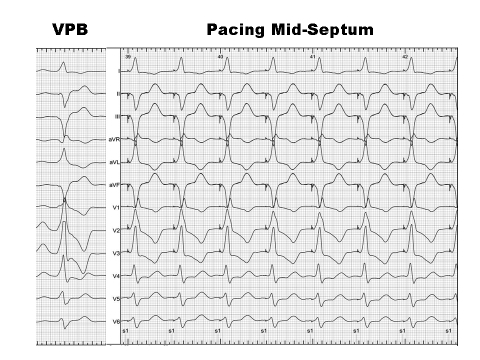
Figure 3.
Confirming the site of origin by pace mapping. Pacing at the mid-septal region demonstrated a good match between the morphology of QRS complexes during pacing and the QRS morphology of the VPBs. Of note, the QRS complexes of VPB showed more initial slurring which may suggest a midmyocardial focus.
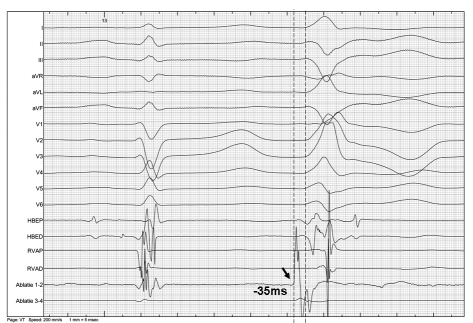
Figure 4.
12-lead electrocardiogram and simultaneous intracardiac electrograms (from His, right ventricle and ablation catheter) recorded during sinus rhythm and a spontaneous VPB. The mapping catheter is positioned in the mid-septal region. During the VPB, the earliest activation (arrow) recorded at the ablation catheter preceded the onset of the QRS complex by 35 ms. Radiofrequency energy was applied in this region which resulted in elimination of VPBs.
One day after the ablation procedure the echocardiogram was repeated to allow more accurate echocardiographic measurements without interference from frequent VPBs. Left ventricular end-diastolic diameter was 56 mm and the ejection fraction was 40%. Two months later she was seen at the outpatient clinic and she reported resolution of her palpitations. The 24-hour Holter recording showed a decrease of the VPB count to six per day. The echocardiogram showed a slight improvement in left ventricular ejection fraction to 45%. An echocardiogram was repeated six months after the ablation. The dimensions of the left ventricle had now decreased to 49 mm and her EF increased to 59%. She had no further complaints of palpitations but mild fatigue persisted. Since palpitations were no longer present and ejection fraction improved, it was decided to discontinue drug treatment.
Case 2
A 45-year-male was seen with complaints of palpitations and mild tiredness. The symptoms had been present for a few years, but the palpitation severity had increased recently. There were no anginal complaints or symptoms of heart failure. There was no familial history of cardiac disease. Physical examination showed a well-controlled blood pressure of 125/80 mmHg. Further cardiorespiratory examination was unremarkable. The 12-lead ECG showed sinus rhythm and frequent VPBs with an inferior axis and left bundle branch block morphology. R/S transition in the precordial leads occurred in lead V3, suggestive of a right ventricular outflow tract origin. An echocardiogram showed a mildly reduced ejection fraction of 40% and an enlarged left end-diastolic diameter of 59 mm. A 24-hour 12-lead Holter recording showed 6844 monomorphic VPBs (7% of all QRS complexes). No (non)-sustained VTs were observed. An exercise test showed a good exercise tolerance. The frequent VPBs disappeared during maximal exercise. A cardiac CT scan showed normal coronary arteries. Cardiac MRI demonstrated no structural abnormalities, especially no overt signs of arrhythmogenic right ventricular dysplasia. Treatment with metoprolol was initiated. During a six-month follow-up, the palpitations got worse, frequent VPBs were still present and LV function did not change. The patient was referred for radiofrequency catheter ablation of the symptomatic VPBs. Clinical ectopy was present at the start of the procedure. A 4 mm tip radiofrequency ablation catheter was used for mapping and ablation. The earliest endocardial activation (25 ms before onset of QRS complex) of the ectopic beat was seen in the septal part of the right ventricular outflow tract. Pace mapping in this region resulted in a 12 out of 12 match of the QRS morphology of paced and spontaneous VPBs complexes. After two applications of radiofrequency energy in this region, no further ectopics were observed in the electrophysiology laboratory.
The patient was seen in the outpatient department one and five months later. The symptoms of palpitations were no longer present and a 24-hour Holter recording showed only three VPBs. In addition, his LV systolic function had gradually increased to 45 and 54%, after the one and five month follow-up, respectively.
Discussion
Ventricular premature beat induced cardiomyopathy
Rhythm disorders such as (incessant) tachyarrhythmias or dissynchronous ventricular activation, i.e. left bundle branch block or right ventricular apex pacing, are well-known causes of dilated cardiomyopathy.14-17 After elimination of the arrhythmia or correction of the ventricular activation sequence, left ventricular dysfunction is often completely reversible.14-17 Recently it was demonstrated that frequent VPBs can cause dilated cardiomyopathy in a subgroup of patients. A causal relation was suggested since after elimination of the VPBs, left ventricular contractile function markedly improved (table 1).4-13
Table 1.
Case reports and case series demonstrating improvement of left ventricular function after successful ablation of ventricular premature beats.
| Author | No. of patients | Age | No. of VPBs | Origin VPBs | EF (%)Before | EF (%)After |
|---|---|---|---|---|---|---|
| Chugh et al.5 | 1 | 26 | 25,000-60,000/d | RVOT | 43 | 58 |
| Satisch et al.8 | 1 | 35 | 1700/h | LV | 40 | 65 |
| Shiraishi et al.10 | 1 | 53 | 50,000/d | RVOT | 25 | Normal |
| Shanmugan et al.9 | 1 | 46 | 3674/d | RVOT | 40 | 51 |
| Yarlagadda et al.13 | 8 | 58±14 | 17,859±13,488/d | RVOT | 39±6% | 62±6% |
| Taieb et al.12 | 6 | 46±18 | 8000-28,000/d | S of V, RVOT, LV | 42±3 | 57±3 |
| Redfearn et al.7 | 1 | 33 | 31,000 | RVOT | 39 | 53 |
| Bogun et al.4 | 22 | 42±12 | 37±13% of QRS | RVOT, LVOT, other | 37±13 | 59±7 |
| Sternick et al.11 | 1 | 55 | 26% of QRS | Posterior Papillary muscle | 38 | 58 |
| Ezzat et al.6 | 1 | 44 | 33,000/d | RVOT | 40 | 60 |
RVOT=right ventricular outflow tract, LV=left ventricle, S of V=sinus of Valsalva, LVOT=left ventricular outflow tract, EF=ejection fraction.
The studies presented so far were case reports or case series, hence the number of patients is only small.4-13 Duffee et al. were the first to describe four patients with presumed idiopathic dilated cardiomyopathy who had an improvement of left ventricular function after elimination of frequent ventricular premature beats by amiodarone treatment.8 In subsequent studies the beneficial effect of radiofrequency catheter ablation was described.4-13 The largest case series was recently published by Bogun et al. who studied a group of 22 patients with dilated cardiomyopathy who were referred for radiofrequency catheter ablation of symptomatic VPBs.4 Additional investigations, including coronary angiogram and/or cardiac MRI, were performed to rule out ischaemic or structural heart disease. The mean number of VPBs was 21% of all QRS complexes on 24-hour Holter recording. Radiofrequency ablation successfully eliminated VPBs in 80% of patients. During a follow-up period of six months, left ventricular ejection fraction normalised in 80% of these patients from 35±13 to 59±7%. Interestingly, they also retrospectively analysed a historical control group with a similar VPB burden and dilated cardiomyopathy. In these ten patients, no ablation was performed. The ejection fraction did not change during follow-up. In addition, the patients with failed ablation also showed no improvement in ejection fraction.4
Although many patients with idiopathic dilated cardiomyopathy have frequent VPBs, it is unclear in what percentage of patients the presence of VPBs initself may be the cause of or contributing factor to left ventricular dysfunction. Vice versa, the prevalence of cardiomyopathy in patients with frequent VPBs was recently studied by Kanei et al. They retrospectively analysed all Holter recordings conducted over a period of five years.19 All patients with identifiable causes of LV dysfunction were excluded. In total, 108 patients were identified with >10 VPBs/hour. Of note, only patients with right ventricular outflow tract VPBs were included. Twenty-four patients had <1000 VPBs/24 hours, 55 patients had 1000 to 10,000 VPBs/24 hours, and 29 patients had ≥10,000 VPBs/24 hours. The prevalence of LV dysfunction was 4, 12, and 34%, respectively. Although these data do not prove causality, they do indicate that in patients with frequent VPBs, left ventricular dysfunction may be fairly common.
Recently Niwano et al. evaluated the prognostic significance of frequent outflow tract VPBs (>1000/day) in a group of patients with no structural heart disease.20 During a 5.6-year observation period no patients experienced serious cardiac events. However in 7% of patients there was a slight decrease (>6%) in ejection fraction. For the prediction of the development of LV dysfunction, VPB prevalence and LVEF at the initial evaluation were independent predicting factors.
The pathophysiological mechanisms underlying VPB-induced cardiomyopathy are unknown.
A VPB results in acute mechanical inefficiency due to inter- and intra-ventricular dissynchronous contraction. In addition, following a VPB, a long compensatory filling phase may result in volume overload of the ventricles. Chronically, this may lead to remodelling of ventricles due to neurohumoral activation and, for example, downregulation of contractile proteins. Indeed, the observation of a reduced LV function early after ablation in our patients suggests true tissue remodelling, rather than a reflection of an acute effect on LV filling pressure or function. Interestingly, the slow time course of recovery (months) of LV function and LV size observed in our two patients is strikingly similar to the response seen in responders to resynchronisation therapy.14,15 Whether this may be the result of the same pathophysiological mechanism remains to be investigated.
On the other hand, most patients with frequent VPBs have normal systolic function,19 suggesting that individual susceptibility and genetic factors are also involved.
Selection of eligible ablation candidates
Recent reports have shown that radiofrequency catheter ablation is a safe and effective treatment strategy for patients with highly symptomatic drug refractory monomorphic VPBs.21,22 In patients with dilated cardiomyopathy and frequent VPBs, ablation therapy may alleviate palpitations and in addition may improve systolic function and reduce clinical symptoms. At present, however, it is incompletely understood which patients with LV dysfunction and frequent VPBs may benefit from radiofrequency ablation therapy. The characteristics of the patients described in the few cases published up till now can be summarised as follows: Patients were relatively young (30 to 50 years), the ejection fraction was mildly reduced (30 to 50%), and Holter recordings showed frequent (>10000) monomorphic VPBs/day (table 1).4-13 The site of origin of the VPB is usually the right ventricular outflow tract, showing the characteristic inferior axis pattern with left bundle branch block morphology. However, other sites such as the left ventricular outflow tract, pulmonary artery, aortic cusps and posteroseptal LV have also been described.4,5,12,13
Conclusion
In the present case report we describe two patients with a dilated cardiomyopathy with symptoms of palpitations due to frequent VPBs. The frequent VPBs were successfully eliminated by radiofrequency catheter ablation, which resulted in alleviation of symptoms. In addition, left ventricular function normalised, demonstrating a causal relation between the two.
The present cases and other recent reports underscore the routine use of 24-hour Holter recordings in patients with presumed idiopathic cardiomyopathy. Detection of frequent monomorphic VPBs may identify a potential reversible form of cardiomyopathy. Vice versa, VPBs are not always benign and the diagnostic evaluation of patients with palpitations and frequent VPBs should at least include an echocardiogram.
Finally, it is important to stress that radiofrequency ablation is not the treatment of choice for the vast majority of patients with frequent VPBs. However, in patients with drug refractory symptomatic frequent monomorphic VPBs and ‘idiopathic’ left ventricular dysfunction, referral for electrophysiological examination should be strongly considered since radiofrequency catheter ablation may offer a potential curative treatment.
References
- 1.Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193-7. [DOI] [PubMed] [Google Scholar]
- 2.Podrid PJ, Fogel RI, Fuchs TT. Ventricular arrhythmia in congestive heart failure. Am J Cardiol. 1992;69:82G-95G; discussion 95G-96G. [DOI] [PubMed] [Google Scholar]
- 3.Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, Brusin MC, et al. Long-term follow-up of right ventricular monomorphic extrasystoles. J Am Coll Cardiol. 2001;38:364-70. [DOI] [PubMed] [Google Scholar]
- 4.Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863-7. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328-9. [DOI] [PubMed] [Google Scholar]
- 6.Ezzat VA, Liew R, Ward DE. Catheter ablation of premature ventricular contraction-induced cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:289-93. [DOI] [PubMed] [Google Scholar]
- 7.Redfearn DP, Hill JD, Keal R, Toff WD, Stafford PJ. Left ventricular dysfunction resulting from frequent unifocal ventricular ectopics with resolution following radiofrequency ablation. Europace. 2003;5:247-50. [DOI] [PubMed] [Google Scholar]
- 8.Satish OS, Yeh KH, Wen MS, Wang CC. Premature ventricular contraction-induced concealed mechanical bradycardia and dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2005;16:88-91. [DOI] [PubMed] [Google Scholar]
- 9.Shanmugam N, Chua TP, Ward D. 'Frequent' ventricular bigeminy--a reversible cause of dilated cardiomyopathy. How frequent is 'frequent'? Eur J Heart Fail. 2006;8:869-73. [DOI] [PubMed] [Google Scholar]
- 10.Shiraishi H, Ishibashi K, Urao N, Tsukamoto M, Hyogo M, Keira N, et al. A case of cardiomyopathy induced by premature ventricular complexes. Circ J. 2002;66:1065-7. [DOI] [PubMed] [Google Scholar]
- 11.Sternick EB, Correa F, Negri R, Scarpelli RB, Gerken LM. Reversible cardiomyopathy provoked by focal ventricular arrhythmia originating from the base of the posterior papillary muscle. J Interv Card Electrophysiol. 2009;25:67-72. [DOI] [PubMed] [Google Scholar]
- 12.Taieb JM, Maury P, Shah D, Duparc A, Galinier M, Delay M, et al. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J Interv Card Electrophysiol. 2007;20:9-13. [DOI] [PubMed] [Google Scholar]
- 13.Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092-7. [DOI] [PubMed] [Google Scholar]
- 14.Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Mortensen PT, Vesterlund T, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-6. [DOI] [PubMed] [Google Scholar]
- 15.Littmann L, Symanski JD. Hemodynamic implications of left bundle branch block. J Electrocardiol. 2000;33(Suppl):115-21. [DOI] [PubMed] [Google Scholar]
- 16.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709-15. [DOI] [PubMed] [Google Scholar]
- 17.Vijgen J, Hill P, Biblo LA, Carlson MD. Tachycardia-induced cardiomyopathy secondary to right ventricular outflow tract ventricular tachycardia: improvement of left ventricular systolic function after radiofrequency catheter ablation of the arrhythmia. J Cardiovasc Electrophysiol. 1997;8:445-50. [DOI] [PubMed] [Google Scholar]
- 18.Duffee DF, Shen WK, Smith HC. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin Proc. 1998;73:430-3. [DOI] [PubMed] [Google Scholar]
- 19.Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2008;13:81-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230-7. [DOI] [PubMed] [Google Scholar]
- 21.Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259-65. [DOI] [PubMed] [Google Scholar]
- 22.Zhu DW, Maloney JD, Simmons TW, Nitta J, Fitzgerald DM, Trohman RG, et al. Radiofrequency catheter ablation for management of symptomatic ventricular ectopic activity. J Am Coll Cardiol. 1995;26:843-9. [DOI] [PubMed] [Google Scholar]