Abstract
Opioids are the most effective analgesics for pain management, and efficient pain control is a therapeutic priority. Herein, we describe the synthesis and pharmacological activities of the 5-benzyl analogue of the μ opioid analgesic 14-methoxymetopon (14-MM). The result of the replacement of the 5-methyl in 14-MM with a benzyl group on in vitro opioid receptor binding and functional profiles, and in vivo behavioural properties, i.e. nociception and motor activity, was investigated. In rodent brain membranes, the 5-benzyl derivative showed high affinity at the μ opioid receptor and decreased interaction with δ and κ receptors, hence displaying a similar binding profile as 14-MM. It displayed potent agonist activity in vitro and in vivo. In in vitro guanosine-5′-O-(3-[35S]thio)-triphosphate ([35S]GTPγS) binding assay, it activated G-proteins in rat brain membranes through a μ opioid receptor-mediated mechanism having significantly enhanced potency compared to DAMGO (d-Ala2,Me-Phe4,Gly-ol5]enkephalin), and to the μ opioid agonist morphinans 14-MM, 14-O-methyloxymorphone (14-OMO) and morphine. In vivo, the 5-benzyl analogue of 14-MM elicited dose-dependent and naloxone-sensitive antinociceptive effects in hot-plate and tail-flick tests in mice after subcutaneous (s.c.) administration. Its analgesic potency was comparable to 14-MM, and was 50-fold higher than that of morphine. Contrary to morphine, 14-MM and 14-OMO, no motor dysfunction was produced by the new opioid in the mouse rotarod test at any of the tested doses. In summary, the 5-benzyl analogue of 14-MM emerged as a novel potent μ opioid antinociceptive agent with reduced propensity to cause unwanted motor impairment.
Keywords: μ Opioid receptor, Opioid, Agonist, 14-Methoxymetopon, Antinociception, Pain, Motor impairment
1. Introduction
Pain constitutes a major public-health problem with an enormous impact on both the individual and the society. Debilitating pain and particularly chronic pain is a constant backdrop to daily life, resulting in personal suffering, reduced work productivity and substantial healthcare costs (Breivik et al., 2006; Marcus et al., 2009). Being a disabling symptom of many medical conditions, effective pain control is one of the most important therapeutic priorities. Among analgesic drugs, opioids are the mainstay for the management of moderate to severe pain (Inturrisi, 2002; Jain, 2004; Benyamin et al., 2008). The opioid class of analgesics includes several clinically used pain relieving agents such as naturally occurring alkaloids (e.g. morphine, codeine), semisynthetic derivatives (e.g. oxycodone, oxymorphone, buprenorphine), and synthetic opioids (e.g. fentanyl, pethidine, tramadol). All these opioid drugs as well as endogenous opioid peptides produce their biological actions by interacting with three receptor types, μ, δ and κ, belonging to the family of G-protein-coupled receptors (GPCRs) (Knapp et al., 1995; Kieffer and Evans, 2009).
Morphine and other opioids produce analgesia primarily through their agonist action at the μ opioid receptor, which is the main type targeted for the pharmacotherapy of pain. However, currently used opioid analgesics also share a number of severe side effects (e.g. respiratory depression, nausea, sedation, dizziness, motor disturbances and constipation), making their clinical usefulness problematic and leading to early discontinuation, under-dosing and inadequate analgesia (Inturrisi, 2002; Jain, 2004; Benyamin et al., 2008). Increasing drug deaths from opioids is a serious issue (Benyamin et al., 2008). Considerable effort has therefore been put forward to understand the appropriate use of opioid analgesics, while medicinal chemistry and opioid pharmacology have been continuously engaged in the search for safer, more efficacious and non-addicting opioid compounds, with the final goal to reduce complications and to improve patient compliance.
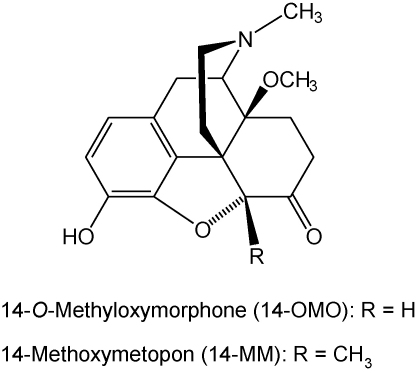
Our research in the morphinan class of opioid analgesics has led us to obtain a number of 14-alkoxymorphinan-6-ones that act as effective antinociceptive agents in various animal models of pain (Schmidhammer et al., 1984, 1990; Fürst et al., 1993, 2005; Greiner et al., 2003; Schütz et al., 2003; Spetea et al., 2004a,b, 2005; Lattanzi et al., 2005; Al-Khrasani et al., 2007; Obara et al., 2007). Previous pharmacological reports described a derivative of oxymorphone, 14-O-methyloxymorphone (14-OMO, Fig. 1), which possesses about 40-fold higher antinociceptive potency than its parent compound, and is up to 400-fold more potent than the ‘gold standard’ for pain therapy, morphine, in rodent models of acute nociception, i.e. hot-plate test (Schmidhammer et al., 1984) and tail-flick test (Fürst et al., 2005; Riba et al., 2010), after subcutaneous (s.c.) administration. However, 14-OMO induces the typical opioid side effects of the conventional μ opioid analgesics including constipation, respiratory depression and physical dependence (Schmidhammer et al., 1984; Lattanzi et al., 2005).
Fig. 1.
Structures of 14-OMO and 14-MM.
Further synthetic and pharmacological work based on the introduction of a methyl group at position 5 of 14-OMO gave rise to 14-methoxymetopon (14-MM, Fig. 1) (Schmidhammer et al., 1990), a potent and high affinity μ opioid receptor agonist in vitro (Fürst et al., 1993; Spetea et al., 2003, 2004a; Mahurter et al., 2006). In vivo, 14-MM retains the high antinociceptive potency of the parent molecule and is considerably more active than morphine in producing analgesia in diverse thermal, chemical and inflammatory pain models, after different routes of administration (s.c., intraperitoneal, intracebroventricular and intrathecal) and in different species (rats and mice) (Schmidhammer et al., 1990; Fürst et al., 1993; Zernig et al., 2000; King et al., 2003; Urigüen et al., 2002; Greiner et al., 2003; Lattanzi et al., 2005; Bileviciute-Ljungar et al., 2006; Riba et al., 2010). It was equipotent to sufentanil in eliciting analgesia in canines after intravenous administration (Freye et al., 2000). Behaviour studies showed that 14-MM causes less pronounced opioid adverse actions in terms of respiratory depression, hypotension, bradycardia, constipation, physical dependence, addiction potential and tolerance (Fürst et al., 1993; Freye et al., 2000; King et al., 2003; Király et al., 2006).
In view of these findings, we aimed in this study to investigate the effect of the replacement of the 5-methyl group in 14-MM with a benzyl substituent (compound 1, Fig. 2) on the pharmacological profile represented by in vitro opioid receptor binding and functional activities, and in vivo behavioural properties, i.e. nociception and motor function, after s.c. administration to mice. Consequently, we have conducted a comparison of the pharmacological activities and physicochemical properties of compound 1 to those of structurally related opioid morphinans, 14-MM and 14-OMO, and of the clinically relevant morphine. Structure–activity relationship (SAR) studies in this class of opioid compounds based on substitution of the 5-methyl group in 14-MM with an arylalkyl group such as benzyl may provide relevant information in the drug discovery process for identification of novel and safer analgesics towards the treatment of pain.
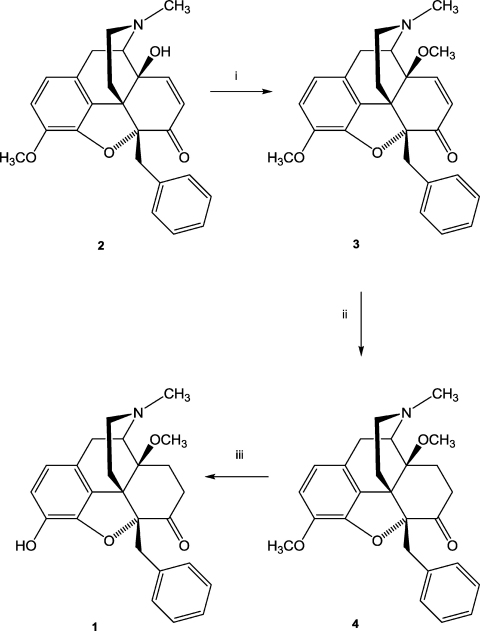
Fig. 2.
Reaction scheme for the synthesis of 5-benzyl substituted morphinans. Reagents and conditions: (i) NaH, (CH3O)2SO2, DMF, 0 °C; (ii) 10% Pd/C, H2, MeOH, 30 psi, r.t.; (iii) 48% HBr, reflux.
2. Materials and methods
2.1. Materials
Thebaine was obtained from Tasmanian Alkaloids Ltd. (Westbury, Tasmania, Australia). Melting points were determined on a Kofler melting point microscope (Reichert, Vienna, Austria) and are uncorrected. 1H NMR spectra were recorded on a Varian Gemini 200 (200 MHz) spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shifts (δ) are reported in ppm (relative to SiMe4 as internal standard), coupling constants (J) in Hz. Mass spectra were recorded on a Finnigan Mat SSQ 7000 apparatus (Finnigan Mat, San Jose, CA, USA). Elemental analysis was performed at the Institute of Physical Chemistry at the University of Vienna, Austria. For thin layer chromatography (TLC), Polygram SIL G/UV254 precoated plastic sheets (Macherey-Nagel, Germany) were used (eluent: methylene chloride/methanol/concentrated ammonia 90:9:1), and for column chromatography, silica gel 60 (230–400 mesh ASTM, Fluka, Switzerland) was used.
Opioid radioligands, [3H][d-Ala2,Me-Phe4,Gly-ol5]enkephalin ([3H]DAMGO), [3H]5α,7α,8β-(-)N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro(4,5)dec-8-yl]benzeneacetamide ([3H]U69,593) and radiolabelled guanosine-5′-O-(3-[35S]thio)-triphosphate ([35S]GTPγS) were purchased from PerkinElmer (Boston, MA, USA). [3H][Ile5,6]deltorphin II was obtained from the Institute of Isotopes Co. Ltd. (Budapest, Hungary). Naloxone hydrochloride (NX), Tris, unlabelled GTPγS, guanosine diphosphate (GDP), DAMGO, d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), nor-binaltorphimine (nor-BNI) and EGTA were obtained from Sigma–Aldrich Inc. (St. Louis, MO, USA). Morphine hydrochloride was obtained from Gatt-Koller GmbH (Innsbruck, Austria). 14-MM (Schmidhammer et al., 1990), 14-OMO (Schmidhammer et al., 1984) and naltrindole (NTI) (Portoghese et al., 1988a,b) were prepared at the Institute of Pharmacy, University of Innsbruck (Innsbruck, Austria) as previously described. All other chemicals were of analytical grade and obtained from standard commercial sources.
2.2. Animals
Sprague–Dawley rat and guinea pig brains used in in vitro assays were obtained from the Institut für Labortierkunde und Laborgenetik, Medizinische Universität Wien (Himberg, Austria). Male C57BL/6J mice (Institut für Labortierkunde und Laborgenetik, Medizinische Universität Wien, Himberg, Austria) weighing 20–25 g were used for in vivo studies. Mice were housed in groups of five and were kept in a temperature-regulated environment under a controlled 12 h light/dark cycle with free access to food and water at all times except during testing. All animal experiments were approved by the Austrian Ethical Commission.
2.3. Synthetic procedures
The 5-benzyl substituted morphinans 1, 3 and 4 were synthesized as illustrated in Fig. 2. Thus, 5β-benzyl-14-hydroxycodeinon (2) prepared from thebaine via 5β-benzylthebaine (Gates et al., 1989) was 14-O-methylated with dimethyl sulfate in the presence of sodium hydride in N,N-dimethylformamide to afford 5β-benzyl-14-methoxycodeinone (3) as a yellow-brown oil. Part of this oil was used for the next synthetic step without further purification while another part was purified by column chromatography and subsequently converted into the hydrochloride salt 3·HCl. Catalytic hydrogenation of 3 in methanol over 10% Pd/C at room temperature gave compound 4. The 3-O-methyl ether of compound 4 was cleaved by refluxing in 48% hydrobromic acid to obtain phenol 1. The base was converted into the hydrochloride salt 1 HCl.
2.3.1. 5β-Benzyl-14-methoxycodeinone hydrochloride (=5-benzyl-7,8-didehydro-4,5α-epoxy-3,14β-dimethoxy-17-methyl-morphinan-6-one hydrochloride) (3·HCl)
Sodium hydride (1.2 g, 50 mmol) (obtained from 2.0 g 60% sodium hydride dispersion in oil by washings with petroleum ether) was added to a stirred solution of 5β-benzyl-14-hydroxycodeinone (2) (Gates et al., 1989) (7.95 g, 19.7 mmol) in 100 ml anhydrous N,N-dimethylformamide under nitrogen at 0 °C (bath temperature). After 15 min, dimethyl sulfate (2.43 ml, 25.6 mmol) was added and the mixture was stirred for 1 h at 0 °C (bath temperature). Excess sodium hydride was destroyed carefully by addition of small pieces of ice. Then the mixture was diluted with water (250 ml) and extracted with methylene chloride (3 × 100 ml); the combined organic layers were washed with water (1 × 100 ml, 3 × 60 ml) and brine (100 ml), dried (sodium sulfate), and evaporated to give 8.3 g of a yellow-brown colored oil. Part of this oil was used for the next synthetic step without further purification while 500 mg of this oil were purified by column chromatography (silica gel, elution with methylene chloride/methanol/concentrated ammonia, 250:2:0.5) to afford a yellowish oil (210 mg). The hydrochloride salt was obtained in the usual way (diethyl ether/HCl) to yield 148 mg of 3·HCl: mp 164–166 °C; IR (KBr): 1680 (CO) cm−1; 1H NMR (DMSO-d6): δ 9.47 (s, broad, +NH), 7.37–7.09 (m, 5 arom. H), 7.04 (d, 1 H, C8–H, J = 10.0 Hz), 6.84 (d, 1 arom. H, J = 8.4 Hz), 6.74 (d, 1 arom. H, J = 8.4 Hz), 6.29 (d, 1 H, C7–H, J = 10.0 Hz), 3.76 (s, 3 H, C3–OCH3), 3.59 (d, 1 H, C5–CH2, J = 15.1 Hz), 3.37 (s, 3 H, C14–OCH3), 3.29 (d, 1 H, C5–CH2), J = 15.1 Hz), 2.88 (d, 3 H, +NCH3, J = 4.6 Hz); MS (CI): m/z 418 (M++1). Anal. Calcd for C26H27NO4·HCl·0.9 H2O: C 66.42, H 6.39, N 2.98. Found: C 66.16, H 6.45, N 2.91.
2.3.2. 5β-Benzyl-4,5α-epoxy-3,14β-dimethoxy-17-methylmorphinan-6-one (4)
A mixture of the yellow-brown colored oil of 3 (6.96 g, 16.7 mmol), 0.7 g 10% Pd/C-catalyst and 150 ml of methanol was hydrogenated at room temperature and 30 psi for 3 h. Then the catalyst was filtered off and the filtrate was evaporated. The residue (slightly brown foam) was crystallized from isopropanol to yield 3.14 g (45%) pure 4: mp 136–138 °C; IR (KBr): 1725 (CO) cm−1; 1H NMR (CDCl3): δ 7.35–7.18 (m, 5 arom. H), 6.66 (d, 1 arom. H, J = 8.2 Hz), 6.57 (d, 1 arom. H, J = 8.2 Hz), 3.90 (s, C3–OCH3), 3.53 (d, 1 H, C5–CH2, J = 14.7 Hz), 3.43 (d, 1 H, C5–CH2, J = 14.7 Hz), 3.34 (s, C14–OCH3), 2.42 (s, NCH3); MS (CI): m/z 420 (M++1). Anal. Calcd for C26H29NO4·0.3 iPrOH: C 73.84, H 7.23, N 3.20. Found: C 73.95, H 6.99, N 3.26.
2.3.3. 5β-Benzyl-4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6-one hydrochloride (1·HCl)
A solution of 4 (2.90 g, 6.9 mmol) in 40 ml of 48% hydrobromic acid was refluxed for 15 min and then evaporated. The residue was dissolved in methanol and again evaporated. The resulting foam (2.50 g) was converted into the base which was purified by column chromatography (silica gel, elution with methylene chloride/methanol/concentrated ammonia, 250:3:0.5) to afford a yellow oil (2.27 g). The hydrochloride salt was obtained in the usual way (diethyl ether/HCl) to yield 2.30 g (75%) of 1·HCl: mp 222–224 °C; IR (KBr): 1725 (CO) cm−1; 1H NMR (DMSO-d6): δ 9.45 (s, OH), 9.38 (s, broad, +NH), 7.37–7.09 (m, 5 arom. H), 6.70 (d, 1 arom. H, J = 8.2 Hz), 6.62 (d, 1 arom. H, J = 8.2 Hz), 3.41 (s, C14-OCH3), 2.89 (d, 3 H, +NCH3, J = 3.4 Hz); MS (CI): m/z 406 (M++1). Anal. Calcd for C25H27NO4·HCl·1.2 H2O: C 64.77, H 6.61, N 3.02. Found: C 65.05, H 6.44, N 2.99.
2.4. In vitro assays
2.4.1. Brain membrane preparations
Membranes were prepared from Sprague–Dawley rat or guinea pig brains as previously described (Spetea et al., 2003). Brains without cerebella were homogenized on ice in 5 volumes/weight of ice-cold 50 mM Tris–HCl buffer (pH 7.4) and diluted in 30 volumes/weight of the same buffer. After centrifugation at 40,000 × g for 20 min at 4 °C, the pellets were resuspended in 30 volumes/weight of 50 mM Tris–HCl buffer (pH 7.4) and incubated at 37 °C for 30 min. The centrifugation step described above was repeated, the final pellets were resuspended in 5 volumes/weight of 50 mM Tris–HCl buffer (pH 7.4) containing 0.32 M sucrose and stored at −80 °C until use. Protein concentration was determined by the method of Bradford using bovine serum albumin as standard (Bradford, 1976).
2.4.2. Opioid receptor binding assays
Binding experiments were performed in 50 mM Tris–HCl buffer (pH 7.4.) in a final volume of 1 ml containing 0.3–0.5 mg protein and at least 10 concentrations of test compound as described (Spetea et al., 2003). Rat brain membranes were incubated either with [3H]DAMGO (1 nM, 45 min, 35 °C) or [3H][Ile5,6]deltorphin II (0.5 nM, 45 min, 35 °C). Guinea pig brain preparations were incubated with [3H]U69,593 (1 nM, 30 min, 30 °C). Reactions were terminated by rapid filtration through Whatman GF glass fiber filters type GF/B pre-soaked in 0.1% polyethylenimine for 1 h at 4 °C for [3H]U69,593, or type GF/C for [3H]DAMGO and [3H][Ile5,6]deltorphin II) using a Brandel M24R Cell Harvester (Gaithersburg, MD, USA). Filters were washed three times with 5 ml of ice-cold 50 mM Tris–HCl buffer (pH 7.4). Non-specific binding was determined in the presence of 10 μM naloxone. The bound radioactivity was measured by liquid scintillation counting using a Beckman Coulter™ LS6500 (Beckman Coulter Inc., Fullerton, CA, USA).
Inhibition constant (Ki) values were calculated from competition binding curves using the non-linear least-square curve fitting by GraphPad Prism software (v3; GraphPad Software Inc., San Diego, CA, USA). All experiments were performed in duplicate and repeated two to six times.
2.4.3. [35S]GTPγS (guanosine-5′-O-(3-[35S]thio)-triphosphate) binding assay
Rat brain membranes (10 μg of protein) were incubated for 60 min at 30 °C in Tris-EGTA buffer (50 mM Tris–HCl buffer, 3 mM MgCl2, 1 mM EGTA, 100 nM NaCl, pH 7.4) containing 30 μM GDP, 0.05 nM [35S]GTPγS and appropriate concentrations of test compound in a final volume of 1 ml as previously described (Spetea et al., 2003). Non-specific binding was measured in the presence of 100 μM unlabelled GTPγS. Reactions were terminated by vacuum filtration through Whatman GF/B glass fiber filters and bound [35S]GTPγS retained on the filters was determined as described for opioid receptor binding assays.
Stimulation of [35S]GTPγS binding produced by the test compound is given as percentage of the basal activity (defined as 100%, measured in the absence of test compound). The EC50 (nM, concentration of ligand to elicit half-maximal effect) and Emax (%, maximum stimulation) were calculated using nonlinear regression analysis and sigmoidal curve fitting with the GraphPad Prism software. All experiments were performed in triplicate and repeated at least three times.
2.5. In vivo testing
2.5.1. Drug administration
Vehicle (saline) or solutions of test compounds prepared in sterile physiological saline (0.9%) were administered s.c. to mice in a volume of 10 μl per 1 g body weight.
2.5.2. Nociceptive assessments
Hot-plate latencies are determined as described (Lattanzi et al., 2005) by placing each mouse on a TSE hot plate V2.0 plate (TSE System Inc., Midland, MI, USA) kept at 55 °C and observing the occurrence of a nociceptive response (licking of a hind paw, jumping). To confine the mice to a certain observation area, a colourless plastic cylinder of 20 cm diameter was placed on the hot-plate. In order to avoid possible tissue injury, a cut-off time of 12 s was used.
The tail-flick test was performed using an UB 37360 Ugo Basile analgesiometer (Ugo Basile s.r.l., Varese, Italy) as described (Greiner et al., 2003). The reaction time required by the mouse to remove its tail due to the radiant heat was measured and defined as the tail-flick latency. A cut-off time of 10 s was used in order to minimize tissue damage.
Hot-plate and tail-flick latencies were measured before (basal latency, BL) and 30 min after drug s.c. administration (test latency, TL). For establishing the dose–response effect, the antinociceptive response was expressed as percent of Maximum Possible Effect (%MPE) = [(TL − BL)/(cut-off time − BL)] × 100 for each dose tested. Each experimental group included five mice. ED50, defined as the opioid dose that produced an analgesic effect equal to 50% MPE, and 95% confidence limits were calculated using linear regression according to the method of Litchfield and Wilcoxon (1949).
2.5.3. Motor activity
The drug effect on motor coordination was tested in the rotarod test using an accelerating rotarod treadmill (Acceler Rota-Rod 7650, Ugo Basile s.r.l., Varese, Italy) for mice (diameter 3.5 cm) (Jones and Roberts, 1968). The treadmill was accelerated from 4 to 40 rpm over a period of 5 min, and the time spent on the drum was recorded for each mouse. A 300 s cut-off time was used. Mice were evaluated prior to and 30 min after drug or vehicle (saline) s.c. administration. Each experimental group included five animals. Percentage (%) changes from the rotarod latencies obtained before (BL) and after drug administration (TL) were calculated as: [(TL − BL)/(300 s − BL)] × 100.
2.6. Determination of physicochemical properties
Lipophilicity of opioid compounds was evaluated at 25 °C by determination of partition (log P) and distribution coefficients (log D) in an immiscible (biphasic) octanol/water medium using the PCA200/Cheqsol instrument (Sirius Analytical Instruments, Sussex, UK) as described (Riba et al., 2010). The instrument was standardized by a four-parameter method as described (Avdeef, 1993). Titrations of the sample solutions having volumes between 6 and 20 ml and molarity between 0.5 and 2.5 mM were started at pH 1.8 (using 0.5 M HCl) and finished at pH 12.2 (using 0.5 M KOH). All titrations were performed in 0.15 M KCl solution under argon gas. The ionization constant (pKa, pH at 50% ionization of the compound) was determined by the shape of the titration curve and partition coefficients were determined by the shift in titration curve of pKa in the presence of octanol. The analysis of pH-metric data was performed by RefinementPro software (v2.0, Sirius, Sussex, UK).
2.7. Statistical analysis
All data are reported as mean ± SEM. Statistical analysis was carried out using one-way analysis of variances (ANOVA), and comparisons among groups were performed by the independent t-test using SPSS software (v15.0; SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
3. Results
3.1. In vitro opioid receptor binding affinities
Binding affinities of compounds 1, 3 and 4 (Fig. 2) at opioid receptors were determined in vitro using competition binding assays in rat brain (μ, δ) and guinea pig brain (κ) membrane preparations, and employing [3H]DAMGO (μ), [3H][Ile5,6]deltorphin II (δ) and [3H]U69,593 (κ) as specific opioid radioligands (Spetea et al., 2003). The μ, δ and κ opioid receptor binding affinities expressed as inhibition constants (Ki) are summarized in Table 1. For comparison purposes, the opioid binding affinity data for 14-MM (Spetea et al., 2003), 14-OMO (Spetea et al., 2004a) and morphine (Spetea et al., 2003) are included.
Table 1.
Binding affinities of investigated opioid compounds at μ, δ and κ opioid receptors.
|
Ki (nM)a |
|||
|---|---|---|---|
| [3H]DAMGO (μ)b | [3H][Ile5,6]deltorphin II (δ)b | [3H]U69,593 (κ)c | |
| 1 | 0.31 ± 0.02 | 13.1 ± 1.7 | 22.8 ± 0.3 |
| 3 | 34.1 ± 6.0 | >10,000 | 1894 ± 453 |
| 4 | 65.4 ± 2.9 | >10,000 | 4414 ± 1229 |
| 14-MMd | 0.15 ± 0.01 | 13.3 ± 0.2 | 25.2 ± 4.9b |
| 14-OMOe | 0.10 ± 0.01 | 4.80 ± 0.22 | 10.2 ± 2.0b |
| Morphined | 6.55 ± 0.74 | 217 ± 19 | 113 ± 9b |
Values are the mean ± SEM of 2–6 independent experiments, all performed in duplicate.
Rat brain membranes were used.
Guinea brain membranes were used.
Data from Spetea et al. (2003).
Data from Spetea et al. (2004a).
In in vitro binding assays, compound 1 was shown to bind with high affinity at the μ opioid receptor (Ki = 0.31 nM), and with two orders of magnitude lower affinities at δ and κ receptors (13.1 and 22.8 nM, respectively), hence displaying a comparable binding profile as the parent compound 14-MM and its 5-unsubstituted analogue 14-OMO (Table 1). Compound 1 exhibited about 20-fold enhanced affinity at the μ receptor in comparison to morphine (Ki = 6.55 nM). The other two 5-benzyl substituted derivatives (3 and 4) showed moderate affinity at the μ opioid receptor (Kis of 34.1 and 65.4 nM, respectively) and significantly reduced interaction with δ and κ receptors (Table 1).
3.2. In vitro stimulation of [35S]GTPγS binding
The effect of the 5-benzyl analogue 1 on G-protein activation was evaluated using the ligand-stimulated [35S]GTPγS binding assay in rat brain membranes (Spetea et al., 2003). Its stimulatory effect was compared with that of 14-MM, 14-OMO, morphine and the prototype μ opioid receptor agonist DAMGO.
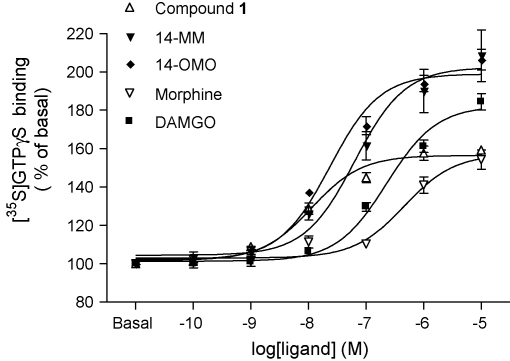
As shown in Fig. 3, compound 1, 14-MM, 14-OMO, morphine and DAMGO produced concentration-dependent increase in [35S]GTPγS binding. Agonist potencies (ED50) and maximal response (Emax) values were determined and are listed in Table 2. The rank order of potencies in stimulating [35S]GTPγS binding was: compound 1 > 14-OMO > 14-MM > DAMGO > morphine. The mean maximal response was higher for 14-MM and 14-OMO and somewhat lower for compound 1 and morphine than that of DAMGO.
Fig. 3.
Concentration-dependent stimulation of [35S]GTPγS binding by compound 1, and 14-MM, 14-OMO, morphine and DAMGO in rat brain membranes. Data are shown as % stimulation over basal [35S]GTPγS binding and represent the mean ± SEM of at least three independent experiments, all performed in triplicate.
Table 2.
Stimulation of [35S]GTPγS binding in response to investigated opioid compounds in rat brain membranes.
| ED50 (nM)a | Emax (%)a | |
|---|---|---|
| 1 | 13.7 ± 2.6d | 158 ± 2c |
| 14-MM | 63.0 ± 6.3d,g | 203 ± 13f |
| 14-OMO | 23.7 ± 2.0d,e | 199 ± 5f |
| Morphine | 462 ± 42f | 157 ± 6b |
| DAMGO | 309 ± 35 | 185 ± 4 |
Values are the mean ± SEM of at least three independent experiments, all performed in triplicate.
p < 0.05.
p < 0.01.
p < 0.001 vs. DAMGO.
p < 0.05.
p < 0.01.
p < 0.001 vs. compound 1.
Compound 1 was shown to be the most potent agonist with an ED50 value of 13.7 nM, whereas its parent compound 14-MM had a potency of 63 nM in stimulating [35S]GTPγS binding in rat brain membranes. It displayed a comparable efficacy to morphine, but it was less efficacious than DAMGO, 14-MM and 14-OMO (Table 2). Also, 14-OMO activated G-proteins as indicated by the concentration-dependent increases in [35S]GTPγS binding (Fig. 3) showing a significantly increased agonist potency (ED50 = 23.7 nM) than 14-MM and DAMGO, while exhibiting comparable efficacy (Table 2).
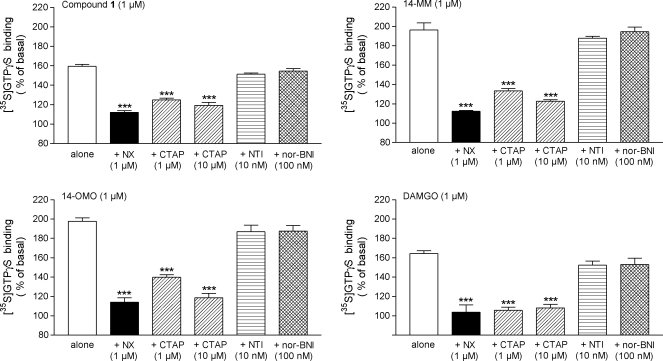
In order to assess the specificity of G-protein activation via opioid receptors by compound 1, agonist-stimulated [35S]GTPγS binding was probed with the opioid antagonist naloxone. Similar to 14-MM, 14-OMO and DAMGO, the increase of [35S]GTPγS binding produced by compound 1 (1 μM) was prevented by naloxone (1 μM) (Fig. 4).
Fig. 4.
Effect of the non-selective opioid antagonist naloxone (NX), and of selective μ (CTAP), δ (NTI) and κ (nor-BNI) opioid receptor antagonists on [35S]GTPγS binding stimulated by compound 1, 14-MM, 14-OMO and DAMGO in rat brain membranes. Assays were performed in the presence of 1 μM of compound 1, 14-MM, 14-OMO or DAMGO alone or in the presence of NX (1 μM), CTAP (1 and 10 μM), NTI (10 nM) and nor-BNI (100 nM). Data are shown as % stimulation over basal [35S]GTPγS binding and represent the mean ± SEM of at least three independent experiments, all performed in triplicate. ***p < 0.001 vs. agonist alone.
Furthermore, to establish the μ opioid receptor specificity of stimulated [35S]GTPγS binding by compound 1, we examined the ability of type selective opioid receptor antagonists to reverse the G-protein activating effects (Fig. 4). [35S]GTPγS binding assays were conducted by incubation of compound 1 (1 μM) in the presence and absence of selective antagonists acting at the μ (CTAP) (Kramer et al., 1989), δ (NTI) (Portoghese et al., 1988a,b) or κ (nor-BNI) (Portoghese et al., 1987) opioid receptors. As shown in Fig. 4, the increase of [35S]GTPγS binding by compound 1 was reversed by co-incubation with CTAP in a concentration-dependent manner. As well, CTAP has caused antagonism of stimulatory actions produced by either 1 μM of 14-MM, 14-OMO or DAMGO. Conversely, NTI (10 nM) and nor-BNI (100 nM) had no significant effect on compound 1-stimulated [35S]GTPγS binding, as also observed with the other μ opioid agonists, 14-MM, 14-OMO and DAMGO (Fig. 4). Concentrations of antagonists, CTAP, NTI and nor-BNI, were chosen based on observations that the corresponding agonist-stimulated [35S]GTPγS binding, i.e. DAMGO (μ) (Fig. 4), SNC80 (δ) and U69,593 (κ) (data not shown), respectively, was selectively inhibited by the antagonist.
3.3. Antinociceptive effects in mice after s.c. administration
Following in vitro studies, compound 1 was investigated in vivo for the antinociceptive properties in mice after s.c. administration using hot-plate (Lattanzi et al., 2005) and tail-flick tests (Greiner et al., 2003), and its activity was compared to that of 14-MM, 14-OMO and morphine (Fig. 5). Antinociceptive ED50 values and 95% confidence limits were calculated and are presented in Table 3.
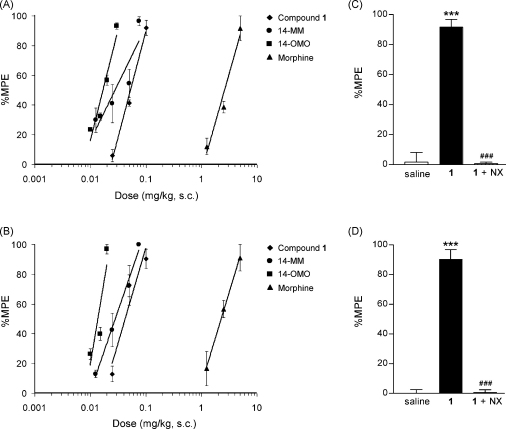
Fig. 5.
Right panel: Dose-dependent antinociceptive effect induced after s.c. administration by compound 1, 14-MM, 14-OMO and morphine in the (A) hot-plate test and (B) tail-flick test in mice. Left panel: Antagonism by naloxone (NX) on the antinociceptive effect of compound 1 in mice after s.c. administration in the (A) hot-plate test and (B) tail-flick test. NX (1 mg/kg) was s.c. injected to mice 10 min before s.c. administration of compound 1 (0.1 mg/kg). Other groups of mice received s.c. injection of saline or compound 1 alone. Hot-plate or tail-flick latencies (as %MPE) were determined 30 min after s.c. administration of the opioid agonist. Data are shown as the mean ± SEM of five mice per experimental group. ***p < 0.001 vs. saline control group; ###p < 0.001 vs. compound 1-treated group.
Table 3.
Antinociceptive potencies in mice after s.c. administration.
| ED50 (mg/kg, s.c.)a |
||
|---|---|---|
| Hot-plate test | Tail-flick test | |
| 1 | 0.053 (0.033–0.066) | 0.043 (0.025–0.073) |
| 14-MM | 0.028 (0.014–0.058) | 0.028 (0.017–0.047) |
| 14-OMO | 0.017 (0.012–0.025) | 0.014 (0.010–0.019) |
| Morphine | 2.63 (1.55–4.39) | 2.29 (1.31–3.80) |
Values in parenthesis are 95% confidence limits.
As shown in Fig. 5, compound 1 produced dose-dependent antinociceptive effects after systemic s.c. administration to mice in both nociceptive tests. The antinociceptive dose necessary to elicit a 50% effect was 0.053 mg/kg in the hot-plate test, and a similar ED50 value of 0.043 mg/kg was determined in the tail-flick test. Compared to morphine, compound 1 showed more than 50-fold increased analgesic potency (Table 3). In line with previous reports (Zernig et al., 2000; King et al., 2003; Greiner et al., 2003; Lattanzi et al., 2005; Riba et al., 2010), 14-MM was found to be 80-fold more potent than morphine in both thermal nociceptive assays in mice following s.c. administration. Similarly, s.c. 14-OMO exhibited over 150-fold enhanced antinociceptive potency than morphine, in agreement with earlier findings in rodents (Schmidhammer et al., 1984; Fürst et al., 2005; Lattanzi et al., 2005; Riba et al., 2010).
To investigate whether the antinociceptive actions of compound 1 are mediated through opioid receptor interactions, we examined the effect of pre-treatment with the opioid antagonist naloxone in mice that subsequently received compound 1. As illustrated in Fig. 5, s.c. dosing of mice with compound 1 (0.1 mg/kg) produced significant increases in both hot-plate and tail-flick responses, which were reversed by naloxone injected 10 min before the agonist (1 mg/kg, s.c.) to exhibit similar values as elicited by saline-treated animals.
3.4. Effect on motor coordination in mice after s.c. administration
Further in vivo pharmacological studies were performed with compound 1 by assessing its effect on motor coordination in mice after s.c. administration using the rotarod test. The potential to depress motor activity was compared to that of morphine, 14-MM and 14-OMO.
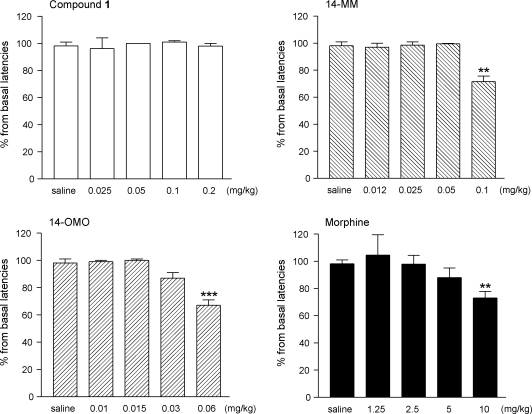
Dose-dependent drug effects on motor performance induced by compound 1, 14-MM, 14-OMO and morphine in the mouse rotarod test are shown in Fig. 6. The highest tested dose in the rotarod assay, which produced maximum MPE% values (i.e. 100%) in antinociception, was selected. Compound 1 provoked no changes in the normal behaviour of mice as demonstrated in rotarod experiments in which no significant alterations in the rotarod latencies were observed over the dose range of 0.025–0.2 mg/kg. A significant decrease in motor coordination compared to saline-treated control mice was recorded after s.c. administration of the μ opioid agonists 14-MM at 0.1 mg/kg and 14-OMO at 0.06 mg/kg (Fig. 6). Also, systemic morphine affected rotarod performance over the dose range of 1.25–10 mg/kg with a significant effect after the 10 mg/kg dose.
Fig. 6.
Effect of opioid compounds on the motor performance in mice as measured using the rotarod test. Mice were tested 30 min after s.c. administration of saline (control) or test compound, and the time the animals remain on the device until falling was recorded. Data are shown as percentage from basal latencies (before drug administration) and each value represents the mean ± SEM of five mice per experimental group. **p < 0.01, ***p < 0.001 vs. saline-treated control group.
3.5. Physicochemical properties
The ionization constants for compound 1 were determined in three independent assays performed at 25 °C. The pKa values for the amine function and the phenolic hydroxyl group, and the value of the octanol–water partition coefficient, log P, a quantitative descriptor of lipophilicity are presented in Table 4. Recently reported physicochemical properties of 14-MM, 14-OMO and morphine (Riba et al., 2010) are included for comparison. As shown in Table 4, the 5-benzyl derivative of 14-MM exhibited higher lipophilicity (log P = 1.49) than its parent compound (log P = 1.12) and an even larger increase when compared to 14-OMO (log P = 0.60). Also, higher values for the distribution coefficient, log D at pH 7.4, was determined for compound 1 than for 14-MM and 14-OMO as well as morphine.
Table 4.
Ionization constants and octanol–water partition (log P) and distribution (log D) coefficients of opioid morphinans at 25 °C.
| Compound | pKa (amine) | pKa (phenol) | log P | log D (pH 7.4) |
|---|---|---|---|---|
| 1 | 8.13 ± 0.04 | 9.40 ± 0.06 | 1.49 ± 0.06 | 0.69 ± 0.13 |
| 14-MMa | 8.36 | 9.39 | 1.12 | 0.11 |
| 14-OMOa | 8.18 | 9.21 | 0.60 | −0.25 |
| Morphinea | 8.15 | 9.29 | 0.88 | 0.06 |
Values are the mean ± SEM of three independent experiments.
Data from Riba et al. (2010).
4. Discussion
In the present study, we have shown by in vitro binding and functional assays compiled with in vivo behavioural approaches that replacement of the methyl group in position 5 in 14-MM with a benzyl substituent leads to qualitative and quantitative differences in the interaction with opioid receptors. The results indicate that the 5-benzyl analogue of 14-MM, compound 1, exhibits high affinity and potent agonism at the μ opioid receptor and shows antinociceptive efficacy through opioid receptor-mediated mechanisms achieved at doses that had no significant effect on motor activity.
Following the pattern of SAR, the in vitro and in vivo pharmacological profile shown by the investigated opioid ligands is related to specific structural features. Substitution of the 5-methyl group in 14-MM with a benzyl group left binding affinities at δ and κ opioid receptors essentially unchanged while retaining the high affinity at the μ receptor (Ki of 0.15 nM for 14-MM vs. 0.31 nM for compound 1). The assessment of binding affinities of 14-OMO and its 5-benzyl analogue 1 revealed that the introduction of an arylalkyl group, i.e. benzyl at position 5, maintained the high affinity at the μ receptor, with only a minor decrease in affinities at δ and κ receptors, hence still displaying μ opioid receptor selectivity. These data confirm and extend previous SAR observations in terms of opioid receptor binding in this series of opioid compounds, when comparing 5-unsubstituted to their 5-methyl substituted analogues (Spetea et al., 2004a; Lattanzi et al., 2005).
When examining the in vitro opioid binding profile of compound 1 and its 3-methoxy analogue 4, it became apparent that the presence of a methoxy group at position 3 has a major effect on binding affinities to all three opioid receptor types. The present observations on the detrimental effect on the interaction with opioid receptors upon substitution at the 3-hydroxy group in the morphinan skeleton support earlier reports (Beyerman et al., 1976; Pasternak et al., 1987; Chen et al., 1991; Mignat et al., 1995; Spetea et al., 1998; Schmidt et al., 2002; Thompson et al., 2004; Crooks et al., 2006). A representative example is the comparison of binding affinities (Ki values in nM) of oxymorphone (μ:δ:κ = 0.97:80.5:61.6) (Lattanzi et al., 2005) with its 3-methoxy analogue oxycodone (μ:δ:κ = 43.6:1087:2658) (Spetea et al., 2005). This phenomenon was also described for morphine having a methylated (i.e. codeine) or glucuronidated (i.e. morphine-3-glucoronide) hydroxyl group at position 3 (Pasternak et al., 1987; Mignat et al., 1995; Thompson et al., 2004).
Similar to the structurally related derivatives, 14-MM and 14-OMO, compound 1 is characterized by agonist activity in vitro and in vivo, while exhibiting a distinct functional profile. In in vitro [35S]GTPγS binding assays, among all investigated opioid ligands, compound 1 was shown to be the most potent agonist in G-protein activation having a 23-fold greater ED50 value than that of the standard μ opioid agonist DAMGO, and producing 85% of the maximum stimulation observed with DAMGO. Although it was as efficacious as morphine in stimulating [35S]GTPγS binding, compound 1 proved to be considerably more potent by more than 30-fold. Comparable ED50 and Emax values to the earlier reported (Spetea et al., 2003) were determined in this study for 14-MM-stimulated [35S]GTPγS binding in rat brain membranes. Notably, here we also report the first data on stimulation of [35S]GTPγS binding by 14-OMO. 14-OMO activated G-proteins as indicated by the concentration-dependent increases in [35S]GTPγS binding showing over 13-fold greater potency than DAMGO, while exhibiting comparable efficacy. We have also established that 14-OMO-stimulated [35S]GTPγS binding is a μ opioid receptor-mediated event like that shown by 14-MM. The enhanced agonist potency of 14-OMO compared to DAMGO as well as to morphine determined by [35S]GTPγS binding assays is in agreement with pharmacological findings in in vitro bioassays using mouse vas deferens (MVD), guinea pig ileum (GPI) and rat vas deferens preparations (Spetea et al., 2004a; Lattanzi et al., 2005; Riba et al., 2010).
In vitro, compound 1 activated G-proteins by specific stimulation of μ opioid receptors in rat brain membranes. This has been demonstrated based on reversibility of the increase of [35S]GTPγS binding by compound 1 in the presence of the selective μ opioid receptor antagonist CTAP (Kramer et al., 1989) and the lack of effect of NTI (Portoghese et al., 1988a,b) and nor-NBI (Portoghese et al., 1987), as selective antagonists at δ and κ opioid receptors, respectively. CTAP (1 and 10 μM), NTI (10 nM) and nor-BNI (100 nM) at tested concentrations blocked stimulation of [35S]GTPγS binding by opioid agonists selective at μ (DAMGO, Fig. 4), δ ([d-Pen2, d-Pen5]enkephalin) and κ opioid receptors (U50,488H), respectively (Mizoguchi et al., 2000).
Based on in vitro functional results, the presence of a benzyl group in position 5 in compound 1 yielded a novel μ opioid agonist, displaying a 5-fold increase in potency compared to its 5-methyl analogue 14-MM, while it appears to be less efficacious in stimulating [35S]GTPγS binding. Although the affinity of analogue 1 for opioid receptors is essentially not different from that of 14-MM, it is possible that the presence of an arylalkyl group like benzyl at position 5 increases the potency of this agonist. A similar profile was observed when comparing agonist activities of the 5-benzyl derivative 1 to its 5-unsubstituted analogue 14-OMO. Methylation at position 5 of 14-OMO resulted in 14-MM, which shows less than 3-fold decrease in potency and similar maximal stimulation of [35S]GTPγS binding. The present observations on 14-MM and 14-OMO as potent agonists corroborate with described in vitro activities in MVD, GPI and RVD bioassays (Spetea et al., 2004a; Lattanzi et al., 2005; Riba et al., 2010).
In vivo, compound 1 appeared to be a potent opioid agonist by exhibiting antinociceptive activity after systemic s.c. administration in mouse models of nociceptive pain. Its antinociceptive effects were reversed by the opioid antagonist naloxone, supporting an opioid receptor-mediated mechanism of action. In both hot-plate and tail-flick tests, compound 1 produced a dose-dependent increase in withdrawal latencies in response to a thermal stimulus giving ED50 values in the μg range, i.e. 0.053 and 0.043 mg/kg. For comparison, s.c. morphine showed ED50 values of 2.63 and 2.29 mg/kg, respectively, in the two nociceptive assessments.
The antinociceptive potency of compound 1 was found to be only 1.9- and 1.5-fold lower than that of 14-MM in the hot-plate and tail-flick tests, respectively, and it was 50-fold higher than that of the ‘gold standard painkiller’ morphine. Thus, replacement of the 5-methyl group in 14-MM with a benzyl substituent appears to be well tolerated by giving rise to a highly potent and efficacious antinociceptive agent. Also, the introduction of a benzyl group at position 5 in 14-OMO, affording compound 1, produced only a modest change (3-fold decrease) in antinociceptive potency. The SAR observations derived in this study from the in vivo pharmacological findings on antinociceptive properties are in qualitative agreement with the in vitro opioid activities of the investigated opioid morphinans.
μ Opioid analgesics including morphine, codeine, oxycodone and fentanyl are known to depress motor activity, a side effect that limits their usefulness (Hayes and Tyers, 1983; Bowdle, 1998; Meert and Vermeirsch, 2005). To further address the behavioural consequences of the μ receptor agonist profile exhibited by the 5-benzyl analogue of 14-MM, its effects on motor coordination using the rotarod test were assessed across an antinociceptive effective range. In addition, the first behavioural data on alterations in motor function following systemic s.c. administration of 14-MM and 14-OMO are reported.
Results from behavioural investigations have shown that s.c. dosing of compound 1 to mice did not significantly affect the performance in the rotarod test over the analgesic dose range of 0.025–0.2 mg/kg. By comparison at equianalgesic doses, systemic morphine caused dose-dependent suppressive effects on motor coordination with significance at 10 mg/kg, a finding consistent with previous reports (Gallantine and Meert, 2005; Jones et al., 2005; Meert and Vermeirsch, 2005). Dosages that produce full antinociceptive efficacy of the μ opioid agonists 14-MM (0.1 mg/kg) and 14-OMO (0.06 mg/kg) were also observed to diminish motor ability in the rotarod test. The mechanism responsible for the impairment of normal motor function has been proposed to be linked to the μ opioid receptor activation in the basal ganglia, nucleus raphe pontis, periaqueductal gray and locus coeruleus (Havemann et al., 1980, 1982; Vankova et al., 1996; Bowdle, 1998). Outcomes of the rotarod experiments compiled with nociceptive behaviour findings indicated that replacing the 5-methyl group with a benzyl substituent in 14-MM led to an opioid ligand, which achieves antinociceptive efficacy at doses that do not significantly influence motor activity.
We experimentally determined the physicochemical properties of compound 1 and found that the substitution of the methyl group in position 5 in 14-MM with a benzyl group leads to a more liphophilic molecule, while also showing increased liphophilicity compared to its 5-unsubstituted analogue 14-OMO. Thus, compound 1 which displays considerable higher liphophilicity than 14-MM, 14-OMO and morphine may represent a feasible candidate for oral and/or transdermal delivery.
In summary, we have described the synthesis and pharmacological characterization of a novel μ opioid receptor ligand, the 5-benzyl analogue of 14-MM, which emerged as a high affinity and potent μ opioid antinociceptive agent with reduced propensity to cause unwanted motor impairment. Our findings revealed that targeting position 5 represents a viable approach for tuning the pharmacological properties of this class of opioids. The results of the present work complement and sharpen the understanding that appropriate molecular manipulations of the morphinan template could afford opioid ligands that besides their scientific value as pharmacological tools, may also have the potential of emerging as novel analgesics with fewer side effects compared to currently available treatments.
Acknowledgements
The authors would like to thank Tasmanian Alkaloids Pty. Ltd., Westbury, Tasmania, Australia, for the generous gift of thebaine and Beatrix Jungwirth for technical assistance. This work was supported by the Austrian Science Fund (FWF): [P12668 and P21350] and AlcaSynn Pharmaceuticals GmbH.
References
- Al-Khrasani, M., Spetea, M., Friedmann, T., Riba, P., Kiral, Y.K., Schmidhammer, H., Fürst S., 2007. DAMGO and 6β-glycine substituted 14-O-methyloxymorphone but not morphine show peripheral, preemptive antinociception after systemic administration in a mouse visceral pain model and high intrinsic efficacy in the isolated rat vas deferens. Brain Res. Bull. 74, 369–375. [DOI] [PubMed]
- Avdeef A. pH-Metric log P. II: Refinement of partition coefficients and ionization constants of multiprotic substances. J. Pharm. Sci. 1993;82:183–190. doi: 10.1002/jps.2600820214. [DOI] [PubMed] [Google Scholar]
- Benyamin R., Trescot A.M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Glaser S.E., Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–120. [PubMed] [Google Scholar]
- Beyerman H.C., Buurman E., Lie T.S., Maat L. Selective removal of phenolic hydroxyl groups in morphinan derivatives. Synthesis of rac. N-Formyl-3-methoxy-6-oxomorphinan. Recl. Trav. Chim. Pays-Bas. 1976;95:43–44. [Google Scholar]
- Bileviciute-Ljungar I., Spetea M., Guo Y., Schütz J., Windisch P., Schmidhammer H. Peripherally mediated antinociception of the μ-opioid receptor agonist 2-[(4,5α-epoxy-3-hydroxy-14β-methoxy-17-methylmorphinan-6β-yl)amino]acetic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J. Pharmacol. Exp. Ther. 2006;317:220–227. doi: 10.1124/jpet.105.096032. [DOI] [PubMed] [Google Scholar]
- Bowdle T.A. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19:173–189. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Chen Z.R., Irvine R.J., Somogyi A.A., Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48:2165–2171. doi: 10.1016/0024-3205(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Crooks P.A., Kottayil S.G., Al-Ghananeem A.M., Byrn S.R., Butterfiled D.A. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorg. Med. Chem. Lett. 2006;16:4291–4295. doi: 10.1016/j.bmcl.2006.05.060. [DOI] [PubMed] [Google Scholar]
- Freye E., Schmidhammer H., Latasch L. 14-Methoxymetopon, a potent opioid, induces no respiratory depression, less sedation, and less bradycardia than sufentanil in the dog. Anesth. Analg. 2000;90:1359–1364. doi: 10.1097/00000539-200006000-00018. [DOI] [PubMed] [Google Scholar]
- Fürst S., Búzás B., Friedmann T., Schmidhammer H., Borsodi A. Highly potent novel opioid receptor agonist in the 14-alkoxymetopon series. Eur. J. Pharmacol. 1993;236:209–215. doi: 10.1016/0014-2999(93)90591-5. [DOI] [PubMed] [Google Scholar]
- Fürst S., Riba P., Friedmann T., Timar J., Al-Khrasani M., Obara I., Makuch W., Spetea M., Schütz J., Przewlocki R., Przewlocka B., Schmidhammer H. Peripheral versus central antinociceptive actions of 6-amino acid-substituted derivatives of 14-O-methyloxymorphone in acute and inflammatory pain in the rat. J. Pharmacol. Exp. Ther. 2005;312:609–618. doi: 10.1124/jpet.104.075176. [DOI] [PubMed] [Google Scholar]
- Gallantine E.L., Meert T.F. A comparison of the antinociceptive and adverse effects of the μ-opioid agonist morphine and the δ-opioid agonist SNC80. Basic Clin. Pharmacol. Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gates M., Boden R.M., Sundararaman P. Derivatives of the thebaine anion. 2. 5-Methylmorphine, 5-methylcodeine, 5-methylheroin, and some related compounds. J. Org. Chem. 1989;54:972–974. [Google Scholar]
- Greiner E., Spetea M., Krassnig R., Schüllner F., Aceto M., Harris L.S., Traynor J.R., Woods J.H., Coop A., Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 18. N-substituted 14-phenylpropyloxymorphinan-6-ones with unanticipated agonist properties: extending the scope of common structure–activity relationships. J. Med. Chem. 2003;46:1758–1763. doi: 10.1021/jm021118o. [DOI] [PubMed] [Google Scholar]
- Havemann U., Turcski L., Kuschinsky K. Role of opioid receptors in the substantia nigra in morphine-induced muscular rigidity. Life Sci. 1982;31:2319–2322. doi: 10.1016/0024-3205(82)90146-1. [DOI] [PubMed] [Google Scholar]
- Havemann U., Winkler M., Kuschinsky K. Opioid receptors in the caudate nucleus can mediate EMG-recorded rigidity in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 1980;313:139–144. doi: 10.1007/BF00498570. [DOI] [PubMed] [Google Scholar]
- Hayes A.G., Tyers M.B. Determination of receptors that mediate opiate side effects in the mouse. Br. J. Pharmacol. 1983;7:731–736. doi: 10.1111/j.1476-5381.1983.tb10011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi C.E. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:S3–13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- Jain, K.K., 2004. Pain Therapeutics—Drugs, Markets and Companies. Jain PharmaBiotech, Basel.
- Jones B.J., Roberts D.J. The quantitative measurement of motor inco-ordination in naive mice using an accelerating rotarod. J. Pharm. Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Jones C.K., Peters S.C., Shannon H.E. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J. Pharmacol. Exp. Ther. 2005;312:726–732. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- Kieffer B.L., Evans C.J. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology. 2009;56:205–212. doi: 10.1016/j.neuropharm.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.A., Su W., Nielan C.L., Chang A.H., Schütz J., Schmidhammer H., Pasternak G.W. 14-Methoxymetopon, a very potent μ-opioid analgesic with an unusual pharmacological profile. Eur. J. Pharmacol. 2003;459:203–209. doi: 10.1016/s0014-2999(02)02821-2. [DOI] [PubMed] [Google Scholar]
- Király K.P., Riba P., D’Addario C., Di Benedetto M., Landuzzi D., Candelotti S., Romualdi P., Fürst S. Alterations in prodynorphin gene expression and dynorphin levels in different brain regions after chronic administration of 14-methoxymetopon and oxycodone-6-oxime. Brain Res. Bull. 2006;70:233–239. doi: 10.1016/j.brainresbull.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Knapp R.J., Malatynska E., Collins N., Fang L., Wang J.Y., Hruby V.J. Molecular biology and pharmacology of cloned opioid receptors. FASEB J. 1995;5:516–525. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- Kramer T.H., Shook J.E., Kazmierski W., Ayres E.A., Wire W.S., Hruby V.J., Burks T.F. Novel peptidic μ-opioid antagonists: Pharmacological characterization in vitro and in vivo. J. Pharmacol. Exp. Ther. 1989;249:544–551. [PubMed] [Google Scholar]
- Lattanzi R., Spetea M., Schüllner F., Rief S.B., Krassnig R., Negri L., Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 22. Influence of the 14-alkoxy group and the substitution in position 5 in 14-alkoxymorphinan-6-ones on in vitro and in vivo activities. J. Med. Chem. 2005;48:3372–3378. doi: 10.1021/jm040894o. [DOI] [PubMed] [Google Scholar]
- Litchfield J.T., Jr., Wilcoxon F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- Mahurter L., Garceau C., Marino J., Schmidhammer H., Toth G., Pasternak G.W. Separation of binding affinity and intrinsic activity of the potent μ-opioid 14-methoxymetopon. J. Pharmacol. Exp. Ther. 2006;319:247–253. doi: 10.1124/jpet.106.105395. [DOI] [PubMed] [Google Scholar]
- Marcus D.A., Cope D.K., Deodhar A., Payne R. Clinical Publishing; Oxford: 2009. Chronic Pain: An Atlas of Investigation and Management. [Google Scholar]
- Meert T.F., Vermeirsch H.A. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol. Biochem. Behav. 2005;80:309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Mignat C., Wille U., Ziegler A. Affinity profiles of morphine, codeine, dihydrocodeine, and their glucuronides at opioid receptor subtypes. Life Sci. 1995;56:793–799. doi: 10.1016/0024-3205(95)00010-4. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., Narita M., Nagase H., Tseng L.F. Activation of G-proteins in the mouse pons/medulla by beta-endorphin is mediated by the stimulation of μ- and putative ɛ-receptors. Life Sci. 2000;67:2733–2743. doi: 10.1016/s0024-3205(00)00852-3. [DOI] [PubMed] [Google Scholar]
- Obara I., Makuch W., Spetea M., Schütz J., Schmidhammer H., Przewlocki R., Przewlocka B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur. J. Pharmacol. 2007;558:60–67. doi: 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Pasternak G.W., Bodnar R.J., Clark J.A., Inturrisi C.E. Morphine-6-glucuronide, a potent μ agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- Portoghese P.S., Lipkowski A.W., Takemori A.E. Binaltorphimine and nor-binaltorphimine, potent and selective κ-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Portoghese P.S., Sultana M., Takemori A.E. Naltrindole, a highly selective and potent non-peptide δ-opioid receptor antagonist. Eur. J. Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Portoghese P.S., Sultana M., Nagase H., Takemori A.E. Application of the message-address concept. In the design of highly potent and selective non-peptide δ opioid receptor antagonists. J. Med. Chem. 1988;31:281–282. doi: 10.1021/jm00397a001. [DOI] [PubMed] [Google Scholar]
- Riba P., Friedmann T., Király K.P., Al-Khrasani M., Sobor M., Asim M.F., Spetea M., Schmidhammer H., Furst S. Novel approach to demonstrate high efficacy of micro opioids in the rat vas deferens: a simple model of predictive value. Brain Res. Bull. 2010;81:178–184. doi: 10.1016/j.brainresbull.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Schmidhammer H., Aeppli L., Atwell L., Fritsch F., Jacobson A.E., Nebuchla M., Sperk G. Synthesis and biological evaluation of 14-alkoxymorphinans. 1. Highly potent opioid agonists in the series of (−)-14-methoxy-N-methylmorphinan-6-ones. J. Med. Chem. 1984;27:1575–1579. doi: 10.1021/jm00378a009. [DOI] [PubMed] [Google Scholar]
- Schmidhammer H., Schratz A., Mitterdorfer J. Synthesis and biological evaluation of 14-alkoxymorphinans. 8. 14-Methoxymetopon, an extremely potent opioid agonist. Helv. Chim. Acta. 1990;73:1784–1787. [Google Scholar]
- Schmidt H., Vormfelde S., Klinder K., Gundert-Remy U., Gleiter C.H., Skopp G., Aderjan R., Fuhr U. Affinities of dihydrocodeine and its metabolites to opioid receptors. Pharmacol. Toxicol. 2002;91:57–63. doi: 10.1034/j.1600-0773.2002.910203.x. [DOI] [PubMed] [Google Scholar]
- Schütz J., Spetea M., Koch M., Aceto M.D., Harris L.S., Coop A., Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 20. 14-Phenylpropoxymetopon—an extremely powerful analgesic. J. Med. Chem. 2003;46:4182–4187. doi: 10.1021/jm030878b. [DOI] [PubMed] [Google Scholar]
- Spetea M., Friedmann T., Riba P., Schütz J., Wunder G., Langer T., Schmidhammer H., Fürst S. In vitro opioid activity profiles of 6-amino acid substituted derivatives of 14-O-methyloxymorphone. Eur. J. Pharmacol. 2004;483:301–308. doi: 10.1016/j.ejphar.2003.10.049. [DOI] [PubMed] [Google Scholar]
- Spetea M., Greiner E., Aceto M.D., Harris L.S., Coop A., Schmidhammer H. Effect of a 6-cyano substituent in 14-oxygenated N-methylmorphinans on opioid receptor binding and antinociceptive potency. J. Med. Chem. 2005;48:5052–5055. doi: 10.1021/jm0580205. [DOI] [PubMed] [Google Scholar]
- Spetea M., Nevin S.T., Hosztafi S., Ronai A.Z., Toth G., Borsodi A. Affinity profiles of novel δ-receptor selective benzofuran derivatives of non-peptide opioids. Neurochem. Res. 1998;23:1211–1216. doi: 10.1023/a:1020738304036. [DOI] [PubMed] [Google Scholar]
- Spetea M., Schüllner F., Moisa R.C., Berzetei-Gurske I.P., Schraml B., Dörfler C., Aceto M.D., Harris L.S., Coop A., Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 21. Novel 4-alkoxy and 14-phenylpropoxy derivatives of the μ opioid receptor antagonist cyprodime. J. Med. Chem. 2004;47:3242–3247. doi: 10.1021/jm031126k. [DOI] [PubMed] [Google Scholar]
- Spetea M., Tóth F., Schütz J., Ötvös F., Tóth G., Benyhe S., Borsodi A., Schmidhammer H. Binding characteristics of [3H]14-methoxymetopon, a high affinity μ-opioid receptor agonist. Eur. J. Neurosci. 2003;18:290–295. doi: 10.1046/j.1460-9568.2003.02744.x. [DOI] [PubMed] [Google Scholar]
- Urigüen L., Fernandez B., Romero E.M., De Pedro N., Delgado M.J., Guaza C., Schmidhammer H., Viveros M. Effects of 14-methoxymetopon, a potent opioid agonist, on the responses to the tail electric stimulation test and plus-maze activity in male rats: neuroendocrine correlates. Brain Res. Bull. 2002;57:661–666. doi: 10.1016/s0361-9230(01)00760-2. [DOI] [PubMed] [Google Scholar]
- Thompson C.M., Wojno H., Greiner E., May E.L., Rice K.C., Selley D.E. Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at μ- and δ-opioid receptors. J. Pharmacol. Exp. Ther. 2004;308:547–554. doi: 10.1124/jpet.103.058602. [DOI] [PubMed] [Google Scholar]
- Vankova M.E., Weinger M.B., Chen D.Y., Brian J.B., Motis V., Koob G.F. Role of central μ, δ and κ-1 opioid receptors in opioid-induced muscle rigidity in the rat. Anesthesiology. 1996;85:574–583. doi: 10.1097/00000542-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Zernig G., Saria A., Krassnig R., Schmidhammer H. Signal transduction efficacy of the highly potent μ opioid agonist 14-methoxymetopon. Life Sci. 2000;66:1871–1877. doi: 10.1016/s0024-3205(00)00510-5. [DOI] [PubMed] [Google Scholar]