Abstract
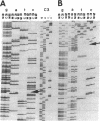
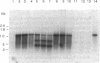
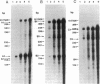
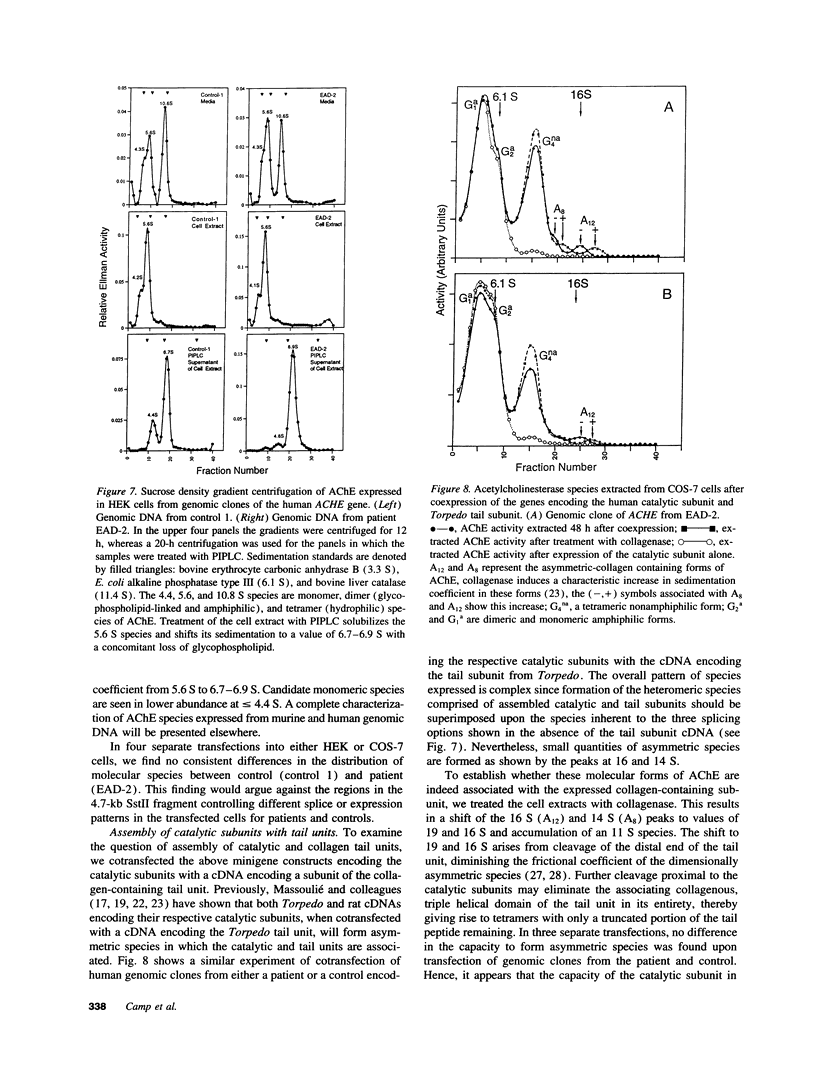
A congenital myasthenic condition has been described in several patients characterized by a deficiency in end-plate acetylcholinesterase (AChE). The characteristic form of AChE in the end-plate basal lamina has the catalytic subunits disulfide linked to a collagen-like tail unit. Southern analysis of the gene encoding the catalytic subunits revealed no differences between patient and control DNA. Genomic DNA clones covering exon 4 and the alternatively spliced exons 5 and 6 were analyzed by nuclease protection and sequencing. Although allelic differences were detected between controls, we found no differences in exonic and intronic areas that might yield distinctive splicing patterns in patients and controls. The ACHE gene was cloned from genomic libraries from a patient and a control. Transfection of the cloned genes revealed identical species of mRNA and expressed AChE. Cotransfection of the genes expressing the catalytic subunits with a cDNA from Torpedo encoding the tail unit yielded asymmetric species that require assembly of catalytic subunits and tail unit. thus the catalytic subunits of AChE expressed in the congenital myasthenic syndrome appear identical in sequence, arise from similar splicing patterns, and assemble normally with a tail unit to form a heteromeric species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Bartels C. F., Zelinski T., Lockridge O. Mutation at codon 322 in the human acetylcholinesterase (ACHE) gene accounts for YT blood group polymorphism. Am J Hum Genet. 1993 May;52(5):928–936. [PMC free article] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagenase sensitivity and aggregation properties of Electrophorus acetylcholinesterase. Eur J Biochem. 1978 Aug 15;89(1):89–94. doi: 10.1111/j.1432-1033.1978.tb20899.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duval N., Krejci E., Grassi J., Coussen F., Massoulié J., Bon S. Molecular architecture of acetylcholinesterase collagen-tailed forms; construction of a glycolipid-tailed tetramer. EMBO J. 1992 Sep;11(9):3255–3261. doi: 10.1002/j.1460-2075.1992.tb05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval N., Massoulié J., Bon S. H and T subunits of acetylcholinesterase from Torpedo, expressed in COS cells, generate all types of globular forms. J Cell Biol. 1992 Aug;118(3):641–653. doi: 10.1083/jcb.118.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G., Viegas-Pequignot E., Ginzberg D., Sindel L., Soreq H., Zakut H. Mapping the human acetylcholinesterase gene to chromosome 7q22 by fluorescent in situ hybridization coupled with selective PCR amplification from a somatic hybrid cell panel and chromosome-sorted DNA libraries. Genomics. 1992 Aug;13(4):1192–1197. doi: 10.1016/0888-7543(92)90037-s. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Gomez M. R. A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol. 1977 Apr;1(4):315–330. doi: 10.1002/ana.410010403. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Getman D. K., Eubanks J. H., Camp S., Evans G. A., Taylor P. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7. Am J Hum Genet. 1992 Jul;51(1):170–177. [PMC free article] [PubMed] [Google Scholar]
- Gibney G., MacPhee-Quigley K., Thompson B., Vedvick T., Low M. G., Taylor S. S., Taylor P. Divergence in primary structure between the molecular forms of acetylcholinesterase. J Biol Chem. 1988 Jan 25;263(3):1140–1145. [PubMed] [Google Scholar]
- Hutchinson D. O., Engel A. G., Walls T. J., Nakano S., Camp S., Taylor P., Harper C. M., Brengman J. M. The spectrum of congenital end-plate acetylcholinesterase deficiency. Ann N Y Acad Sci. 1993 Jun 21;681:469–486. doi: 10.1111/j.1749-6632.1993.tb22931.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson D. O., Walls T. J., Nakano S., Camp S., Taylor P., Harper C. M., Groover R. V., Peterson H. A., Jamieson D. G., Engel A. G. Congenital endplate acetylcholinesterase deficiency. Brain. 1993 Jun;116(Pt 3):633–653. doi: 10.1093/brain/116.3.633. [DOI] [PubMed] [Google Scholar]
- Jennekens F. G., Hesselmans L. F., Veldman H., Jansen E. N., Spaans F., Molenaar P. C. Deficiency of acetylcholine receptors in a case of end-plate acetylcholinesterase deficiency: a histochemical investigation. Muscle Nerve. 1992 Jan;15(1):63–72. doi: 10.1002/mus.880150112. [DOI] [PubMed] [Google Scholar]
- Karpel R., Ben Aziz-Aloya R., Sternfeld M., Ehrlich G., Ginzberg D., Tarroni P., Clementi F., Zakut H., Soreq H. Expression of three alternative acetylcholinesterase messenger RNAs in human tumor cell lines of different tissue origins. Exp Cell Res. 1994 Feb;210(2):268–277. doi: 10.1006/excr.1994.1039. [DOI] [PubMed] [Google Scholar]
- Krejci E., Coussen F., Duval N., Chatel J. M., Legay C., Puype M., Vandekerckhove J., Cartaud J., Bon S., Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 1991 May;10(5):1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. L., Taylor P. Structural characterization of the asymmetric (17 + 13) S species of acetylcholinesterase from Torpedo. II. Component peptides obtained by selective proteolysis and disulfide bond reduction. J Biol Chem. 1982 Oct 25;257(20):12292–12301. [PubMed] [Google Scholar]
- Legay C., Bon S., Massoulié J. Expression of a cDNA encoding the glycolipid-anchored form of rat acetylcholinesterase. FEBS Lett. 1993 Jan 4;315(2):163–166. doi: 10.1016/0014-5793(93)81155-s. [DOI] [PubMed] [Google Scholar]
- Legay C., Bon S., Vernier P., Coussen F., Massoulié J. Cloning and expression of a rat acetylcholinesterase subunit: generation of multiple molecular forms and complementarity with a Torpedo collagenic subunit. J Neurochem. 1993 Jan;60(1):337–346. doi: 10.1111/j.1471-4159.1993.tb05856.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Camp S., Rachinsky T. L., Bongiorno C., Taylor P. Promoter elements and transcriptional control of the mouse acetylcholinesterase gene. J Biol Chem. 1993 Feb 15;268(5):3563–3572. [PubMed] [Google Scholar]
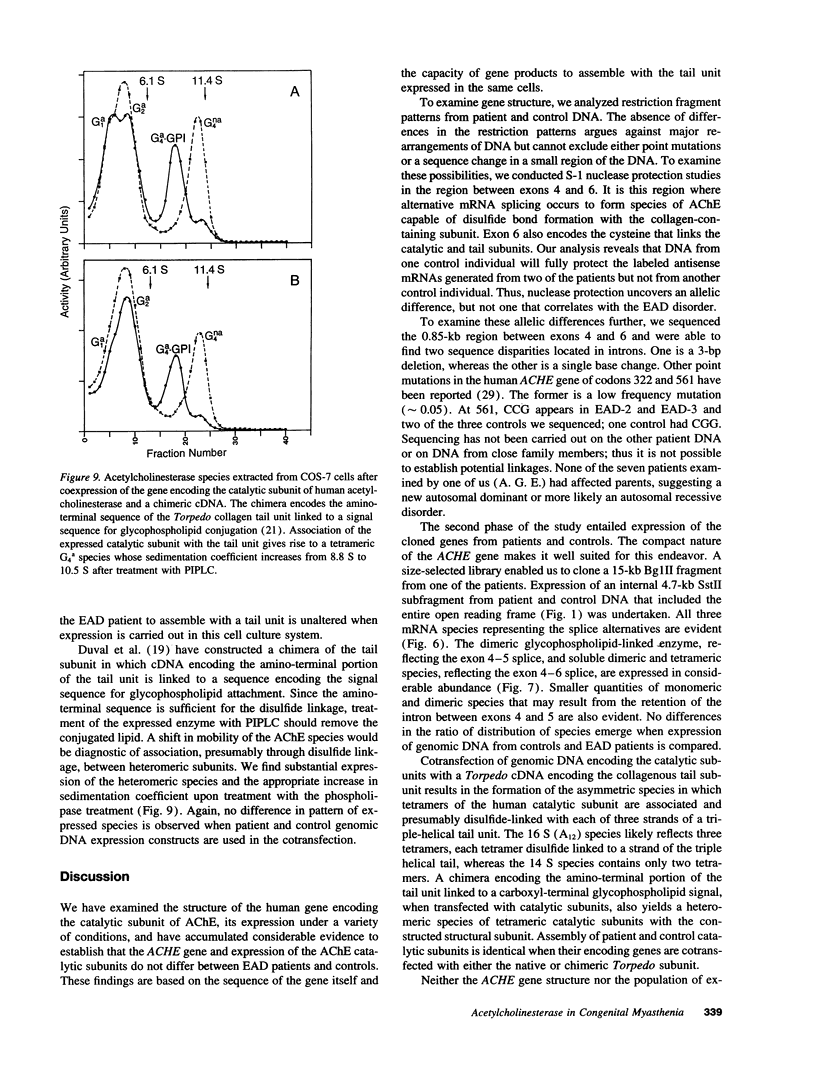
- Li Y., Camp S., Rachinsky T. L., Getman D., Taylor P. Gene structure of mammalian acetylcholinesterase. Alternative exons dictate tissue-specific expression. J Biol Chem. 1991 Dec 5;266(34):23083–23090. [PubMed] [Google Scholar]
- Li Y., Camp S., Taylor P. Tissue-specific expression and alternative mRNA processing of the mammalian acetylcholinesterase gene. J Biol Chem. 1993 Mar 15;268(8):5790–5797. [PubMed] [Google Scholar]
- MacPhee-Quigley K., Vedvick T. S., Taylor P., Taylor S. S. Profile of the disulfide bonds in acetylcholinesterase. J Biol Chem. 1986 Oct 15;261(29):13565–13570. [PubMed] [Google Scholar]
- Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993 Jul;41(1):31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- Maulet Y., Camp S., Gibney G., Rachinsky T. L., Ekström T. J., Taylor P. Single gene encodes glycophospholipid-anchored and asymmetric acetylcholinesterase forms: alternative coding exons contain inverted repeat sequences. Neuron. 1990 Feb;4(2):289–301. doi: 10.1016/0896-6273(90)90103-m. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Scott W., Moss D. J. Cell relationships in transformation of human leukocytes by Epstein-Barr virus. Int J Cancer. 1974 Jul 15;14(1):122–129. doi: 10.1002/ijc.2910140115. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Sikorav J. L., Duval N., Anselmet A., Bon S., Krejci E., Legay C., Osterlund M., Reimund B., Massoulié J. Complex alternative splicing of acetylcholinesterase transcripts in Torpedo electric organ; primary structure of the precursor of the glycolipid-anchored dimeric form. EMBO J. 1988 Oct;7(10):2983–2993. doi: 10.1002/j.1460-2075.1988.tb03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P., Radić Z. The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol. 1994;34:281–320. doi: 10.1146/annurev.pa.34.040194.001433. [DOI] [PubMed] [Google Scholar]