Abstract
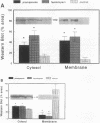
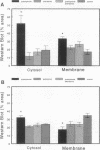
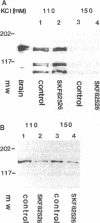
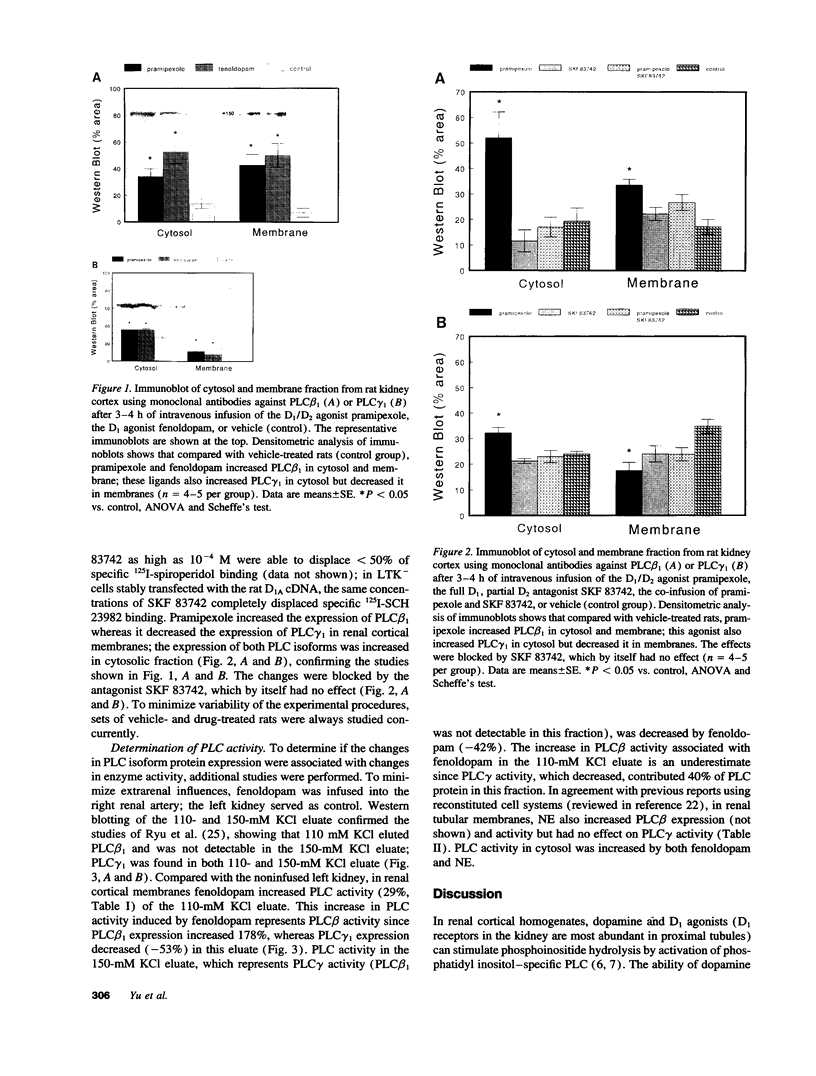
Dopamine and D1 agonists and NE all increase phosphatidyl inositol-specific phospholipase C (PLC) activity, but whereas dopamine produces a natriuresis, NE has an antinatriuretic effect. To determine if catecholamines differentially regulate the expression of PLC isoforms, we infused fenoldopam, a D1 agonist, or pramipexole, a D1/D2 agonist, intravenously or infused fenoldopam or NE into the renal artery of anesthetized rats. After 3-4 h of infusion, when the expected natriuresis (fenoldopam or pramipexole) or antinatriuresis (NE) occurred, the kidneys were removed for analysis of PLC isoform protein expression activity. Western blot analysis revealed that in renal cortical membranes, fenoldopam and pramipexole increased expression of PLC beta 1 and decreased expression of PLC gamma 1; PLC delta was unchanged. In the cytosol, pramipexole and fenoldopam increased expression of both PLC beta 1 and PLC gamma 1. No effects were noted in the medulla. A preferential D1 antagonist, SKF 83742, which by itself had no effect, blocked the effects of pramipexole, thus confirming the involvement of the D1 receptor. In contrast, NE also increased PLC beta 1 but did not affect PLC gamma 1 protein expression in membranes. The changes in PLC isoform expression were accompanied by similar changes in PLC isoform activity. These studies demonstrate for the first time differential regulation of PLC isoforms by catecholamines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Ibarra F., Svensson L. B., Klee C., Greengard P. Calcineurin mediates alpha-adrenergic stimulation of Na+,K(+)-ATPase activity in renal tubule cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7394–7397. doi: 10.1073/pnas.89.16.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berstein G., Blank J. L., Smrcka A. V., Higashijima T., Sternweis P. C., Exton J. H., Ross E. M. Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-beta 1. J Biol Chem. 1992 Apr 25;267(12):8081–8088. [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Na+-K+-ATPase is an effector protein for protein kinase C in renal proximal tubule cells. Am J Physiol. 1989 Feb;256(2 Pt 2):F370–F373. doi: 10.1152/ajprenal.1989.256.2.F370. [DOI] [PubMed] [Google Scholar]
- Blank J. L., Brattain K. A., Exton J. H. Activation of cytosolic phosphoinositide phospholipase C by G-protein beta gamma subunits. J Biol Chem. 1992 Nov 15;267(32):23069–23075. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown G., Douglas J. Effect of angiotensin II infusion in rats on Na,K-ATPase activity in renal cortical microsomal preparations. Arch Biochem Biophys. 1989 Nov 15;275(1):236–243. doi: 10.1016/0003-9861(89)90369-x. [DOI] [PubMed] [Google Scholar]
- Carter H. R., Wallace M. A., Fain J. N. Purification and characterization of PLC-beta m, a muscarinic cholinergic regulated phospholipase C from rabbit brain membrane. Biochim Biophys Acta. 1990 Aug 13;1054(1):119–128. doi: 10.1016/0167-4889(90)90213-w. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Chabre O., Wong Y. H., Federman A. D., Bourne H. R. Recombinant Gq alpha. Mutational activation and coupling to receptors and phospholipase C. J Biol Chem. 1992 Jan 5;267(1):31–34. [PubMed] [Google Scholar]
- Felder C. C., Albrecht F. E., Campbell T., Eisner G. M., Jose P. A. cAMP-independent, G protein-linked inhibition of Na+/H+ exchange in renal brush border by D1 dopamine agonists. Am J Physiol. 1993 Jun;264(6 Pt 2):F1032–F1037. doi: 10.1152/ajprenal.1993.264.6.F1032. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Blecher M., Jose P. A. Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem. 1989 May 25;264(15):8739–8745. [PubMed] [Google Scholar]
- Felder C. C., Jose P. A., Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989 Jan;248(1):171–175. [PubMed] [Google Scholar]
- Gingrich J. A., Caron M. G. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Hedge S. S., Ricci A., Amenta F., Lokhandwala M. F. Evidence from functional and autoradiographic studies for the presence of tubular dopamine-1 receptors and their involvement in the renal effects of fenoldopam. J Pharmacol Exp Ther. 1989 Dec;251(3):1237–1245. [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Albrecht F., Asico L. D., Eisner G. M., Robillard J. E., Jose P. A. Ontogeny of DA1 receptor-mediated natriuresis in the rat: in vivo and in vitro correlations. Am J Physiol. 1992 Sep;263(3 Pt 2):R631–R638. doi: 10.1152/ajpregu.1992.263.3.R631. [DOI] [PubMed] [Google Scholar]
- Liu Y. F., Civelli O., Zhou Q. Y., Albert P. R. Cholera toxin-sensitive 3',5'-cyclic adenosine monophosphate and calcium signals of the human dopamine-D1 receptor: selective potentiation by protein kinase A. Mol Endocrinol. 1992 Nov;6(11):1815–1824. doi: 10.1210/mend.6.11.1282671. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Wilson P. B., Blackmore P. F., Exton J. H. The hormone-sensitive hepatic Na+-pump. Evidence for regulation by diacylglycerol and tumor promoters. J Biol Chem. 1986 Nov 5;261(31):14551–14556. [PubMed] [Google Scholar]
- Mah S. J., Ades A. M., Mir R., Siemens I. R., Williamson J. R., Fluharty S. J. Association of solubilized angiotensin II receptors with phospholipase C-alpha in murine neuroblastoma NIE-115 cells. Mol Pharmacol. 1992 Aug;42(2):217–226. [PubMed] [Google Scholar]
- Mahan L. C., Burch R. M., Monsma F. J., Jr, Sibley D. R. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero M. B., Paxton W. G., Duff J. L., Berk B. C., Bernstein K. E. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-gamma 1 in vascular smooth muscle cells. J Biol Chem. 1994 Apr 8;269(14):10935–10939. [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Ohnishi J., Ishido M., Shibata T., Inagami T., Murakami K., Miyazaki H. The rat angiotensin II AT1A receptor couples with three different signal transduction pathways. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1094–1101. doi: 10.1016/0006-291x(92)90859-j. [DOI] [PubMed] [Google Scholar]
- Park D., Jhon D. Y., Kriz R., Knopf J., Rhee S. G. Cloning, sequencing, expression, and Gq-independent activation of phospholipase C-beta 2. J Biol Chem. 1992 Aug 15;267(23):16048–16055. [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Multiple forms of phospholipase C isozymes and their activation mechanisms. Adv Second Messenger Phosphoprotein Res. 1992;26:35–61. [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Ribeiro C. P., Mandel L. J. Parathyroid hormone inhibits proximal tubule Na(+)-K(+)-ATPase activity. Am J Physiol. 1992 Feb;262(2 Pt 2):F209–F216. doi: 10.1152/ajprenal.1992.262.2.F209. [DOI] [PubMed] [Google Scholar]
- Rodrigues P. dos S., Dowling J. E. Dopamine induces neurite retraction in retinal horizontal cells via diacylglycerol and protein kinase C. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9693–9697. doi: 10.1073/pnas.87.24.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12511–12518. [PubMed] [Google Scholar]
- Satoh T., Cohen H. T., Katz A. I. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. Am J Physiol. 1993 Sep;265(3 Pt 2):F399–F405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- Schelling J. R., Hanson A. S., Marzec R., Linas S. L. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992 Dec;90(6):2472–2480. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Hamad D., Wang Y. P., Jo O. D., Yanagawa N. Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol. 1993 Apr;264(4 Pt 2):F737–F743. doi: 10.1152/ajprenal.1993.264.4.F737. [DOI] [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Smrcka A. V., Sternweis P. C. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C beta by G protein alpha and beta gamma subunits. J Biol Chem. 1993 May 5;268(13):9667–9674. [PubMed] [Google Scholar]
- Spiegel A. M., Shenker A., Weinstein L. S. Receptor-effector coupling by G proteins: implications for normal and abnormal signal transduction. Endocr Rev. 1992 Aug;13(3):536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Choi W. C., Lee K. Y., Rhee S. G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988 Oct 5;263(28):14497–14504. [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Undie A. S., Friedman E. Selective dopaminergic mechanism of dopamine and SKF38393 stimulation of inositol phosphate formation in rat brain. Eur J Pharmacol. 1992 Aug 3;226(4):297–302. doi: 10.1016/0922-4106(92)90046-x. [DOI] [PubMed] [Google Scholar]
- Vyas S. J., Eichberg J., Lokhandwala M. F. Characterization of receptors involved in dopamine-induced activation of phospholipase-C in rat renal cortex. J Pharmacol Exp Ther. 1992 Jan;260(1):134–139. [PubMed] [Google Scholar]
- Vyas S. J., Jadhav A. L., Eichberg J., Lokhandwala M. F. Dopamine receptor-mediated activation of phospholipase C is associated with natriuresis during high salt intake. Am J Physiol. 1992 Mar;262(3 Pt 2):F494–F498. doi: 10.1152/ajprenal.1992.262.3.F494. [DOI] [PubMed] [Google Scholar]
- Wange R. L., Smrcka A. V., Sternweis P. C., Exton J. H. Photoaffinity labeling of two rat liver plasma membrane proteins with [32P]gamma-azidoanilido GTP in response to vasopressin. Immunologic identification as alpha subunits of the Gq class of G proteins. J Biol Chem. 1991 Jun 25;266(18):11409–11412. [PubMed] [Google Scholar]
- Wu D., Katz A., Lee C. H., Simon M. I. Activation of phospholipase C by alpha 1-adrenergic receptors is mediated by the alpha subunits of Gq family. J Biol Chem. 1992 Dec 25;267(36):25798–25802. [PubMed] [Google Scholar]