Abstract
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular hypertrophy, increased ventricular stiffness and impaired diastolic filling. We investigated to what extent myocardial functional defects can be explained by alterations in the passive and active properties of human cardiac myofibrils. Skinned ventricular myocytes were prepared from patients with obstructive HCM (two patients with MYBPC3 mutations, one with a MYH7 mutation, and three with no mutation in either gene) and from four donors. Passive stiffness, viscous properties, and titin isoform expression were similar in HCM myocytes and donor myocytes. Maximal Ca2+-activated force was much lower in HCM myocytes (14 ± 1 kN/m2) than in donor myocytes (23 ± 3 kN/m2; P < 0.01), though cross-bridge kinetics (ktr) during maximal Ca2+ activation were 10% faster in HCM myocytes. Myofibrillar Ca2+ sensitivity in HCM myocytes (pCa50 = 6.40 ± 0.05) was higher than for donor myocytes (pCa50 = 6.09 ± 0.02; P < 0.001) and was associated with reduced phosphorylation of troponin-I (ser-23/24) and MyBP-C (ser-282) in HCM myocytes. These characteristics were common to all six HCM patients and may therefore represent a secondary consequence of the known and unknown underlying genetic variants. Some HCM patients did however exhibit an altered relationship between force and cross-bridge kinetics at submaximal Ca2+ concentrations, which may reflect the primary mutation. We conclude that the passive viscoelastic properties of the myocytes are unlikely to account for the increased stiffness of the HCM ventricle. However, the low maximum Ca2+-activated force and high Ca2+ sensitivity of the myofilaments are likely to contribute substantially to any systolic and diastolic dysfunction, respectively, in hearts of HCM patients.
Keywords: Hypertrophic cardiomyopathy, Skinned cardiac myocytes, Viscoelasticity, Ca2+ sensitivity, Cross-bridge kinetics
Research Highlights
► The passive stiffness of skinned HCM cardiac myocytes was similar to that of normal (donor) myocytes. ► Maximum Ca-activated force production was reduced by 40% in HCM vs donor myocytes. ► This loss of force could contribute to systolic dysfunction in HCM hearts. ► Myofibrillar Ca sensitivity was higher in HCM than in donor myocytes. ► The enhanced Ca sensitivity could compensate for the smaller maximum force but would tend to cause diastolic dysfunction. ► These characteristics were common to all HCM patients studied, suggesting the changes were secondary consequence of the underlying genetic variants.
1. Introduction
Hypertrophic cardiomyopathy (HCM) is a genetically-determined disease that affects 1 in 500 people and which is characterized by ventricular hypertrophy, interstitial fibrosis, myocyte disarray and diastolic dysfunction [1]. Its clinical outcomes range from entirely asymptomatic disease to chronic progressive heart failure, arrhythmias and sudden cardiac death [2]. Molecular genetic studies have led to the concept of HCM as a disease of the sarcomere, since almost all of the known HCM disease genes encode sarcomeric proteins, in particular MYH7 (which encodes β-myosin heavy chain, β-MyHC) and MYBPC3 (encoding cardiac myosin binding protein-C, cMyBP-C), with each accounting for about one-third of the known HCM-associated mutations [1–3]. However, it remains the case that 40–60% of HCM patients screened appear to have no mutation in any sarcomeric protein so far implicated in HCM [4,5].
The mechanisms linking myocardial dysfunction in human HCM, either directly or indirectly, with changes in sarcomeric proteins are unclear. The characteristic diastolic dysfunction (compromised myocardial relaxation and passive filling) is largely a consequence of increased ventricular stiffness, which may be due to the ventricular hypertrophy, disarray of myocytes, interstitial fibrosis, or possible myocardial ischaemia [3,6]. However, there could also be an increased intrinsic stiffness of the myocytes. Myocyte passive stiffness is largely due to titin in the sarcomere [7–9] and an increase in the proportion of the stiffer (N2B) isoform of titin relative to the more compliant (N2BA) isoform was reported in an animal model of hypertrophy [10] and in patients exhibiting diastolic heart failure with concentric LV hypertrophy [11]. In addition, myocyte viscoelasticity, which would contribute to the dynamic stiffness of the myocardium during chamber filling, was increased in pressure-overload hypertrophy in animal models [12]. However there has been no detailed investigation of the passive viscoelasticity of human myocytes to examine whether this may contribute to the increased stiffness of the HCM myocardium.
Systolic performance too is often compromised in HCM, particularly during tachycardia. Cardiac output may be reduced by the impairment of diastolic filling. In addition, in approximately 25% of HCM patients there is upper septal hypertrophy associated with mitral valve dysfunction (systolic anterior motion), which obstructs the outflow tract (hypertrophic obstructive cardiomyopathy, HOCM). It is not clear whether there are also changes in the contractile properties of myocytes that could either contribute to, or help to compensate for, the altered cardiac function in HCM. Results from studies with transgenic animals or isolated proteins have generally reported that HCM is associated with increased myofilament Ca2+ sensitivity, with variable effects on maximum force or ATPase activity [13], though a common (and perhaps unifying) feature is an increased energetic cost of contraction [14]. In the only previous study (to our knowledge) with myocytes from human HCM hearts, permeabilized (“skinned”) ventricular myocytes from patients with one of two truncation mutations in the MYBPC3 gene showed a reduced maximum steady-state force production but elevated myofibrillar Ca2+ sensitivity [15]. However, it was not clear whether these changes were specific to the two MYBPC3 mutations studied or are a general characteristic of the myocardium in all HCM patients. Furthermore, cross-bridge cycling kinetics were not determined, so no inference could be made about the dynamics of myocardial contraction and relaxation in HCM.
To investigate the role of the myocytes in determining the passive and active properties of the HCM myocardium, we examined in detail the steady-state and dynamic characteristics of passive stiffness and active force production in the myocytes from a representative group of HCM patients (with mutations in MYBPC3, MYH7, or neither gene), compared with myocytes from non-diseased hearts. By comparing results between individual patients, we explored whether the changes in contractile phenotype were gene-specific or were common to all the HCM patients. While passive viscoelasticity was not changed significantly, we found consistent alterations in the active properties of the HCM myocytes that likely contribute to the pathophysiology of the HCM myocardium. Most of these changes are likely to be secondary consequences of the disease process.
2. Methods
Methods were modified from those described previously [16]. Details are given in the online Data Supplement. In brief, hypertrophied tissue was obtained from septal tissue removed during surgical myectomy in HCM patients and donor tissue was obtained from the LV free wall of unused donor hearts [17]. All tissue samples were flash-frozen in liquid N2. It is unlikely that HCM patients are a homogeneous population (due to their different genotypes, drug treatments, co-morbidities, etc.), so we studied sufficient myocytes from each patient to allow comparison between individual patients.
Mutation screening of the coding regions and splice sites of MYH7 and MYBPC3, the predominant HCM-associated genes, was carried out [18]. Mutations were identified in patients M15 (MYBPC3 T2604A+C deletion at 2605, predicted to encode a C-terminally truncated protein), MA (MYBPC3 R502W), and ML (MYH7 R719Q), but the other three HCM patients had no mutations in either gene.
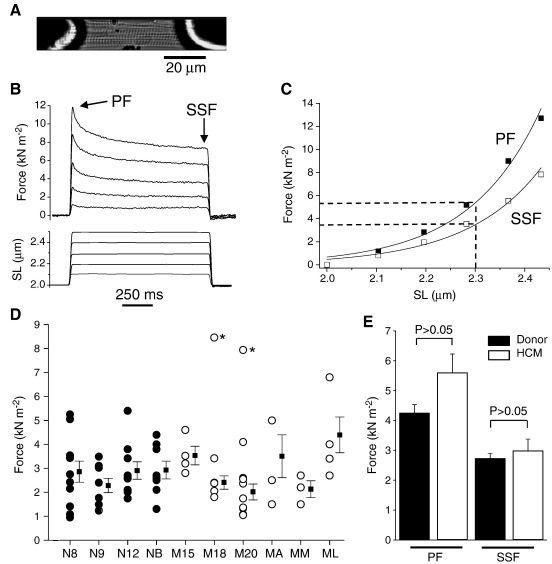
Single skinned myocytes were prepared from the frozen tissue, were glued between a force transducer and a motor (Fig. 1A) and set to a sarcomere length (SL) of 2.0 μm in relaxing solution at 15 °C. SL was measured by online video analysis. Myocyte passive viscoelasticity and its SL dependence were measured using step increases of cell length of 1 s duration (Fig. 1B). Each stretch induced a rapid rise in force to a peak, followed by a slower decay in force (stress relaxation) to reach a quasi-steady-state force (here termed “steady-state force”) after 1 s. Stress relaxation is due to the viscoelastic properties of the myocyte (i.e. of titin chiefly), while steady-state force is determined by its elastic properties [19]. Since the SL reached in each length step varied from myocyte to myocyte, we compared the force–SL extension curves of different myocytes in two ways. In the first (Fig. 1C), we used the measured force–SL relationship in each myocyte to estimate the force at SL = 2.3 μm by interpolation. This force was used as a measure of stiffness in each myocyte. In the second approach we grouped the values of SL reached in all myocytes during the length step into bins of 0.1 μm wide; this was used to determine the mean force–SL relationship and the parameters of stress relaxation (Fig. 2).
Fig. 1.
Passive force in skinned myocytes. (A) Typical donor skinned myocyte attached to the force transducer and servomotor. (B) Example of stretch protocol and force response. PF, peak force; SSF, steady-state force. (C) Force–SL extension curves in a typical myocyte. Stiffness was assessed from the force at SL = 2.3 μm. (D) Passive stiffness (here measured from steady-state force) in 33 individual myocytes from 4 donor hearts (filled circles) and 30 from 6 HCM hearts (open circles). Squares show mean ± SEM for each heart. Asterisks indicate myocytes excluded from the calculation of means. (E) Mean stiffness data from peak force and steady-state force measurements in human donor myocytes (n = 4) and HCM myocytes (n = 6).
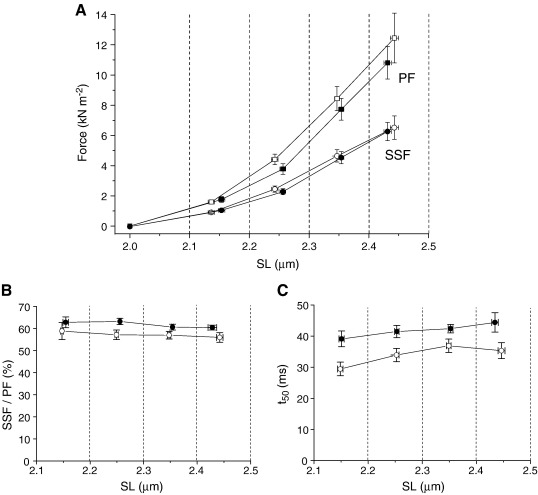
Fig. 2.
Sarcomere length dependence of passive force and viscoelasticity in skinned donor myocytes (filled symbols) and HCM myocytes (open symbols). Data were grouped into 0.1-μm-wide ‘bins’ according to the SL reached during the length step and the averages were calculated for each heart. Symbols show mean ± SE from 4 donor and 6 HCM hearts. (A) Peak and steady-state forces. (B) Amplitude of force decay during stress relaxation, expressed as the steady-state force divided by the peak force. (C) Mean half-time of force decay (t50).
Ca2+-activated force and the rate constant of force redevelopment (ktr) were determined during maintained Ca2+ activation of the myocytes. The ktr value is an indirect measure of cross-bridge cycling kinetics that reflects the rate of cross-bridge reattachment and transition to the force-generating state(s) [20]. The activated myocyte was subjected to a rapid release/restretch protocol and ktr was determined from a single exponential fit to the force redevelopment trace. Maximal force was normalized to myocyte cross-sectional area.
Force and ktr data were averaged from 3–10 myocytes from each heart and this mean value was taken as a single datum point for the calculation of the overall means. Unless stated otherwise, data points show mean ± SE for tissue from n = 4 donor hearts and n = 6 HCM hearts.
3. Results
3.1. Passive stiffness and stress relaxation
Passive force was compared in skinned human donor and HCM myectomy myocytes that were subject to a series of step stretches (Fig. 1B). From the resulting force–SL relationship for each myocyte (e.g. Fig. 1C) we estimated the peak and steady-state forces at 2.3 μm. Fig. 1D shows the results for steady-state force in individual cells from each donor and HCM patient. There was some variability between myocytes taken from the same patient. Two myocytes had atypically high stiffness, possibly due to adherent collagen (see later) and were excluded from further analysis. The mean data (Fig. 1E) showed no significant difference between the stiffness of HCM and donor myocytes, whether measured from peak force or steady-state force.
To examine the SL dependence of the time-independent (elastic) and time-dependent (viscoelastic) components of the force response, we grouped the SL values reached during the length steps into 0.1-μm-wide bins (Fig. 2). The resulting force–SL relationships confirmed that the steady-state force increase was the same in HCM and donors over the entire SL range, although there was a (non-significant) tendency for peak force to be higher in the HCM than donor myocytes at SLs > 2.15 μm. The viscoelastic properties of the myocytes were assessed from the rate and extent of stress relaxation during the 1 s length step. Viscoelastic behavior caused passive force to fall by 40% during this step (Fig. 2B). The half-time (t50) and relative magnitude of stress relaxation were found to be independent of SL and were not significantly different between donor and HCM myocytes (Fig. 2B and C), although there was a trend for t50 to be smaller (faster stress relaxation) in HCM myocytes. We conclude that myocyte viscoelastic properties were not altered significantly in the HCM myocytes.
Titin is the major determinant of the passive viscoelasticity of myocytes [7–9]. Titin protein isoform expression in the human donor and HCM myocardium was therefore analyzed (Supplementary Data). As found previously, human tissue contained both the N2B and N2BA isoforms. The relative proportion of N2B (the shorter, stiffer isoform) was not significantly different in HCM and donor myocardium (69 ± 2%, n = 6 and 61 ± 2%, n = 4, respectively). There was also no difference between HCM and donor myocardium in terms of titin degradation, measured by the T2 band density relative to total titin (20 ± 5% in HCM vs. 23 ± 5% in donor) and total titin protein, expressed relative to the total expression of MyHC protein (0.53 ± 0.02 vs. 0.47 ± 0.02, P = 0.93).
3.2. Maximum Ca2+-activated force and cross-bridge kinetics
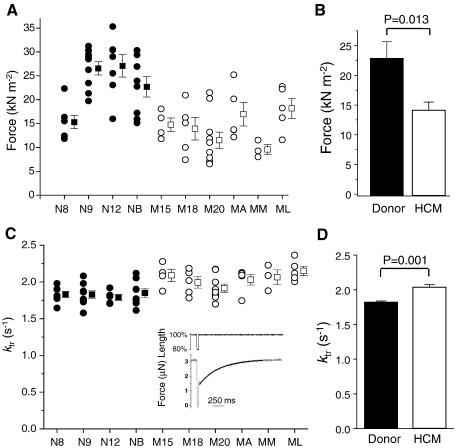
Fig. 3 shows the maximum Ca2+-activated force production for the individual myocytes from donor and HCM hearts (panel A) and the averaged data (panel B). Maximal isometric force in HCM myocytes (14.2 ± 1.3 kN/m2) was 40% smaller than in donor myocytes (23.4 ± 4.0 kN/m2). Myocytes from donor N8 exhibited a smaller force than the other donors but we found no other functional or biochemical differences in N8 compared with the other donors, so this sample was included in the calculation of mean force (Fig. 3B); without this inclusion, the difference in maximum force between HCM and donor myocytes would have been even greater.
Fig. 3.
Isometric force and cross-bridge kinetics in skinned myocytes at maximal Ca2+ activation (pCa 4.5). (A) Data from individual myocytes (circles) and mean data (squares). Symbols as in Fig. 1. (B) Mean force data (n = 4 donor hearts and 6 HCM hearts). (C) Rate constant of force redevelopment (ktr). Inset: example of force redevelopment in a skinned donor myocyte after rapid release/restretch, and fitted single exponential curve. (D) Mean ktr data.
We investigated possible reasons for this large loss of force in HCM myocytes compared with donor myocytes. One possibility was that the kinetics of cross-bridge cycling were altered substantially, e.g. force would be reduced if attachment of cross-bridges to actin were slower, or the detachment faster. To assess cross-bridge kinetics, we measured the rate of force redevelopment (ktr) after forced detachment of the cross-bridges. The ktr values at pCa 4.5 were consistent within individual patients (Fig. 3C) and were 10% faster in HCM myocytes than in donor myocytes (Fig. 3D). The force and ktr data are consistent with a faster cross-bridge detachment, rather than slower attachment, in HCM myocytes (see Discussion).
3.3. Structure of myocytes
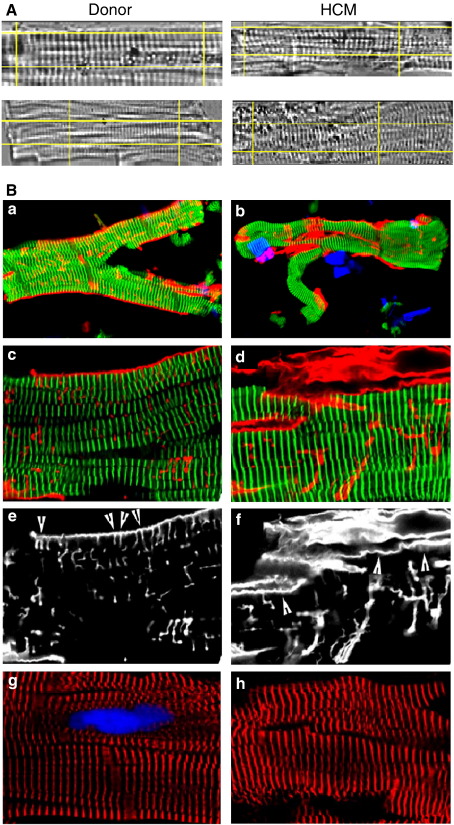
Another possible reason for the reduced force was disruption or loss of myofibrils in HCM myocytes. Videomicroscopy of skinned myocytes (Fig. 4A) revealed a somewhat distorted striation pattern in HCM myocytes and a slightly shorter resting SL (1.73 ± 0.01 μm) than in donor myocytes (1.78 ± 0.01 μm; n = 4, 21 myocytes each; P < 0.001). Confocal microscopy (Fig. 4B) confirmed the slight disorder of the sarcomeres in HCM myocytes. However, staining with antibodies against titin or sarcomeric α-actinin provided no evidence for fewer myofibrils in the HCM myocytes, although precise quantitative studies could not be carried out. This suggests that the large reduction of maximum force could not be explained by loss of myofibrils.
Fig. 4.
Structure of typical donor myocytes (patient N12) and HCM myocytes (patient M15). (A) Myocyte video images from the computer screen. (B) Confocal images using primary antibodies as follows: a–d (a and b overview; c and d high magnification), polyclonal rabbit anti-titin m8 to visualize the myofibrils (green), monoclonal mouse anti-collagen IV to stain for extracellular matrix (red), DAPI (blue) for nucleus; e and f, collagen IV (same field as c and d); g and h, sarcomeric α-actinin (red), nucleus (blue). HCM myocytes showed increased collagen IV around the myocytes but an absence of the regular arrangement of collagen IV seen on the surface of donor myocytes (compare arrowheads in e and f).
Interestingly, in the donor samples collagen IV fibers were often associated intimately with the myocytes and exhibited a partially striated pattern at the level of the Z-disc, reflecting the arrangement of costameres (arrowheads, Fig. 4B, panels e and f). It is possible that similar collagen, running along the length of the myocyte, may have accounted for the abnormally high stiffness seen in some myocytes in Fig. 1D. Although there was generally more collagen IV in the HCM myocytes, this tended to be found in clumps and the association with costameric structures was much less pronounced than in donor myocytes.
3.4. Ca2+ sensitivity of force and cross-bridge cycling kinetics
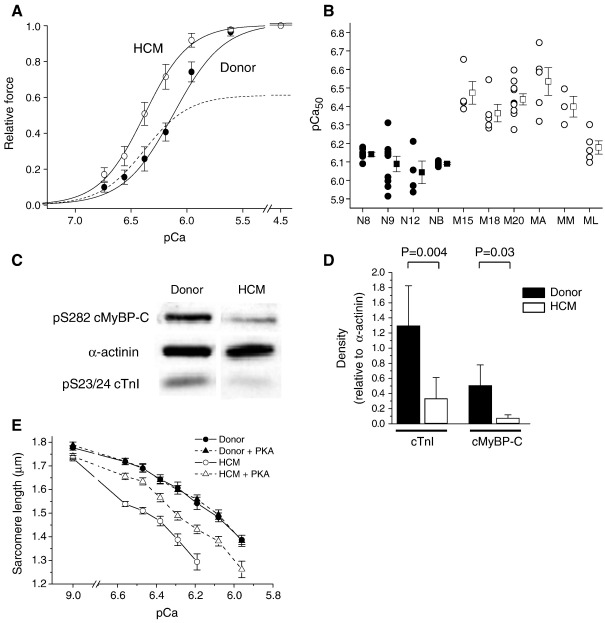
In intact cardiac myocytes the Ca2+ transient does not activate the myofibrils fully, so it is essential to determine myofibrillar properties at submaximal levels of activation. The Ca2+ sensitivity was consistently higher for the HCM skinned myocytes than for the donors, as shown by the average force–pCa curves (Fig. 5A) and the individual pCa50 values (Fig. 5B). The mean pCa50 values were 6.40 ± 0.05 (n = 6) for HCM myocytes and 6.09 ± 0.02 (n = 4) for donor (P = 0.001), while the steepness of the force–pCa relationship was unchanged (nH values of 3.73 ± 0.56 and 2.95 ± 0.12, respectively; P > 0.05). This increase in Ca2+ sensitivity causes the inhibitory effect of the reduced maximum force to be overcome for pCa values above ~ 6.2 (dashed line, Fig. 5A).
Fig. 5.
Ca2+ sensitivity of force in donor myocytes (filled symbols) and HCM myocytes (open symbols). (A) Mean force–pCa curves for the 4 donor patients (28 myocytes) and HCM patients (32 myocytes). Force was normalized to the maximum force (pCa 4.5). Dashed line shows the mean HCM curve factoring in the depression of maximum force. (B) Individual and mean pCa50 values for the individual patients. (C) Representative Western blots for phosphorylated cTnI (ser-23/24), cMyBP-C (ser-282) and α-actinin (loading control) in one donor and one HCM sample. (D) Mean data. (E) Mean shortening–pCa relationships in unrestrained myocytes (n = 14–16 myocytes per group).
Myofibrillar Ca2+ sensitivity is influenced by protein kinase A (PKA)-mediated phosphorylation of troponin-I and possibly MyBP-C. Analysis using phosphorylation-specific antibodies revealed that phosphorylation of TnI (ser-23/24) sites and of MyBP-C (ser282) were significantly lower in HCM myocardium than in donor myocytes (Fig. 5C and D). To investigate whether the lower phosphorylation could account for the higher Ca2+ sensitivity of the HCM myocytes, we compared the Ca2+-shortening relationships in skinned, unattached myocytes with and without incubation in PKA. With HCM myocytes, PKA phosphorylated TnI and MyBP-C (see Data Supplement) and decreased myofibrillar Ca2+ sensitivity (Fig. 5E), whereas with donor myocytes there was little change in phosphorylation or myofibrillar Ca2+ sensitivity, leading to the difference in Ca2+ sensitivity between the HCM and donor myocytes being reduced by about one-half. Thus the differing Ca2+ sensitivity of the myocytes under basal conditions was partly due to differences in phosphorylation at PKA sites.
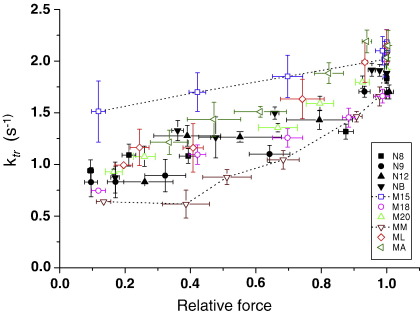
Fig. 6 shows how both cross-bridge kinetics (ktr) and force (i.e. the level of thin filament activation) changed as the bathing Ca2+ concentration was altered. As found previously with human myofibrils [16], ktr increased as [Ca2+] (and force) was raised, and this was true for both donor and HCM myocytes. The ktr–force relationships for the HCM patients at submaximal Ca2+ concentrations were generally similar to those for donor myocytes. However, patient M15 noticeably showed greater ktr values at submaximal force levels (i.e. faster cross-bridge kinetics for a given level of thin filament activation), whereas patient MM tended to have the slowest kinetics. Overall, there was no consistent difference between the Ca2+ regulation of cross-bridge kinetics between HCM and donor patients.
Fig. 6.
Relationship between cross-bridge cycling kinetics and force in donor myocytes (filled symbols) and HCM myocytes (open symbols) at different levels of Ca2+ activation. Each point shows the force and ktr values (with standard errors) measured in myocytes from the same heart at each Ca2+ concentration. Averages of 4 donor hearts and 6 HCM hearts. The force–ktr relationships were similar in donor and HCM myocytes, except for myocytes from two HCM patients (dashed lines).
3.5. Analysis of contractile proteins
Analysis of 12.5% polyacrylamide gels showed that, relative to actin, the expression of TnT, MLC-2 and MyHC protein did not differ significantly between donor myocardium and HCM myocardium, nor did the phosphorylation level of MLC-2 (see Supplement).
4. Discussion
This study is the first to examine both the steady-state and dynamic properties of passive viscoelasticity and Ca2+-activated contractions in skinned myocytes from patients with obstructive HCM, although van Dijk et al. [15] have previously reported steady-state force measurements in myocytes from a set of HCM patients with truncation mutations in the MYBPC3 gene. Our HCM unselected cohort contained two patients with mutations in MYBPC3, one with a mutation in MYH7, and three with no mutation in either gene. These ratios are representative of HCM patients in general, in whom mutations in MYBPC3 and MYH7 have been found in 16–26% and 14–19%, respectively, of patients, while 40–60% of patients had no mutation in the genes for the main sarcomeric proteins [4,5]. Furthermore, we obtained sufficient data from individual patients to allow us to compare between them, in order to examine whether the changes we observed were specific to the particular MYBPC3 or MYH7 mutation, or were common to all the HCM patients. We found the passive elastic and viscous properties of the HCM myocytes were similar between patients and were not significantly different from those of donor myocytes. In contrast, the maximum Ca2+-activated force of myocytes from HCM patients was decreased by 40% compared with donor myocytes, while the Ca2+ sensitivity of force production was substantially greater. These functional changes, which could adversely affect both diastolic and systolic function, were seen in all our HCM patients, suggesting they are secondary consequences of the underlying genetic differences between patients. However there was some evidence that differences between the HCM patients could be revealed by cross-bridge cycling kinetics at submaximal Ca2+ activation.
4.1. Myocyte viscoelasticity
While measurements in vivo have shown an increase in chamber stiffness in the hypertrophied heart [21], it is not known whether this is due in part to changes in the passive properties of the myocytes. During diastolic filling myocyte length increases continuously, so the passive stiffness of the myocytes during this period depends upon both their elasticity (length-dependent) and viscoelasticity (velocity-dependent). Previous studies have reported that pressure-overload hypertrophy in animals and humans is associated with increased myocyte stiffness resulting from an increased titin N2B:N2BA ratio [10,11] or with increased myocyte viscosity resulting from microtubule proliferation [12]. Our results demonstrate that passive stiffness and viscosity were not significantly different between HCM and donor myocytes (Figs. 1 and 2). The passive viscoelastic behavior of the myocyte is thought to be due to the unfolding of titin's globular domains [9] and to titin's interactions with actin [22]. Consistent with the functional data, there was no significant difference in the titin N2B/N2BA isoform ratio between HCM and donor myocytes. Thus the myocytes from our HCM patients exhibited no enhancement of dynamic or static stiffness that could contribute to the increased diastolic stiffness characteristic of hypertrophied myocardium. This contrasts with other studies with ischemic or dilated cardiomyopathy, in which changes in stiffness and the titin isoform ratio were reported to either cause, or compensate for, changes in the elastic component of tissue stiffness [23]. We conclude that the higher stiffness of the HCM myocardium is due to factors other than myocyte viscoelasticity, such as the disarray of myocytes and increased tissue fibrosis [6].
4.2. Maximum force production
A major difference in the active properties of the HCM and donor myocytes was found with maximum Ca2+-activated force, which was decreased by about 40% in HCM compared with donor myocytes (Fig. 3). The large loss of force-producing capacity can account for the smaller contraction of intact myocytes from similar myectomy samples [17] and would tend to cause systolic dysfunction in the intact heart.
We found no change in protein content of the myofibrils that could explain this large loss of force. Although there was no evidence for a loss of myofibrils (Fig. 4), HCM myocytes did have a somewhat less organized sarcomere alignment than donor myocytes. Depending on the precise derangements at the molecular level, this might cause a loss of force, though further work is needed to explore this. Another possibility is that the decrease in maximal force was due to a reduction in the number of attached, force-producing cross-bridges per sarcomere, caused by either a smaller rate of cross-bridge attachment and transition to force-producing states (fapp) or a greater rate of detachment (gapp). Maximum ktr (which is ∝ fapp + gapp) [20] was ~ 10% faster in HCM myocytes (Fig. 3D), which is not consistent with a slower fapp. A faster gapp would however both increase ktr and decrease force. If we assume that 50% of cross-bridges (∝ fapp/[fapp + gapp]) are attached at maximal Ca2+ (so fapp = gapp), then the 10% increase in ktr could be caused by a 20% increase in gapp, which would lower force by 9%. Thus a faster gapp could explain some of the reduced force in HCM myocytes but there must be additional factors, such as fewer cycling cross-bridges or a decrease in the force produced per cross-bridge. Using myosin isolated from myectomies similar to ours, in an in vitro motility assay the fraction of filaments motile was reduced by 19% [17], which suggested a fall in the population of active myosins due to alterations in myosin itself; a similar fall in the number of active cross-bridges would contribute substantially to the decrease in force observed here.
An important question is whether the decrease in maximum force is directly due to the mutations in the HCM patients, or to secondary changes. This question cannot be answered definitively with human studies, since human tissue is necessarily obtained only during surgery on patients who are already symptomatic and in whom any primary defect has likely caused many secondary changes. Nevertheless, our finding (Figs. 3 and 5) that a similar contractile phenotype is seen in myocytes from six unrelated HCM patients (with different mutations in MYBPC3 or MYH7, or neither of these), measured under identical conditions, suggests that the contractile deficiency is a common consequence of HCM, secondary to the initial abnormalities produced by the known and unknown genetic variant(s) underlying the cardiomyopathy [17]. By analogy, we suggest that similar secondary changes may account for the reduced maximum force production observed in HCM myocytes with another MYBPC3 mutation [15] or in HCM myofibrils with the MYH7 R403Q mutation [24].
4.3. Myofibrillar Ca2+ sensitivity
HCM myocytes were more Ca2+-sensitive than donor myocytes, with pCa50 elevated by 0.31 units, corresponding to a 50% reduction in the [Ca2+] required for half-maximal activation of force (Fig. 5). This disparity was due, at least in part, to the very low levels of phosphorylation in TnI at ser-23/24 sites and MyBP-C at ser-282 (Fig. 5C and D), since the difference in Ca2+ sensitivity was much reduced after incubation in PKA (Fig. 5E). Whether the difference remaining after PKA reflects a primary influence of an underlying mutation, as has been found in animal models of HCM [13], remains to be established. Our results differ somewhat from those of van Dijk et al. [15], who reported a reduction in TnI phosphorylation but unchanged MyBP-C phosphorylation in HCM patients with truncation mutations in MYBPC3. van Dijk et al. suggested that the contractile dysfunction might be caused by the differential phosphorylation of TnI and MyBP-C, but this explanation is unlikely to apply to our patients, who exhibited reduced phosphorylation of both TnI and MyBP-C.
One caveat with all studies like these is that the phosphorylation status of the biopsies may be influenced by the drug treatment of the patients immediately prior to biopsy retrieval. In particular, the donor hearts may show elevated phosphorylation due to inotropic drug support prior to cardiac explantation [25], which may exaggerate the difference in Ca2+ sensitivity observed here. Nevertheless, it seems likely that the high Ca2+ sensitivity observed with HCM myocytes reflects the situation in vivo. This elevated Ca2+ sensitivity would tend to maintain contractile strength in the face of the reduced contractility (dashed line, Fig. 5A), which may help explain why systolic function may not be compromised in HCM patients. On the other hand, this increase in Ca2+-responsiveness would, by causing a large relative enhancement of force at Ca2+ concentrations just above threshold (Fig. 5A), tend to slow ventricular relaxation and enhance diastolic stiffness in intact myocytes. This myofilament-based factor could thus contribute to poor diastolic function of the HCM myocardium in vivo. Finally, a raised Ca2+ sensitivity may increase the risk of arrhythmias [26], which is a characteristic feature of HCM.
4.4. Cross-bridge kinetics
The cycling rate of activated cross-bridges is a major determinant of the kinetics of force development and relaxation in the myocardium [27], and is therefore a key factor regulating the contractility of the heart. Cross-bridge cycling kinetics (ktr) during maximal Ca2+ activation were 10% faster in HCM myocytes than in donors (Fig. 3D). This distinguishes the contractile phenotype from end-stage heart failure, where the cross-bridges are usually slower than normal, due to a decrease in the α:β MyHC isoform ratio [28]. It has been shown that re-expression of atrial light chain-1 (ALC-1) in hypertrophied ventricular myocardium is associated with a faster ktr [29]. While we did not detect expression of ALC-1 in our HCM biopsies using 1-D gels, increased ALC-1 expression was detected in similar HCM biopsies using 2-D gels [17], so this is a possible cause of the faster maximum cross-bridge cycling kinetics in our experiments.
More appropriate for the intact heart are cross-bridge kinetics under the physiologically relevant conditions of submaximal Ca2+ activation (Fig. 6). In most HCM patients the relative force–ktr relationship was similar to donors, implying no inherent change in the Ca2+ regulation of cross-bridge cycling kinetics. (Note that for the analysis in Fig. 6 we assume that the observed reduction in maximum Ca2+ activated force is due to fewer active cross-bridges, with no change in force per attached cross-bridge). However, patient M15 exhibited submaximal ktr values above those for the other human samples. Interestingly, this mutation (MYBPC3 T2604A+C deletion at 2605) encodes for a C-terminally truncated cMyBP-C that leads to a 35% reduction of cMyBP-C content in this patient [18]. There is evidence that cMyBP-C functions as a phosphorylation-sensitive “brake” on cross-bridge movement towards the thin filament, because cross-bridge cycling is increased by phosphorylation of cMyBP-C or by the absence of cMyBP-C in KO mice [30]. It therefore possible that the faster cross-bridge kinetics in M15 results from the MyBP-C haploinsufficiency in this patient. There was no clear acceleration of kinetics with the other MYBPC3 mutation, but this patient (MA) has a missense mutation that is associated with a much smaller (17%) reduction in cMyBP-C than with patient M15 [18].
The third mutation we found (MYH7 R719Q) was in patient ML. This mutation has previously been shown to reduce sliding velocity in a motility assay with Dictyostelium myosin [31] or increase it in human slow muscle fibers [32]. We found that this mutation led to no obvious alteration in isometric cross-bridge cycling kinetics at physiological levels of Ca2+ activation. Thus findings from model systems should be extrapolated to the human myocardium with care.
It should be noted that our kinetic data do not confirm or refute the concept that HCM is associated with inefficient ATP utilization by the myofilaments [14]. In some of our HCM samples the cross-bridge kinetics at submaximal Ca2+ activation tended to be faster than in donors and in some they tended to be slower. However simultaneous measurements of ATPase activity and force would be required to directly assess efficiency of chemomechanical transduction in these patients.
In summary, this study reports the static and dynamic properties of skinned myocytes from a representative group of patients with hypertrophic cardiomyopathy. Myocyte passive properties were no different in HCM and donor myocytes, which suggests that other factors are responsible for the increased stiffness of the intact ventricle. HCM myocytes exhibited a large decrease in maximum Ca2+ activated force, which if representative of myocytes in the intact myocardium, would tend to cause systolic dysfunction. Counteracting this would be the enhanced myofibrillar Ca2+ sensitivity in HCM myocytes, but this factor would tend to slow relaxation and contribute to diastolic dysfunction in the intact heart. Thus the properties of the myocytes likely contribute to both the systolic and diastolic contractile dysfunction of the HCM heart.
5. Disclosures
None declared.
Acknowledgments
We are grateful to Dr. Lucie Carrier (University of Hamburg) for the kind gift of the MyBP-C ser282 antibody. This work was supported by the British Heart Foundation (PG/07/067/23323), Department of Health NIHR Biomedical Research Centres funding scheme, and FP6 LSHM-CT-2005-018833 EUGeneHeart.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yjmcc.2010.06.006.
Appendix A. Supplementary data
References
- 1.Watkins H., Seidman J.G., Seidman C.E. Familial hypertrophic cardiomyopathy: a genetic model of cardiac hypertrophy. Hum Mol Genet. 1995;4:1721–1727. doi: 10.1093/hmg/4.suppl_1.1721. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Elliott P., McKenna W.J. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 4.Van Driest S.L., Vasile V.C., Ommen S.R., Will M.L., Tajik A.J., Gersh B.J. Myosin binding protein C mutations and compound heterozygosity in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:1903–1910. doi: 10.1016/j.jacc.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Theis J.L., Bos J.M., Theis J.D., Miller D.V., Dearani J.A., Schaff H.V. Expression patterns of cardiac myofilament proteins—genomic and protein analysis of surgical myectomy tissue from patients with obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2009;2:325–333. doi: 10.1161/CIRCHEARTFAILURE.108.789735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varnava A.M., Elliott P.M., Sharma S., McKenna W.J., Davies M.J. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–482. doi: 10.1136/heart.84.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linke W.A., Popov V.I., Pollack G.H. Passive and active tension in single cardiac myofibrils. Biophys J. 1994;67:782–792. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazorla O., Freiburg A., Helmes M., Centner T., McNabb M., Wu Y. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Minajeva A., Kulke M., Fernandez J.M., Linke W.A. Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys J. 2001;80:1442–1451. doi: 10.1016/S0006-3495(01)76116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren C.M., Jordan M.C., Roos K.P., Krzesinski P.R., Greaser M.L. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res. 2003;59:86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 11.Borbély A., van der Velden J., Papp Z., Bronzwaer J.G.F., Edes I., Stienen G.J.M. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 12.Harris T.S., Baicu C.F., Conrad C.H., Koide M., Buckley J.M., Barnes M. Constitutive properties of hypertrophied myocardium: cellular contribution to changes in myocardial stiffness. Am J Physiol. 2002;282:H2173–H2182. doi: 10.1152/ajpheart.00480.2001. [DOI] [PubMed] [Google Scholar]
- 13.Tardiff J. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 14.Ashrafian H., Redwood C., Blair E., Watkins H. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet. 2003;19:263–268. doi: 10.1016/S0168-9525(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk S.J., Dooijes D., dos Remedios C., Michels M., Lamers J.M.J., Winegrad S. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119:1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 16.Herron T.J., Rostkova E., Kunst G., Chaturvedi R., Gautel M., Kentish J.C. Activation of myocardial contraction by the N-terminal domains of myosin binding protein-C. Circ Res. 2006;98:1290–1298. doi: 10.1161/01.RES.0000222059.54917.ef. [DOI] [PubMed] [Google Scholar]
- 17.Jacques A.M., Briceno N., Messer A.E., Gallon C.E., Jalilzadeh S., Garcia E. The molecular phenotype of human cardiac myosin associated with hypertrophic obstructive cardiomyopathy. Cardiovasc Res. 2008;79:481–491. doi: 10.1093/cvr/cvn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marston S., Copeland O., Jacques A., Livesey K., Tsang V., McKenna W.J. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res. 2009;105:219–222. doi: 10.1161/CIRCRESAHA.109.202440. [DOI] [PubMed] [Google Scholar]
- 19.Mutungi G., Ranatunga K.W. The viscous, viscoelastic and elastic characteristics of resting fast and slow mammalian (rat) muscle fibres. J Physiol-London. 1996;496:827–836. doi: 10.1113/jphysiol.1996.sp021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman W., McLaurin L.P., Stefadouros M.A. Left ventricular stiffness associated with chronic pressure and volume overloads in Man. Circ Res. 1974;35:793–800. doi: 10.1161/01.res.35.5.793. [DOI] [PubMed] [Google Scholar]
- 22.Kulke M., Fujita-Becker S., Rostkova E., Neagoe C., Labeit D., Manstein D.J. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res. 2001;89:874–881. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 23.Krüger M., Linke W.A. Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46:490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Belus A., Piroddi N., Scellini B., Tesi C., Amati G.D., Girolami F. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol. 2008;586:3639–3644. doi: 10.1113/jphysiol.2008.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marston S.B., de Tombe P.P. Point/Counterpoint. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008;45:603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudenbacher F., Schober T., Pinto J.R., Sidorov V.Y., Hilliard F., Solaro R.J. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer S., Kentish J.C. Roles of Ca2+ and crossbridge kinetics in determining the maximum rates of Ca2+ activation and relaxation in rat and guinea pig skinned trabeculae. Circ Res. 1998;83:179–186. doi: 10.1161/01.res.83.2.179. [DOI] [PubMed] [Google Scholar]
- 28.de Tombe P.P. Altered contractile function in heart failure. Cardiovasc Res. 1998;37:367–380. doi: 10.1016/s0008-6363(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 29.Morano M., Zacharzowski U., Maier M., Lange P.E., exi-Meskishvili V., Haase H. Regulation of human heart contractility by essential myosin light chain isoforms. J Clin Invest. 1996;98:467–473. doi: 10.1172/JCI118813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stelzer J.E., Fitzsimons D.P., Moss R.L. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J. 2006;90:4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita H., Sugiura S., Momomura S., Omata M., Sugi H., Sutoh K. Characterization of mutant myosins of Dictyostelium discoideum equivalent to human familial hypertrophic cardiomyopathy mutants. J Clin Invest. 1997;99:1010–1015. doi: 10.1172/JCI119228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poetter K., Jiang H., Hassanzadeh S., Master S.R., Chang A., Dalakas M.C. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.