Abstract
Currently used antiretroviral therapy is highly successful but there is still a need for new effective and safe prophylactics and therapeutics. We have previously identified and characterized a human engineered antibody domain (eAd), m36, which exhibits potent broadly neutralizing activity against HIV-1 by targeting a highly conserved CD4 binding-induced (CD4i) structure on the viral envelope glycoprotein (Env) gp120. m36 has very small size (~15 kDa) but is highly specific and is likely to be safe in long-term use thus representing a novel class of potentially promising HIV-1 inhibitors. Major problems with the development of m36 as a candidate therapeutic are possible short serum half life and lack of effector functions that could be important for effective protection in vivo. Fusion of m36 to human IgG1 Fc resulted in dramatically diminished neutralization potency most likely due to the sterically restricted nature of the m36 epitope that limits access of large molecules. To confer effector functions and simultaneously increase the potency, we first matured m36 by panning and screening a mutant library for mutants with increased binding to gp120. We next fused m36 and its mutants with the first two domains (soluble CD4, sCD4) of the human CD4 by using a polypeptide linker. Our results showed that the selected m36 mutants and the sCD4-fusion proteins exhibited more potent antiviral activities than m36. The m36-sCD4 fusion proteins with human IgG1 Fc showed even higher potency likely due to their bivalency and increased avidity although with a greater increase in molecular size. Our data suggest that m36 derivatives are promising HIV-1 candidate therapeutics and tools to study highly conserved gp120 structures with implications for understanding mechanisms of entry and design of vaccine immunogens and small molecule inhibitors.
Keywords: HIV-1, engineered antibody domain, soluble CD4, fusion protein, half life, effector function
1. Introduction
Human immunodeficiency virus type 1 (HIV-1) entry is triggered by interaction of the viral envelope glycoprotein (Env) gp120 with cellular receptor CD4. Binding of CD4 induces extensive conformational changes in gp120 leading to formation and/or exposure of highly conserved structures. These structures are functionally important and, therefore, are potential targets for therapeutics including monoclonal antibodies (mAbs) (Choudhry et al., 2006; Chen and Dimitrov 2009).
The coreceptor-binding site (CoRbs) on the viral spike, of which the “bridging sheet” of gp120 is a critical component, contains determinants that are highly conserved across genetically diverse HIV-1 isolates from different clades (Kwong et al., 1998). It is highly immunogenic eliciting many antibodies in vivo. The CoRbs is typically hidden or unformed on free virons. It becomes exposed or formed after attachment of viruses to target cells through CD4 binding that induces conformational changes in gp120. Therefore, antibodies to the CoRbs are called CD4-induced (CD4i) antibodies. A representative of CD4i mAbs is 17b, which was derived from B cells of an HIV-1-infected individual, was extensively studied, and helped for the solution of the X-ray crystallographic structure of gp120 complexed with the first two domains (soluble CD4, sCD4) of the human CD4 (Kwong et al., 1998). Other CD4i mAbs include E51(Xiang et al., 2003), 412d (Choe et al., 2003), 21c (Xiang et al., 2002), X5 (Moulard et al., 2002) and its improved version, m9 (Zhang et al., 2004). Some of these antibodies, as antibody fragments such as single-chain Fv fragments (scFvs) and antigen-binding fragments (Fabs), exhibit potent broadly neutralizing activities in vitro. However, when they are converted to full-length antibodies such as IgG1s, neutralization could be dramatically decreased or totally lost (Labrijn et al., 2003). According to a previously published model (Labrijn et al., 2003), the size-dependent neutralization by CD4i antibodies could be due to steric restriction for antibody access to CD4i epitopes. One of the reasons could be that after cellular CD4 binds to the virus, the available space between the virus and the target cell surface is not sufficient to accommodate a whole antibody molecule but is adequate for antibody fragments. We, therefore, hypothesized that the smallest independently folded antibody domains can be engineered (engineered antibody domains, eAds) (size, 11–15 kDa) to exhibit exceptionally potent and broad neutralizing activity by targeting hidden conserved epitopes that are not accessible by larger antibodies (Chen et al., 2008a). This led to our discovery of the first reported human eAd against HIV-1, m36, which targets a highly conserved CD4i epitope on gp120 and efficiently neutralized HIV-1 strains from genetically diverse groups in a pseudovirus-cell line-based assay (Chen et al., 2008a). m36 also efficiently neutralized a panel of primary isolates from clade B in a PBMC-based assay and inhibited HIV-1 Env-mediated cell-cell fusion (Chen, et al., unpublished work). Several single heavy chain variable domains (referred to as VHHs) have been recently selected from llama immunized with HIV-1 gp120 and characterized as potent HIV-1 entry inhibitors, interfering with virus binding to CD4 (Forsman et al., 2008).
Two major issues need to be addressed before eAds or VHHs can be suited for in-vivo use which are similar for other small-size antibody fragments including scFvs and Fabs. These include their short half-life in circulation and lack of biological effector functions. Long half life is important for long-lasting antiviral activity; antibody Fc-mediated effector functions have been demonstrated to contribute to sterilizing protection of animals against simian/human immunodeficiency virus (SHIV) challenge (Hessell et al., 2007). A possible solution to these issues is to fuse antibody fragments with human antibody Fc. However, development of m36-Fc fusion proteins with preserved neutralization potency and breath is far more challenging because the increase in the molecular size resulted in dramatic decreases in antiviral activity most likely due to the steric occlusion of CD4i epitopes on HIV-1 gp120 (Chen et al., 2008a). In this study, we address these issues by maturing m36 for improved binding to gp120 in the absence of CD4 and recruiting sCD4 in the m36-Fc fusion proteins, aiming at circumventing the physical constrains established by the cellular CD4. Our results show that the new constructs have on average higher potency and possibly broader neutralizing activity than m36. Due to the presence of Fc, favorable serum half life and effector functions such as antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are also expected.
2. Materials and methods
2.1. Cells, viruses, plasmids, gp120s, gp140s, and antibodies
We purchased the 293T cells from ATCC. Other cell lines and plasmids used for expression of various HIV-1 Envs were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (ARRRP). Gp140SC and gp140CAP were produced in our laboratory; their genes were codon optimized from the Env sequences of a clade-B and a clade-C HIV-1 isolate, respectively, and synthesized. The codon-optimized genes were cloned into the mammalian expression vector pSecTagB. The hexahistidine-tagged proteins were expressed in stably transfected CHO-K1 cells and purified by immobilized metal ion affinity chromatography (IMAC) by using Ni-NTA resin (Qiagen, Valencia, CA) according to manufacturer’s protocols. Gp140JRFL was a gift from B.F. Haynes (Duke University Medical Center, Durham, NC). Gp120Bal and the single-chain fusion protein gp120Bal-CD4 (Fouts et al., 2000) were gifts from T. Fouts (Institute of Human Virology, Baltimore; currently at Profectus, Baltimore, MD). Horseradish peroxidase (HRP)-conjugated anti-FLAG tag antibody and HRP-conjugated anti-human IgG (Fc-specific) antibody were purchased from Sigma-Aldrich (St. Louis).
2.2. Library construction and selection of m36 mutants
A phage-displayed library (size, ~108 members) of m36 was constructed by random mutagenesis. To introduce point mutations, we performed random DNA mutagenesis with the Gene-Morph PCR Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. m36 gene fragments with mutations were PCR amplified by using m36-encoding plasmid as a template and primers m36F1 (5′-TGGTTTCGCTACCGTGGCCCAGGCGGCCCAGGTGCAGCTGGTG- 3) (sense) and HISR (5′-GTCGCCGTGGTGGTGGTGGTGGTGGCCGGCCTGGCCACTTG-3′) (antisense). The PCR products were gel-purified, digested with SfiI, and gel-purified again. The purified fragments were then cloned into the phagemid pComb3X linearized by SfiI. A phage library was prepared by electroporation of Escherichia coli (E. coli) strain TG1 electroporation-competent cells (Stratagene, La Jolla, CA) with desalted and concentrated ligation, as described previously (Chen et al., 2008b).
The library (phage) was used for selection of m36 mutants against HIV-1 antigens conjugated to magnetic beads (Dynabeads M-270 epoxy; DYNAL Inc., New Hyde Park, NY) as described previously (Zhu et al., 2006). For sequential panning, 5, 2.5 and 0.5 μg of gp120Bal were used in the first, third and fifth rounds, respectively; antigens were alternated with 5, 2.5 and 0.5 μg of gp140JRFL during the second, fourth and sixth rounds. Clones that bound to HIV-1 antigens were identified from the sixth round of panning by using soluble expression-based monoclonal ELISA (semELISA) as described (Chen et al., 2010).
2.3. Cloning of the fusion proteins of m36 and its mutant
The following primers were used: m36F, 5′-TGGTTTCGCTACCGTGGCCCAGCCGGCCCAGGTGCAGCTGGTG-3′ (sense); m36R1, 5′-GTGAGTTTTGTCGGGCCCTGAGGAGACGGTGAC-3′ (antisense); CD4F1, 5′-GGTGGCTCTGGTTCCGGTAAGAAGGTGGTGCTGGGC-3′ (sense); CD4R1, 5′-CGGGTTTAAACTCAGCCCTTATCGTCATCGTCCTTGTAGTCGCCGTGGTGGTG GTGGTGGTGGGCCAGCACCACGATGTC-3′ (antisense); CD4R2, 5′-GGAGGGCCC GGCCAGCACCACGATGTC-3′ (antisense); LinkerF1, 5′-GGAGGGCCCGGCGGCGGCTCTGGTGGTGGTTCTGGTGGCGGCTCTGAGGGTGGTGGCTCTGAG-3′ (sense); LinkerF2, 5′-GGAGGGCCCGAGGGTGGTGGCTCTGAG-3′ (sense); LinkerR1, 5′-ACCGGAACCAGAGCCACCACCGGAACCGCCTCCCTCAGAGCCGCCACCCTCA GAACCGCCACCCTCAGAGCCACCACCCTC-3′ (antisense); CD4FcF, 5′-TGGTTTCGCTACCGTGGCCCAGCCGGCCAAGAAGGTGGTGCTGGGC-3′ (sense); CD4FcR, 5′-GTGAGTTTTGTCGGGCCCGGCCAGCACCACGATGTC-3′ (antisense).
To construct the Fc-fusion protein (m36.4h1Fc) of m36 mutant m36.4, the m36.4 fragment was PCR amplified by using primers m36F and m36R1, digested with SfiI and ApaI, and cloned into pSecTagB-Fc. For generation of m36-sCD4 fusion proteins, linkers L1 (primers: LinkerF1 and LinkerR1) and L2 (primers: LinkerF2 and LinkerR1) were PCR amplified without a template. The human sCD4 gene fragment was amplified by PCR (primers: CD4F1 and CD4R1) with sCD4-encoding plasmid pD1D2 as a template. The linker L1 was joined to sCD4 by overlapping PCR performed in a volume of 50 μl by using both templates (in the same molarities) for 7 cycles in the absence of primers and 15 additional cycles in the presence of primers (500 pM of LinkerF1 and CD4R1, respectively). The resultant product appended with ApaI and PmeI restriction sites on both sides was digested and cloned into the pSecTagB-Fc vector-based m36h1Fc-encoding plasmid (Chen et al., 2008a) to generate m36L1CD4. In the same way m36L2CD4 was constructed except for the use of linker L2 as one of the templates and primers LinkerF2 and CD4R1 in the overlapping PCR. m36.4L2CD4 was generated by replacing the Fc portion in the m36.4h1Fc with L2-sCD4 gene fragment, which was obtained for cloning of m36L2CD4 as described above. For construction of m36L2CD4Fc, L2-sCD4 fragment was PCR (primers: LinkerF2 and CD4R2) amplified with m36L2CD4-encoding plasmid as a template. The PCR product containing ApaI restriction site on both sides was digested, gel-purified and cloned into the m36h1Fc-encoding pSecTagB-Fc plasmid linearized by ApaI. m36.4L2CD4Fc was generated by cloning the same L2-sCD4 fragment as for m36L2CD4Fc construction into the plasmid encoding m36.4h1Fc. sCD4Fc was made by amplifying (primers: CD4FcF and CD4FcR) sCD4 fragment, which was subsequently digested with SfiI and ApaI, and cloned into pSecTagB-Fc.
2.4. Expression and purification of m36, its mutants and their fusion proteins
m36 and its mutants were expressed in E. coli HB2151; all the fusion proteins were produced in 293 free style cells as described previously (Chen et al., 2008a). The m36 mutants and the sCD4-fusion proteins (excluding sCD4Fc and the m36-sCD4-Fc-fusion constructs), which were tagged with hexahistidine and FLAG at their C-terminus, were purified from the soluble fraction of HB2151 periplasm and the 293 cell culture supernatants, respectively, by immobilized metal ion affinity chromatography (IMAC) by using Ni-NTA resin (Qiagen, Valencia, CA) according to manufacturer’s protocols. The Fc-fusion proteins were purified from the 293 cell culture supernatants by using nProtein A Sepharose 4 Fast Flow.
2.5. ELISA
ELISA was performed as described (Chen et al., 2008a). Bound m36, m36 mutants and the sCD4-fusion proteins (excluding sCD4Fc and the m36-sCD4-Fc-fusion constructs) were detected by HRP-conjugated anti-FLAG tag antibody (Sigma-Aldrich). The fusion proteins with human IgG1 Fc were detected by HRP-conjugated anti-human IgG (Fc-specific) antibody (Sigma-Aldrich). The half-maximal binding (EC50) was calculated by fitting the data to the Langmuir adsorption isotherm.
2.6. Pseudovirus neutralization assay
Pseudoviruses were derived from 293T cells and neutralization assay was performed in duplicate by using HOS-CD4-CCR5 (for all R5 and dual tropic viruses) or HOS-CD4-CXCR4 cell lines as described previously (Chen et al., 2008a). Percentage neutralization was calculated by the following formula: (1 − average RLU of antibody-containing wells/average RLU of virus-only wells) × 100. IC50 and IC90 of neutralization were assigned for the antibody concentration at which 50% and 90% neutralization were observed, respectively.
3. Results
3.1. Construction of a library of random m36 mutants and selection of highest-affinity binders
Lack of or weak binding to gp120 and dramatically increased binding after engagement of CD4 are striking features of CD4i antibodies. Attachment of virions to cellular receptor CD4, however, creates steric occlusion between viral spike and target cell surface which could strongly decrease neutralizing activities of CD4i antibodies by limiting access of large antibody molecules (Labrijn et al., 2003). We therefore hypothesize that maturation of CD4i antibodies by improving binding to gp120 in the absence of CD4 could, to certain extent, compromise the steric occlusion and endow antibodies with more potent neutralization when the antibodies are converted to larger molecules for purposes such as gain of long half life in circulation.
To test this hypothesis, we constructed a phage-displayed library (size, ~108 members) of m36 where point mutations were introduced by random mutagenesis through error-prone PCR. The library was panned sequentially against two different Envs from clade B isolates, gp120Bal and gp140JRFL, in order that enriched m36 mutants could preserve cross-reactivity. To identify individual antibody that specifically bound to both antigens, clones were randomly selected after six rounds of panning and subjected to semELISA. Sequencing of a number of positive clones revealed that they represented four different clones, designated m36.1, m36.2, m36.4 and m36.5, respectively (Fig. 1). These clones were also selected by panning the library sequentially with gp140SC (clade B) and gp140CAP (clade C). Notably, three (m36.1, m36.4 and m36.5) of them acquired the same mutation (44Q/E) to an acidic residue in the framework (FR) 2 (FR2) compared to m36; the other one (m36.2) also carried an acidic residue substitution (45A/D) at a close position. Besides m36.4, the other three mutants contained additional mutations in various positions. In ELISA-based assays, these mutants showed specific and significantly higher binding than m36 to gp120Bal (Fig. 2A) and gp140JRFL (Fig. 2B) in the absence of CD4; they also bound much better to gp140SC (Fig. 2C) and gp140CAP (data not shown). Although these antibodies were selected against Envs only, slightly increased interaction with gp120Bal-CD4 complex was also observed with some of the mutants (Fig. 2D).
Fig. 1.
Amino acid sequence alignment of m36 mutants with m36. The sequences are numbered and antibody FRs and CDRs are indicated according to the ImMunoGeneTics (IMGT) numbering system (http://imgt.cines.fr/IMGT_vquest/vquest?livret=0&Option=humanIg). The residues in the mutants, which are identical to those in m36, are indicated by dots.
Fig. 2.
ELISA binding of m36 and its mutants to gp120Bal (A), gp140JRFL (B), gp140SC (C) and gp120Bal-CD4 (D). Antibody specificity is determined by using an unrelated antigen, bovine serum albumin (BSA).
3.2. Improved neutralization by the selected m36 mutants and their larger size fusion proteins
To see whether the increase in binding could result in more potent neutralization, m36.4, which contained only a single mutation compared to m36, was chosen and tested with a small panel of HIV-1 Env-pseudotyped viruses from genetically diverse primary isolates. As shown in Tables 1, m36.4 exhibited slightly higher potency than m36 - on average both IC50s and IC90s were decreased. Higher decreases in IC50s were observed with 92UG037.8 (clade A), 89.6 (clade B) and CM243 (clade E). In a previous study we generated a fusion protein (m36h1Fc) of m36 with human IgG1 Fc for possible avidity effects and long half life in vivo (Chen et al., 2008a). m36h1Fc exhibited higher binding to Envs than m36. However, there was a dramatic decrease in neutralization against most of the isolates tested likely because of the sterically restricted nature of the m36 epitope that limits access of large antibody derivatives, representing a strategy that HIV-1 has evolved to escape from antibodies generated by the human immune system; the finding that in the presence of low concentration of sCD4, m36h1Fc restored potent neutralization against these isolates supports this possible mechanism (Chen et al., 2008a). To find out whether the increased binding of m36.4 to gp120s could decrease the effects of the size constrain, we made the same fusion protein for m36.4, designated m36.4h1Fc. It was tested side by side with m36h1Fc against three isolates, Bal, JRFL and 89.6, of which the first two were barely neutralized and the last one was efficiently neutralized by m36h1Fc in a previous study (Chen et al., 2008a). The results showed that m36.4h1Fc exhibited slightly better neutralization than m36h1Fc to Bal and JRFL while having a much greater increase in antiviral activity against 89.6 (Fig. 3).
Table 1.
Neutralization of HIV-1 pseudotyped from different clades by m36, m36.4 and their fusion proteins.
| Virus | Clade | Tropism | m36 |
m36.4 |
m36L2CD4 |
m36.4L2CD4 |
m36L2CD4Fc |
m36.4L2CD4Fc |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50a | IC90b | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |||
| 92UG037.8 | A | R5 | 205 ± 3 | 1580 ± 156 | 18 ± 2 | 1300 ± 24 | 17 ± 0.5 | 98 ± 11 | 15 ± 3 | 90 ± 35 | 15 ± 2 | 200 ± 16 | 24 ± 7 | 110 ± 9 |
| Bal | B | R5 | 19 ± 5 | 79 ± 8 | 11 ± 0.2 | 64 ± 10 | 2.6 ± 0.3 | 23 ± 7 | 1.7 ± 0.2 | 19 ± 1 | 0.8 ± 0.4 | 5.7 ± 2.9 | 0.5 ± 0.3 | 2.2 ± 1.4 |
| JRFL | B | R5 | 78 ± 12 | 336 ± 20 | 27 ± 6 | 169 ± 28 | 7.8 ± 3.1 | 85 ± 9 | 7.5 ± 4.4 | 76 ± 15 | 2.3 ± 1.6 | 50 ± 17 | 3 ± 4 | 66 ± 24 |
| JRCSF | B | R5 | 33 ± 10 | 148 ± 24 | 22 ± 3 | 106 ± 7 | 23 ± 8 | 110 ± 15 | 41 ± 28 | 196 ± 20 | 13 ± 1 | 110 ± 9 | 26 ± 11 | 122 ± 6 |
| R2 | B | R5 | 23 ± 3 | 139 ± 18 | 12 ± 2 | 101 ± 5 | 1.5 ± 0.6 | 37 ± 4 | 7.2 ± 3.3 | 65 ± 16 | 1.2 ± 0.8 | 25 ± 8 | 0.5 ± 0.2 | 21 ± 1 |
| AD8 | B | R5 | 19 ± 2 | 183 ± 21 | 11 ± 4 | 99 ± 15 | 70 ± 10 | 255 ± 43 | 64 ± 9 | 237 ± 21 | 18 ± 6 | 105 ± 17 | 19 ± 10 | 111 ± 23 |
| IIIB | B | X4 | 1.7 ± 0.5 | 24 ± 4 | 0.8 ± 0.1 | 17 ± 2 | <0.2 | 0.2 ± 0.1 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| NL4-3 | B | X4 | 0.3 ± 0.1 | 2.0 ± 0.7 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| 89.6 | B | R5X4 | 16 ± 4 | 127 ± 32 | <0.2 | 13 ± 9 | 1.8 ± 1.5 | 21 ± 6 | 5.5 ± 3.9 | 12 ± 8 | 1.1 ± 0.3 | 16 ± 2 | 0.4 ± 0.3 | 19 ± 5 |
| 92HT | B | R5X4 | 0.3 ± 0.1 | 5.9 ± 2.5 | 4.7 ± 0.8 | 36 ± 6 | 7.1 ± 1.2 | 68 ± 13 | 4.9 ± 0.5 | 87 ± 8 | 7.6 ± 0.4 | 185 ± 20 | 8.3 ± 2.1 | 201 ± 14 |
| GXC-44 | C | R5 | 4.2 ± 1.3 | 80 ± 9 | 6.5 ± 2.1 | 87 ± 11 | 1.8 ± 0.6 | 69 ± 7 | 0.9 ± 0.5 | 21 ± 3 | 1.3 ± 0.5 | 74 ± 8 | 1.0 ± 1.3 | 36 ± 5 |
| Z2Z6 | D | R5 | 480 ± 34 | 1810 ± 57 | 225 ± 16 | 1090 ± 81 | 274 ± 33 | 1620 ± 130 | 220 ± 10 | 1235 ± 98 | 37 ± 9 | 980 ± 35 | 231 ± 20 | 1170 ± 40 |
| CM243 | E | R5 | 645 ± 42 | 1083 ± 25 | 190 ± 24 | 1130 ± 66 | 7.0 ± 2.9 | 198 ± 35 | 6.3 ± 3.4 | 200 ± 16 | 1.7 ± 0.5 | 744 ± 51 | 3.6 ± 1.0 | 890 ± 42 |
| GXE | E | R5 | -c | - | 1371 ± 60 | >2000 | 180 ± 30 | 1845 ± 92 | 144 ± 18 | 1120 ± 75 | 55 ± 9 | 833 ± 40 | 210 ± 14 | 1230 ± 78 |
| Arithmetic meand | 117 | 431 | 41 | 324 | 32 | 199 | 29 | 172 | 7.6 | 192 | 24 | 211 | ||
| Geometric meand | 17 | 118 | 7.3 | 71 | 4.6 | 35 | 5.0 | 31 | 2.3 | 31 | 2.6 | 28 | ||
Antibody concentration (nM) resulting in 50% inhibition of virus infection.
Antibody concentration (nM) resulting in 90% inhibition of virus infection.
No significant neutralization at the highest antibody concentration (2000 nM) tested.
Arithmetic and geometric means were calculated for 13 (except GXE that m36 did not significantly neutralize at the highest concentration tested) of 14 viruses including those with values <0.2 nM, which were assigned a value of 0.1.
Fig. 3.
Dose-dependent neutralization of Bal, JRFL and 89.6 by m36h1Fc and m36.4h1Fc. The pseudotyped viruses were generated from 293T cells and the assays were performed on HOS-CD4-CCR5 cells.
3.3. Generation of fusion proteins of m36 and m36.4 with sCD4 or sCD4-Fc
Synergy in neutralization between sCD4 and CD4i antibodies has been observed in several studies (Dey et al., 2003; Lagenaur et al., 2010; West et al., 2010); the major mechanism of action is that sCD4 induces conformational changes in gp120 that result in formation and/or exposure of CD4i epitopes without involvement of cellular CD4. Thus, the antibodies could better anchor free virons before attachment, which could help solve the accessibility problem with large antibody molecules.
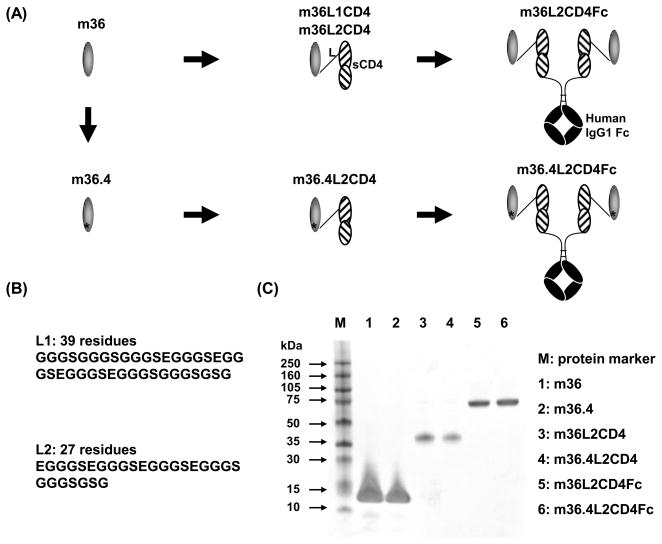
We, therefore, generated fusion proteins of m36 with human sCD4. First, we determined the appropriate order of the antibody and sCD4 in the single-chain chimeric fusion proteins. In a separate experiment, we made two homodimers of m36. In one construct (m36d1), two m36 molecules were covalently linked by a polypeptide composed of three repeats of G4S motif. In the other construct (m36d2), a single cysteine was introduced to a polypeptide tail at the C-terminus of m36 and dimerization of the purified protein via a disulfide bond was determined by size-exclusion chromatography, non-reducing and reducing SDS-PAGE. We found that binding to gp120s and neutralization of m36d1 were decreased by ~16 folds and ~3 folds, respectively, compared to those of m36 while m36d2 showed comparable or better binding and neutralization than m36 (Chen et al., unpublished work). These results suggest that in m36d1, the linker could interrupt recognition of the second m36 molecule probably because the linker is in close proximicity to the antigen-binding site of the second m36. Therefore, we decided to join m36 to the N-terminus of the human sCD4 (Fig. 4A). Because the N-terminus of sCD4 is relatively far away from the binding site of gp120 according to the crystal structure of a gp120-sCD4 complex (Kwong et al., 1998), we assume that these polypeptide linkers may not interfere with interaction of sCD4 with gp120. Second, we tried a natural polypeptide linker derived from the M13 bacteriophage where the linker connects the second and third domains of capsid protein pIII; the linker is expected to provide remarkable flexibility and stability as it is naturally designed for such purposes and proved to be capable of efficiently displaying a variety of foreign proteins in phage display technology (Hoogenboom et al., 1998). To explore the effects of linker length, we used the full-length bacteriophage pIII linker (L1, 39 residues) in one construct (m36L1CD4) and an empirically shortened linker (L2, 27 residues) in the other construct (m36L2CD4) (Fig. 4A and B).
Fig. 4.
Design of fusion proteins of m36 and its mutants. (A) Schematic representation of fusion protein architectures. (B) Sequences of polypeptide linkers connecting m36 or m36.4 with sCD4. (C) Reducing SDS-PAGE of m36, m36.4 and their fusion proteins.
m36L1CD4 and m36L2CD4 were expressed in transiently transfected 293 free style cells; the proteins were secreted into the shaking culture supernatants with yield of ~1.6 mg l−1. Both proteins ran on reducing SDS-PAGE with an apparent molecular weight (MWa) of ~40 kDa, which was close to the calculated MW (MWc) (37.192 kDa for m36L1CD4 and 36.417 kDa for m36L2CD4, including the His and FLAG tags) (Fig. 4C and data not shown). In an ELISA assay, they bound to gp120Bal much better than m36 or sCD4 alone, or a combination of m36 and sCD4 (with the same molarity), suggesting the contribution of synergistic and/or avidity effects (Fig. 5A). Notably, m36L2CD4 bound even better than m36L1CD4. However, we did not observe obvious difference in neutralization potencies between two constructs against several isolates tested (data not shown). We used linker L2 for generation of additional m36-based fusion proteins.
Fig. 5.
Comparative analysis of ELISA binding. (A) m36-sCD4 fusion proteins with linkers of different length are compared to m36 or sCD4 alone, and unlinked m36 plus sCD4, for binding to gp120Bal. (B) Difference in binding of m36L2CD4 and m36L2CD4Fc to gp120Bal. (C) Dramatic increase in binding of m36L2CD4Fc compared to that of m36h1Fc and sCD4Fc. (D) Comparison between the fusion proteins of m36 and m36.4 for binding to gp120Bal.
To further increase avidity effects and serum half life and confer biological effector functions, we fused m36L2CD4 to human IgG1 Fc (Fig. 4A and C). The new construct, designated m36L2CD4Fc, was well expressed and easily purified from the shaking 293 free style cell culture supernatant with a yield of ~4.2 mg l−1. It bound to gp120Bal with an EC50 (8 nM) higher than that (25 nM) of m36L2CD4 (Fig. 5B). To rule out the possibility that the strong binding was resulted mainly from the dimerization of either m36 or sCD4, we made fusion protein (sCD4Fc) of sCD4 with the human IgG1 Fc and compared binding of m36L2CD4Fc to those of m36h1Fc and sCD4Fc (Fig. 5C). The results showed that although sCD4Fc and m36h1Fc exhibited stronger binding to gp120Bal than monomeric sCD4 and m36, respectively, their binding strengths were much lower than that of m36L2CD4Fc.
We then made similar constructs by replacing m36 with m36.4. The resultant proteins, m36.4L2CD4 and m36.4L2CD4Fc showed slightly higher binding to gp120Bal than their parent counterparts, respectively (Fig. 5D).
3.4. Efficient neutralization of HIV-1 by the sCD4- and sCD4-Fc-fusion proteins of m36 and m36.4
To determine the potency and breadth of HIV-1 neutralization by these fusion proteins, we conducted cell line-based pseudovirus neutralization assays with 14 HIV-1 isolates from different clades including those which were categorized as tier 1 (e.g., Bal and IIIB) or tier 2 (e.g., JRFL and JRCSF) viruses in terms of sensitivity to broadly neutralizing antibodies. Although there was an increase in molecular size, all fusion proteins were more effective than m36 against almost all the isolates tested, having IC50s and IC90s on average several folds lower than those of m36 (Tables 1). m36L2CD4Fc and m36.4L2CD4Fc exhibited even more potent neutralization than m36L2CD4 and m36.4L2CD4 against some isolates while a slight decrease in potency was observed with GXE (clade E) for m36.4L2CD4Fc. Overall, no significant difference in potency was seen between m36L2CD4 and m36.4L2CD4, and between m36L2CD4Fc and m36.4L2CD4Fc whereas some isolates could be slightly more efficiently neutralized by one antibody and others could be better affected by the other antibody. Of particular note, GXE (clade E), which was insensitive to m36, could be relatively potently neutralized by the fusion proteins (Tables 1) suggesting that the neutralizing activities of the fusion proteins could be broader than that of m36.
We next compared m36L2CD4Fc to m36h1Fc and sCD4Fc in neutralization against four isolates (Fig. 6). m36h1Fc did not or poorly inhibited three isolates (92UG037.8, Bal and JRFL) while efficiently neutralized 89.6, in agreement with our previous study (Chen et al., 2008a). sCD4Fc was highly efficient in neutralizing all the isolates with IC50s less than 40 nM. As expected, even more potent neutralization occurred with m36L2CD4Fc; the IC50s with Bal and 89.6 were at least 9-fold lower than those for sCD4Fc; about 5 and 2 folds decreases in IC50s with 92UG037.8 and JRFL, respectively, were also observed for m36L2CD4Fc compared to that for sCD4Fc. These results also confirmed that the increased potency of m36 and m36.4 after fusion with sCD4 or sCD4-Fc was attributed mainly to the synergistic and/or avidity effects between the antibody and sCD4 but not due to the dimerization of the antibody or sCD4.
Fig. 6.
Dose-dependent inhibition of 92UG037.8, Bal, JRFL and 89.6 by m36h1Fc, sCD4Fc and m36L2CD4Fc, respectively. The pseudotyped viruses were generated from 293T cells and the assays were performed on HOS-CD4-CCR5 cells
4. Discussion
Isolated stable human eAds have been described (Holt et al., 2003) and are promising candidate therapeutics against cancer and autoimmune diseases. Recently, one eAd (against TNFα) was successfully evaluated in a phase I clinical trial and a phase II trial has commenced (http://www.arana.com); a VHH from camelid origin (called nanobody) was also safe (http://www.ablynx.com). In our previous study (Chen et al., 2008a), we described the discovery and potent anti-HIV-1 activity of the first reported human eAd, m36. The small size (11–15 kDa) of eAds endows them with two major unique beneficial properties - much better penetration into densely packed tissues (e.g., lymphoid tissues where HIV-1 predominantly replicates) compared to the conventional antibodies (150 kDa for a human IgG1) and ability to access functionally important structures on the surface of some molecules (e.g., HIV-1 Envs) that are not accessible by full-length antibodies. Other excellent biophysical properties of eAds include high yield (low production cost) from bacterial cells, solubility, stability and ease for engineering such as affinity maturation. As reagents for diagnosis, eAds could be also useful because they can be rapidly cleared and exhibit relatively low or no toxicity and immunogenecity. However, due to their small size and lack of binding to the Fc receptors, eAds exhibit short half life in circulation and lack of biological effector functions that are critical for eliminating both free agents (e.g., virus particles) and aberrant cells (e.g., virus-infected cells) in vivo. A possible solution is to fuse eAds with Fc (Chen and Dimitrov, 2009) (see also http://www.arana.com); although it brings eAds back to medium MW (~75 kDa for fusion with human IgG1 Fc) reagents, the fusion proteins could still promise more efficient penetration than full-length antibodies (~150 kDa for a human IgG1). This solution, however, may not be applicable to m36 because it targets a sterically restricted structure formed during HIV-1 entry and therefore, the increase in antibody size could dramatically diminish neutralization potency, as has been demonstrated in our previous study (Chen et al., 2008a). To address these issues, we used two strategies in this study – affinity maturation for enhanced binding to gp120 without CD4 and fusion to sCD4 and Fc. We found that sCD4-Fc fusion proteins of m36 and its matured version exhibit a profound increase in antiviral activity compared to m36 alone, albeit with about 9-fold increase in MWs.
Our finding that a single mutation in the FR2 of m36 (m36.4) significantly improves the antibody affinity and neutralization could also help understand how the antibody interacts with its highly conserved hidden epitope on gp120. One possible explanation for the increased binding is that the mutation could play a critical role in adjustment of the antibody tertiary structure that results in adoption of a better-fit antigen-binding site. It is noteworthy, however, that three of the mutants (m36.1, m36.4 and m36.5) carry the same mutation (44Q/E), which is acidic; the other one (m36.2) also has an acidic amino acid substitution (45A/D) at a very close position (Fig. 1). Crystallographic analysis (Kwong et al., 1998) of the sCD4-gp120-17b complex shows that the hydrophobic and acidic surface of the 17b heavy chain accounts for its interaction with gp120 by mimicking the tyrosine-rich, acidic N-terminal region of CCR5; possible electrostatic interactions occur between the basic surface of the gp120 “bridging sheet” and the acidic antibody residues (all known CD4i mAbs show unusually high concentration of acidic residues in heavy chain CDR loops) (Huang et al., 2004). These results suggest an alternative explanation that the acquired acidic residues in the FR2 of m36 mutants could be part of the binding interface, enhancing the electrostatic interactions with basic residues on gp120. It is theoretically possible that eAds could use FRs to compensate the loss of antigen-interacting surface contributed by the hypervariable loops of either VH or VL. If the second possibility proves to be true, m36.4 could be further matured by screening a mutant library with introduction of additional acidic and hydrophobic mutations to the interface where the 44Q/E mutation occurs. The newly identified mutants and those described in this study could be potentially useful not only as potent candidate therapeutics but can also help probe the highly conserved structures around the CoRbs with implications for the development of small-molecule inhibitors and elucidation of mechanisms of viral entry and evasion of immune responses.
Linkage between sCD4 and CD4i mAbs for synergistic and/or avidity effects on neutralization has been previously described (Dey et al., 2003; Lagenaur et al., 2010; West et al., 2010). In these studies, a single or multi-repeat G4S motif, which was artificially designed, was exclusively used as the linker between sCD4 and antibody. Here, we selected as a linker a polypeptide derived from M13 bacteriophage where the peptide connects the second and third domains of capsid protein pIII. This naturally occurring peptide may be more resistant to cleavage by proteinases and therefore, could confer a higher level of stability of the fusion proteins. However, peptides from non-human sources may be immunogenic in humans; there will be a need to evaluate such a possibility or to select human-derived linkers to reduce possible immunogenicity. The compositions of the phage pIII linker are almost the same as those of the G4S linker, except that the former contains additionally glutamines, and glycine and serine are not regularly distributed (Fig. 4B). It will be of interest to test whether such an arrangement could really delay the degradation of fusion proteins in vivo. In one of the previous studies (Lagenaur et al., 2010), effects of different linker length were explored. The results showed that no obvious difference in neutralization potency was observed among three constructs with linker of 35 residues (7 repeats of G4S motif), 40 residues (8 repeats of G4S motif) and 55 residues (11 repeats of G4S motif), respectively, whereas the use of a linker composed of a single G4S motif gave a much weaker potency suggesting a length spanning five amino acid residues may not be sufficient for simultaneous binding of the sCD4 and antibody moieties to a single gp120 subunit. In agreement with the recent study (Lagenaur et al., 2010), our results showed that m36L1CD4 (linker, 39 residues) and m36L2CD4 (linker, 27 residues) had indistinguishable potencies in neutralizing several isolates tested (data not shown), although the latter exhibited higher binding to gp120Bal (Fig. 5A). A possible explanation is that bifunctional binding molecules can potentially display enhanced activities even when the two binding moieties are incapable of interacting simultaneously with a single target molecule. Lagenaur et al. (Lagenaur et al., 2010) showed that a sCD4-17b fusion protein linked by a single G4S motif, which was not expected to enable simultaneous binding of two moieties, could be more effective against some isolates than the mixture of unlinked sCD4 plus 17b. West Jr. et al. (West et al., 2010) demonstrated that a sCD4 fusion protein with the anti-HIV carbohydrate antibody 2G12 also resulted in a potent neutralizing reagent with more broadly neutralizing activity than 2G12 alone.
Some isolates (e.g., Z2Z6 and GXE) were not neutralized very effectively by the newly constructed fusion proteins (Tables 1). Two likely reasons could be suggested for this observation. First, sCD4 does not bind well to gp120s from some isolates especially primary isolates as was demonstrated previously (Moore et al., 1992); in the trimeric envelope spike, engagement of multiple CD4 molecules might be required to elicit the conformational changes that expose the CoRbs. Our results showed that sCD4 bound very weakly to gp120Bal and only slightly enhanced m36 binding when the two components were not covalently linked (Fig. 5A) while sCD4 had a high affinity (EC50, ~6 nM) with gp140JRFL (data not shown). Interestingly, the fusion proteins exhibited dramatic increases in binding to gp120Bal (Fig. 5) suggesting that the benefits from avidity effects could be significantly higher than those (synergistic effects) due to the enhanced exposure/formation of the m36 epitope by sCD4. sCD4 could have even weaker binding to other gp120s than to gp120Bal. Second, the linker we used may not provide sufficient flexibility for efficient binding to some isolates because the slight deviations in the angles, at which sCD4 and antibody approach gp120, could result in substantial differences in the requirement for linker flexibility. As a result, neither avidity nor synergistic effects could be expected when sCD4 is fused with antibodies and the antiviral potency of the fusion proteins can be significantly compromised by the increased antibody size. Thus, additional work is desirable to determine optimal linker lengths and compositions.
The fusion proteins of m36 and its mutants described in this study could be useful not only as candidate HIV-1 therapeutics adding a new member to the growing family of bifunctional entry inhibitors, but also as a tool to explore mechanisms of entry and to understand how HIV-1 guards its conserved structures and evades neutralizing immune responses.
Acknowledgments
We thank Christopher Broder, Gerald Quinnan, Dennis Burton, Barton Haynes and Tim Fouts for providing reagents. This project was supported by the Intramural AIDS Targeted Antiviral Program of the National Institutes of Health (NIH), by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, by federal funds from the National Cancer Institute, NIH, under contract N01-CO-12400, and by the Gates Foundation (D.S.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Chen W, Zhu Z, Feng Y, Dimitrov DS. Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc Natl Acad Sci USA. 2008a;105:17121–17126. doi: 10.1073/pnas.0805297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu Z, Feng Y, Xiao X, Dimitrov DS. Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J Mol Biol. 2008b;382:779–789. doi: 10.1016/j.jmb.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Dimitrov DS. Human monoclonal antibodies and engineered antibody domains as HIV-1 entry inhibitors. Curr Opin HIV AIDS. 2009;4:112–117. doi: 10.1097/COH.0b013e328322f95e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu Z, Feng Y, Dimitrov DS. A large human domain antibody library combining heavy and light chain CDR3 diversity. Mol Immunol. 2010;47:912–921. doi: 10.1016/j.molimm.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang CC, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell. 2003;114:161–170. doi: 10.1016/s0092-8674(03)00508-7. [DOI] [PubMed] [Google Scholar]
- Choudhry V, Zhang MY, Dimitrova D, Prabakaran P, Dimitrov AS, Fouts TR, Dimitrov DS. Antibody-based inhibitors of HIV infection. Expert Opin Biol Ther. 2006;6:523–531. doi: 10.1517/14712598.6.5.523. [DOI] [PubMed] [Google Scholar]
- Dey B, Del Castillo CS, Berger EA. Neutralization of human immunodeficiency virus type 1 by sCD4-17b, a single-chain chimeric protein, based on sequential interaction of gp120 with CD4 and coreceptor. J Virol. 2003;77:2859–2865. doi: 10.1128/JVI.77.5.2859-2865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman A, Beirnaert E, asa-Chapman MM, Hoorelbeke B, Hijazi K, Koh W, Tack V, Szynol A, Kelly C, McKnight A, Verrips T, de HH, Weiss RA. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J Virol. 2008;82:12069–12081. doi: 10.1128/JVI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts TR, Tuskan R, Godfrey R, Reitz M, Hone D, Lewis GK, DeVico AL. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol. 2000;74:11427–11436. doi: 10.1128/jvi.74.24.11427-11436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM. Domain antibodies: proteins for therapy. Trends Biotechnol. 2003;21:484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hoogenboom HR, De Bruine AP, Hufton SE, Hoet RM, Arends JW, Roovers RC. Antibody phage display technology and its applications. Immunotechnology. 1998;4:1–20. doi: 10.1016/s1380-2933(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, Wyatt R, Choe H, Farzan M, Kwong PD. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci USA. 2004;101:2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur LA, Villarroel VA, Bundoc V, Dey B, Berger EA. sCD4-17b bifunctional protein: Extremely broad and potent neutralization of HIV-1 Env pseudotyped viruses from genetically diverse primary isolates. Retrovirology. 2010;7:11. doi: 10.1186/1742-4690-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, McKeating JA, Huang YX, Ashkenazi A, Ho DD. Virons of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M, Phogat SK, Shu Y, Labrijn AF, Xiao X, Binley JM, Zhang MY, Sidorov IA, Broder CC, Robinson J, Parren PW, Burton DR, Dimitrov DS. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci USA. 2002;99:6913–6918. doi: 10.1073/pnas.102562599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Jr, Galimidi RP, Foglesong CP, Gnanapragasam PN, Klein JS, Bjorkman PJ. Evaluation of CD4-CD4i antibody architectures yields potent, broadly cross-reactive anti-human immunodeficiency virus reagents. J Virol. 2010;84:261–269. doi: 10.1128/JVI.01528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SH, Doka N, Choudhary RK, Sodroski J, Robinson JE. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res Hum Retroviruses. 2002;18:1207–1217. doi: 10.1089/08892220260387959. [DOI] [PubMed] [Google Scholar]
- Xiang SH, Wang L, Abreu M, Huang CC, Kwong PD, Rosenberg E, Robinson JE, Sodroski J. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology. 2003;315:124–134. doi: 10.1016/s0042-6822(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Zhang MY, Shu Y, Rudolph D, Prabakaran P, Labrijn AF, Zwick MB, Lal RB, Dimitrov DS. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J Mol Biol. 2004;335:209–219. doi: 10.1016/j.jmb.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Dimitrov AS, Bossart KN, Crameri G, Bishop KA, Choudhry V, Mungall BA, Feng YR, Choudhary A, Zhang MY, Feng Y, Wang LF, Xiao X, Eaton BT, Broder CC, Dimitrov DS. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol. 2006;80:891–899. doi: 10.1128/JVI.80.2.891-899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]