Abstract
Introduction
Sudden arrhythmogenic cardiac death is a major cause of mortality in patients with congestive heart failure due to adverse electrical remodelling. To establish whether abnormal conduction is responsible for arrhythmogenic remodelling in progressed stages of heart failure, we have monitored functional, structural and electrical remodelling in a murine model of heart failure, induced by longstanding pressure overload.
Methods
Mice were subjected to transverse aortic constriction (TAC; n=18) or sham operated (n=19) and monitored biweekly by echocardiography and electrocardiography. At the 16-week endpoint, electrical mapping was performed to measure epicardial conduction velocity and susceptibility to arrhythmias. Finally, tissue sections were stained for Cx43 and fibrosis.
Results
In TAC mice, fractional shortening decreased gradually and was significantly lower compared with sham at 16 weeks. Left ventricular hypertrophy was significant after six weeks. TAC mice developed PQ prolongation after 12 weeks, QT prolongation after 16 weeks and QRS prolongation after two weeks. Right ventricular conduction velocity was slowed parallel to fibre orientation. In 8/18 TAC hearts, polymorphic ventricular tachyarrhythmias were provoked and none in sham hearts. TAC mice had more interstitial fibrosis than sham. Immunohistology showed that Cx43 levels were similar but highly heterogeneous in TAC mice. All parameters were comparable in TAC mice with and without arrhythmias, except for Cx43 heterogeneity, which was significantly higher in arrhythmogenic TAC mice.
Conclusion.
Chronic pressure overload resulted in rapid structural and electrical remodelling. Arrhythmias were related to heterogeneous expression of Cx43. This may lead to functional block and unstable reentry, giving rise to polymorphic ventricular tachyarrhythmias. (Neth Heart J 2010;18:509–15.)
Keywords: Heart Failure, Arrhythmias, Conduction
Heart failure (HF) is a major burden of disease in industrialised countries with an associated lifetime risk of about 20% in both men and women.1 By definition, heart failure is a functional disease in which cardiac output is unable to meet the demands of the organs. In spite of this, a significant part of mortality in HF patients is caused by sudden cardiac death (SCD), predominantly the outcome of ventricular arrhythmias.1 Nevertheless, mechanisms underlying the evolution of increased vulnerability to arrhythmias during the development of heart failure are not fully comprehended.
The propensity for cardiac arrhythmias is thought to be determined by three factors: 1) presence of an arrhythmogenic substrate, 2) occurrence of initiating triggers, and 3) modulating factors, such as the autonomic nervous system or drugs that can either ameliorate or deteriorate both substrate and triggers.2,3 In the early compensated stages of HF (NYHA class I and II), triggered arrhythmias due to repolarisation abnormalities are the main cause of SCD. However, in the later decompensated stages of the disease, a wide QRS complex (>120 ms) has been reported to be a predictor of mortality in CHF patients.4,5 A wide QRS complex may be caused by slow impulse conduction, which is an important parameter for reentrant arrhythmias. In turn, conduction slowing may be due to several factors, which include reduced electrical coupling in the ventricular myocardium due to reduced expression of the main gap junction protein Connexin43 (Cx43), reduced excitability mediated by the sodium channel and enhanced collagen deposition (fibrosis).3
In this study, we have biweekly followed functional, structural and electrical remodelling in a murine model of heart failure, which was induced by longstanding pressure overload up to 16 weeks through transverse aortic constriction (TAC). The aim was to establish whether abnormal conduction is responsible for arrhythmogenic remodelling in progressed stages of heart failure.
Results
Functional remodelling
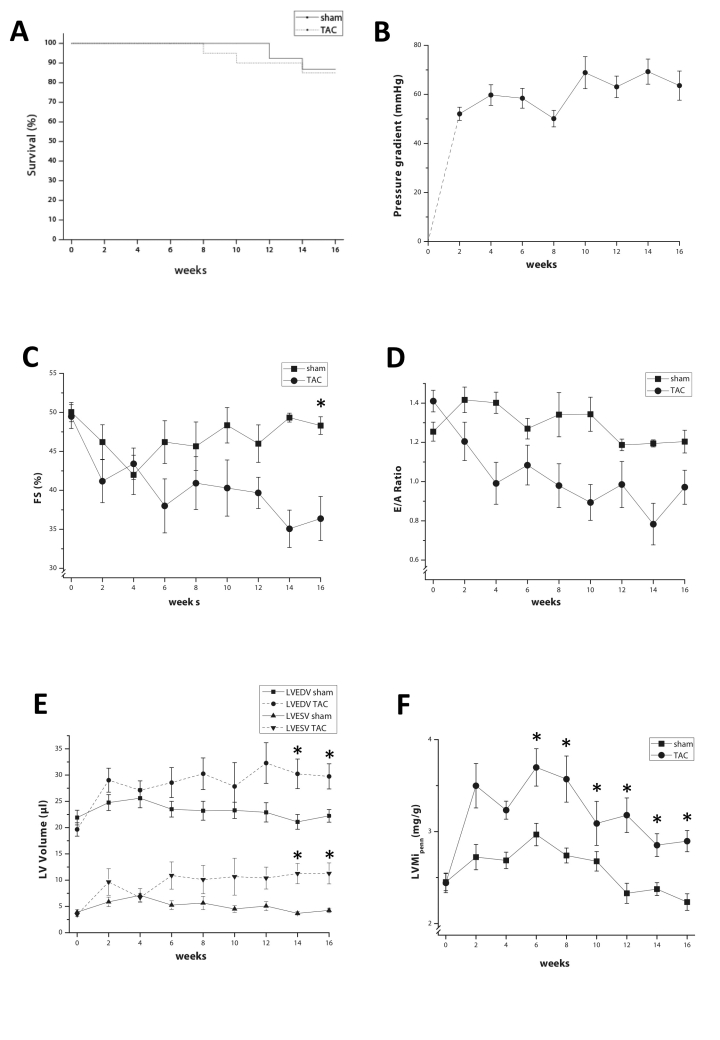
Mortality in aorta constricted (TAC) mice was slightly higher compared with mice with control operation (sham), but was not significantly different, as shown by the Kaplan-Maier curve (figure 1A). Immediately after the operation, pressure gradient across the constriction was 52.8±3.2 mmHg and increased further to reach 61.4±7.5 mmHg at four months (figure 1B).
Figure 1.
A) Kaplan-Maier plot of 16-week survival in TAC and sham mice. B) Development of the pressure gradient across the aortic constriction as determined by Doppler echo. C) Fractional shortening (%) during the 16-week follow-up period in TAC and sham mice. D) Evolution of E/A ratio over time. E) Left ventricular end-diastolic volumes (LVEDV) and end-systolic volumes (LVESV) in TAC and sham mice. F) Left ventricular mass index in TAC and sham mice as calculated from B-mode echocardiography. Asterisks indicate p≤0.05.
Two weeks after operation, left ventricular (LV) systolic function (measured as relative fractional shortening) decreased in both groups, but recovered in sham and remained stable until 16 weeks (figure 1C). However, in TAC, left ventricular systolic function deteriorated persistently and was significantly decreased (fractional shortening 38.5±2.6% in TAC vs. 47.2±1.5% in sham; p<0.05). Diastolic dysfunction (E to A ratio) developed rapidly and remained stable after four weeks, albeit not significantly different from sham mice (figure 1D).
Already after two weeks, LV end-systolic and end-diastolic volumes were increased in TAC mice, which were significantly higher compared with sham at 14 and 16 weeks (figure 1E). The increase in LV mass index (LVMi = LV mass / body weight), showed a similar pattern and was significantly higher compared with sham (figure 1F).
The echocardiographic parameters indicated that TAC hearts became hypertrophic and dilated, which was confirmed at sacrifice (16 weeks), at which both heart weight and heart weight to body weight ratio were markedly increased in TAC by ∽35%. In TAC, mean heart weight was 168.6±0.009 vs. 124.8±0.004 mg in sham (p<0.001), while heart weight to body weight ratio measured 7.1±0.4 vs. 5.2±0.1 mg/g (p<0.001).
Electrical remodelling
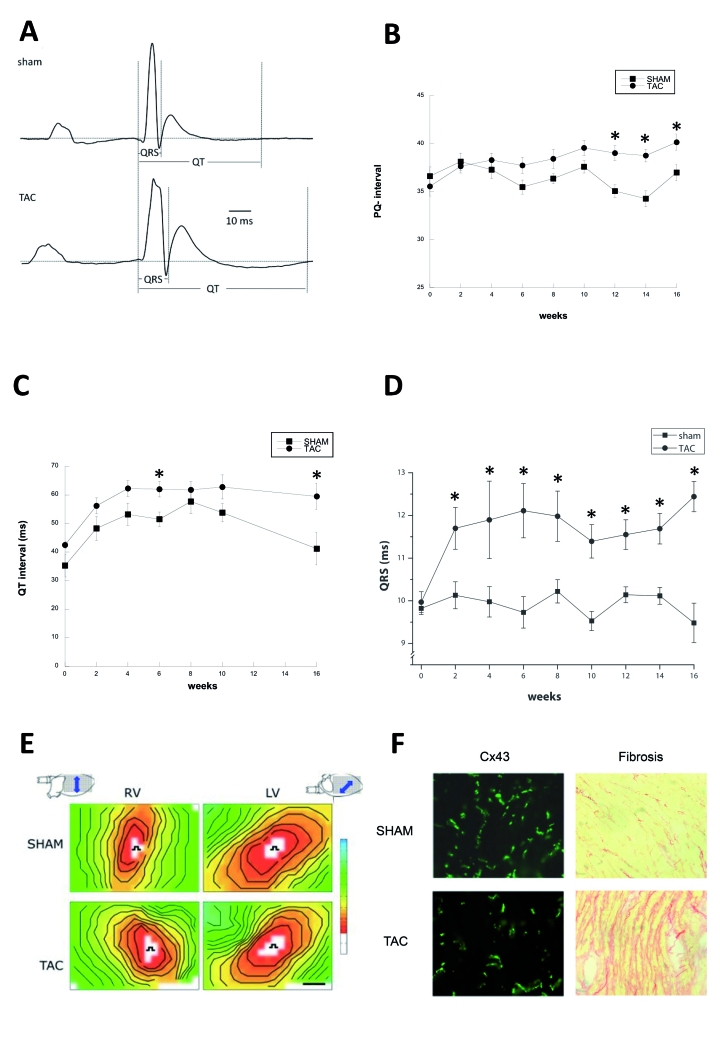
The electrocardiographic recordings of TAC and sham mice showed specific differences at 16 weeks (figure 2A). P-wave duration was unchanged, but PQ (40.1±0.85 vs. 37.0±0.86 ms, p<0.05), QRS duration (12.4±0.36 vs. 9.5±0.46 ms, p<0.05), and the QT interval (59.5±6.6 vs. 41.2±5.7 ms, p<0.05) were significantly higher in TAC compared with sham. The evolution of these changes is depicted in figures 2B to D. Evolution of PQ interval showed a divergent pattern between TAC and sham, reaching significance as from 12 weeks. Interestingly, already at two weeks, the QT interval was increased in TAC compared with sham and remained stable over time. More striking, QRS duration was significantly prolonged already at week 2 in TAC.
Figure 2.
Typical ECG traces of TAC (lower tracing) and sham (upper tracing) operated mice at 16 weeks. B) Evolution of PQ interval during 16-week follow-up in TAC and sham mice. C) The QT interval over time D. The evolution of the QRS duration over time. E) Typical examples of epicardial activation maps showing conduction slowing in TAC, most prominently in RV. F) Immunohistochemical staining of Cx43 and picro-sirius red staining of collagen. Asterisks indicate p≤0.05.
Mapping of electrical activity
At the 16-week endpoint, the animals were sacrificed and the hearts were quickly excorporated and Langendorff perfused. Mapping of epicardial electrical activity was performed with a 13x19 unipolar electrode grid. The hearts were stimulated from the centre of the grid at a basic cycle length of 150 ms. Unipolar electrograms were converted to activation maps to determine epicardial conduction velocities (figure 2E). Left ventricular conduction velocities longitudinal (CVL) and transversal (CVT) to the fibre orientation (indicated by the orientation of the arrow in the heart pictograms of figure 2E) were not different between TAC and sham (in TAC vs. sham, respectively: CVL: 41.3±2.2 vs. 46.6±3.2 cm/s; CVT: 22.9±1.2 vs. 23.4±1.3 cm/s). In the right ventricle (RV), CVL was significantly slowed (TAC vs. sham, respectively: 39.0±1.8 vs. 50.9±2.7; p<0.05). CVT was, however, unchanged in RV (TAC vs. sham, respectively: 25.9±1.9 vs. 28.0±2.2 cm/s). Using stimulus trains of 16 basic cycles of 150 ms, followed by one premature stimulus with decrementing coupling intervals, the effective refractory period (ERP) was determined. In TAC, ERP was longer in both LV (78.7±6.0 vs. 63.1±8.0 ms) and RV (56.7±5.8 vs. 46.0±3.5 ms), albeit non-significantly. The vulnerability to arrhythmias was tested by progressively more aggressive stimulation with one or three premature stimuli or by burst pacing. In 8/18 (44%) TAC hearts, polymorphic ventricular tachyarrhythmias were provoked and none in sham hearts (0/19; Χ2: p<0.05).
After the electrophysiological procedure, hearts were frozen, sectioned, and examined for the expression of the gap junction protein Cx43 by immunohistochemistry and for the amount of collagen by picro-sirius red staining. The total amount of Cx43 was not different between RV (LV) of TAC and sham [1.4±0.2(1.9±0.5) vs. 2.1±0.2(2.6±0.4) %, respectively], but Cx43 was clearly more heterogeneously expressed (Figure 2F, left panels). Note that in TAC mice areas with close to normal Cx43 expression neighbour areas virtually deprived of Cx43 proteins. In TAC, the amount of interstitial fibrosis was significantly higher in both ventricles (TAC vs. sham, respectively: LV: 15±2 vs. 9.3±1%; RV: 13±2 vs. 8.5±1%, p<0.05).
Discussion
Long standing pressure overload in the mouse heart resulted in progression towards decompensated cardiac hypertrophy with high susceptibility to induction of ventricular arrhythmias.
The initial response after aortic constriction was cardiac hypertrophy. Already at the first observation two weeks after constriction, heart weight - body weight ratio increased significantly by one third. This response might have occurred even faster, as significant left ventricular hypertrophy was already observed after seven days in another study.6 Cardiac systolic function was well compensated for the first four weeks after TAC, but gradually deteriorated thereafter, which is comparable with other studies in which decompensation also occurred after four to five weeks.7-9 Systolic function was lower in TAC mice with arrhythmias (TAC+), compared with TAC mice without arrhythmias (TAC-), albeit not significant (35.0±2.7% vs. 42.2±3.6% in TAC- and TAC+, respectively; p=0.14). Interestingly, lung weight was significantly higher in TAC+, suggesting that the amount of backward failure may play a proarrhythmic role (0.261±0.043g vs. 0.178±0.005g in TAC- and TAC+, respectively).
Strikingly, the time course of electrical remodelling better paralleled hypertrophic response, i.e. structural remodelling, rather than functional remodelling. Both QT and QRS duration were increased after two weeks and remained different thereafter. Increase in QT duration is known to occur rapidly in TAC mice: already after seven days of TAC, a significant QT prolongation was observed, which correlated to lower potassium current densities.6 Parenthetically, QT times were not different between TAC mice with and without arrhythmias.
Generally, a broadened QRS complex indicates delayed activation of the ventricles, which may be due to either slowed impulse conduction or increased path length (due to enhanced heart size), or both.10 At two weeks, heart weight was increased and remained higher in TAC compared with sham, which suggested increased path length. In conjunction, we have established that conduction velocity was slowed after 16 weeks.
Conduction slowing is regarded arrhythmogenic, as it reduces wave length (= conduction velocity times ERP) of a reentrant circuit in the leading circle concept.11 As increased QRS duration may reflect slowed conduction, it is expected that QRS duration would be further increased in TAC mice with arrhythmias. However, QRS duration was not different between TAC+ and TAC- mice. Likewise, there was no difference in conduction velocity, nor ERP, nor wave length between TAC+ and TAC- mice.
In TAC mice, increased levels of collagen (fibrosis) and a heterogeneous expression of Cx43 were found. Fibrosis is associated with conduction slowing, especially in the transversal conduction.12 This was not observed in this mouse model. Longitudinal conduction slowing is typically associated with depressed sodium current (for a review see Kleber et al.)3 In this study, RNA levels of the sodium current defining protein Nav1.5 were not different between TAC and sham (data not shown), supporting the notion that alterations in the sodium current do not play a pivotal role in this model.
Heterogeneous expression of Cx43, however, has been shown to be associated with enhanced susceptibility to arrhythmias in patients,13 dogs14,15 and mice.16 Spatial heterogeneity in Cx43 expression in our study was quantified (as described before)17 and showed a significantly higher heterogeneous Cx43 expression in TAC operated compared with sham-operated mice. Areas almost deprived of Cx43 protein neighboured areas with normal Cx43 expression. Most interestingly, heterogeneity in Cx43 expression was significantly higher in TAC+ compared with TAC- mice. The areas with low levels of Cx43 are presumably prone to functional block during VTs, when the safety factor of impulse conduction is challenged. Multiple or variable locations of functional block may lead to unstable and meandering reentrant circuits, producing polymorphic VTs as observed in TAC mice. Increased collagen expression may in turn amplify the effect of conduction slowing in areas deprived of Cx43.
Conclusion
Chronic pressure overload led to rapid development of hypertrophy with a gradual depression of systolic function. Electrical remodelling occurred early and led to QT and QRS prolongation. After 16 weeks, 44% of the TAC mice were arrhythmogenic. Arrhythmogeneity was not associated with QRS prolongation, nor with a decreased macroscopic conduction velocity and cardiac dysfunction. Also, fibrosis and absolute lower levels of Cx43 expression did not appear to be discriminative. However, spatial heterogeneous Cx43 expression was more prominent in TAC mice with arrhythmias. Heterogeneous Cx43 expression may lead to functional block and unstable reentry, giving rise to polymorphic ventricular tachyarrhythmias.
Acknowledgements
This work was supported by the Netherlands Heart Foundation (Grant number M96.001).
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25-146. [DOI] [PubMed] [Google Scholar]
- 2.Coumel P. The management of clinical arrhythmias. An overview on invasive versus non-invasive electrophysiology. Eur Heart J. 1987;8:92-9. [DOI] [PubMed] [Google Scholar]
- 3.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431-88. [DOI] [PubMed] [Google Scholar]
- 4.Anastasiou-Nana MI, Nanas JN, Karagounis LA, Tsagalou EP, Alexopoulos GE, Toumanidis S, et al. Relation of dispersion of qrs and qt in patients with advanced congestive heart failure to cardiac and sudden death mortality. Am J Cardiol. 2000;85:1212-7. [DOI] [PubMed] [Google Scholar]
- 5.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. Qrs duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085-91. [DOI] [PubMed] [Google Scholar]
- 6.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing k+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao Y, Ishikura F, Beppu S, Asakura M, Takashima S, Asanuma H, et al. Echocardiographic assessment of lv hypertrophy and function in aortic-banded mice: Necropsy validation. Am J Physiol Heart Circ Physiol. 2002;282:H1703-1708. [DOI] [PubMed] [Google Scholar]
- 8.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129s1/svimj and c57bl/6j mice: Temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2007;292:H2119-2130. [DOI] [PubMed] [Google Scholar]
- 9.Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, et al. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied Heart. Circ Res. 2009;104:365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegerinck RF, Verkerk AO, Belterman CN, van Veen TA, Baartscheer A, Opthof T, et al. Larger cell size in rabbits with heart failure increases myocardial conduction velocity and qrs duration. Circulation. 2006;113:806-13. [DOI] [PubMed] [Google Scholar]
- 11.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. Iii. The "Leading circle" Concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977;41:9-18. [DOI] [PubMed] [Google Scholar]
- 12.van Veen TA, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, et al. Impaired impulse propagation in scn5a-knockout mice. Combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation. 2005;112:1927-35. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, et al. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865-70. [DOI] [PubMed] [Google Scholar]
- 14.Cabo C, Yao JA, Boyden PA, Chen S, Hussain W, Duffy HS, et al. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res. 2006;72:241-9. [DOI] [PubMed] [Google Scholar]
- 15.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988-96. [DOI] [PubMed] [Google Scholar]
- 16.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, et al. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194-9. [DOI] [PubMed] [Google Scholar]
- 17.Boulaksil M, Winckels SK, Engelen MA, Stein M, van Veen TA, Jansen JA, et al. Heterogeneous connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12:913-21. [DOI] [PubMed] [Google Scholar]