SUMMARY
West Nile virus (WNV) is the most common arthropod-borne flavivirus in the United States; however, the vector ligand(s) that participate in infection are not known. We now show that an Aedes aegypti C-type lectin, mosGCTL-1, is induced by WNV, interacts with WNV in a calcium-dependent manner, and facilitates infection in vivo and in vitro. A mosquito homologue of human CD45 in A. aegypti, designated mosPTP-1, recruits mosGCTL-1 to enable viral attachment to cells, and to enhance viral entry. In vivo experiments show that mosGCTL-1 and mosPTP-1 function as part of the same pathway and are critical for WNV infection of mosquitoes. A similar phenomenon was also observed in Culex quinquefasciatus, a natural vector of WNV, further demonstrating that these genes participate in WNV infection. During the mosquito blood-feeding process, WNV infection was blocked in vivo with mosGCTL-1 antibodies. A molecular understanding of flaviviral-arthropod interactions may lead to strategies to control viral dissemination in nature.
Keywords: West Nile virus, C-type lectin, protein tyrosine phosphatases, mosquito, arthropod-based vaccine
INTRODUCTION
West Nile virus (WNV) is maintained in a bird-mosquito transmission cycle and has become the most common arthropod-borne flavivirus in the United States. Humans, horses and other non-avian vertebrates are incidental hosts (Monath and Heinz, 1996). Infection in man can result in fever, meningitis, or encephalitis, among other symptoms (Hubálek and Halouzka, 1999; Leis et al., 2002). Approved human vaccines or therapeutics are not available and preventive measures largely focus upon mosquito control (Reisen and Brault, 2007).
The ability of different mosquito species to transmit WNV varies widely. Culex spp. are the major vectors for WNV worldwide (Nir et al., 1968; Turell et al., 2000). In the United States, the most important vectors are Culex pipiens in the East, Culex tarsalis in the Midwest and West, and Culex quinquefasciatus in the Southeast (Hayes et al., 2005). WNV has also been isolated from Aedes, Ochlerotatus and Culisetam mosquitoes (http://www.cdc.gov/ncidod/dvbid/westnile/mosquitoSpecies.htm). Aedes aegypti, a member of the Culicinae subfamily, is a major vector for numerous flaviviruses (Gould and Solomon, 2008). A. aegypti is ideal for viral pathogenesis studies because these mosquitoes are easy to cultivate and the genome has been characterized (Gubler, 1998; Nene et al., 2007; Halstead, 2008). A. aegypti is readily susceptibility to infection with WNV in the laboratory, and the virus rapidly disseminates throughout most of the mosquito after the blood meal. As with Culex spp., A. aegypti is a threat for the transmission of WNV to humans (Vanlandingham et al., 2007).
C-type lectins are a group of carbohydrate-binding proteins (Zelensky and Gready, 2005). Several members of this family are highly expressed by immune cells, including monocytes, macrophages and dendritic cells (DCs), and play a central role in activating host defenses (Robinson et al., 2006). Human mannose-binding lectin (MBL) is a pattern recognition molecule of the innate immune system that binds to sugars on the surface of invading pathogens, leading to opsonization, phagocytosis, and activation of the complement pathway (Neth et al., 2002). In contrast, some C-type lectins are recruited to facilitate flaviviral infection. In mammals, 2 membrane C-type lectins, DC-SIGN (CD209) and L-SIGN (CD209L), interact with flaviviruses via high mannose glycans on viral glycoproteins (Klimstra et al., 2003), and are essential host cell factors exploited by dengue virus (DENV) and WNV to invade immature DCs and macrophages (Geijtenbeek et al., 2000; Soilleux et al., 2002; Tassaneetrithep et al., 2003; Davis et al., 2006). Another C-type lectin, the mannose receptor (MR), also interacts with the DENV envelope protein, and may enhance viral attachment to phagocytes (Miller et al., 2008). A recent study identified C-type lectin domain family 5, member A (CLEC5A), as a DENV receptor. The association between CLEC5A and DENV does not result in viral entry, but rather stimulates the release of pro-inflammatory cytokines, potentially contributing to the pathogenesis of dengue hemorrhagic fever (Chen et al., 2008).
Protein tyrosine phosphatases (PTPs) remove phosphate groups from phosphorylated tyrosine residues, and play critical roles in cell communication, shape, motility, proliferation and differentiation (Alonso et al., 2004; Mustelin et al., 2005). One well-known PTP, protein tyrosine phosphatase receptor type C (PTPRC, CD45), is important for thymocyte development and T cell activation (Byth et al., 1996; Trowbridge and Thomas, 1994), and is expressed on all nucleated cells of hemopoietic origin (Thomas, 1989). The association between MBL and CD45 in immature T cells influences thymocyte development (Baldwin and Ostergaard, 2001).
Genome-wide RNA interference (RNAi) screening studies have revealed several hundred host factors that influence WNV or DENV infection in human or drosophila cell lines, and identified cellular pathways that have a role in viral internalization, replication, assembly or secretion (Krishnan et al., 2008; Sessions et al., 2009). The relationship, however, between flaviviruses and mosquitoes is not well understood. We now examine WNV-mosquito interactions using A. aegypti and Culex quinquefasciatus, characterize the expression profile of mosquito homologues of human susceptibility proteins (Krishnan et al., 2008) in response to viral infection, and identify a lectin-based pathway that is critical for viral infection of mosquitoes.
RESULTS
A recent RNAi screening study characterized 283 human proteins that facilitate WNV infection (Krishnan et al., 2008). We have now identified 215 homologues of these genes in A. aegypti. The expression of 32 genes was altered by the presence of WNV in mosquitoes, potentially suggesting a role in viral infection of the arthropod (Table S1). To examine their function, each of these 32 genes was therefore silenced in mosquitoes using RNAi, and the effect on viral load assessed. Knockdown of 13 genes significantly altered the viral burden in the vector (Table S1). One of these genes, AAEL000563, exhibited the most dramatic reduction in viral load after silencing, and was selected as the target for further investigation. AAEL000563 belongs to the A. aegypti galactose-specific binding C-type lectin family, shares 26% amino acid identity with human mannose-binding lectin, and was designated as mosGCTL-1 (mosquito Galactose-specific binding C-Type Lectin).
mosGCTL-1 expression increases during WNV infection of A. aegypti
To further investigate the relationship between WNV infection and mosGCTL-1 expression, we determined the viral load and mosGCTL-1 level in selected A. aegypti tissues following the inoculation of WNV into female mosquitoes. WNV was readily detectable 4 days post-infection and the viral level subsequently increased. The salivary glands and hemolymph had the highest levels of WNV, while the viral load in the midgut was substantially lower (Figure 1A). mosGCTL-1 expression was induced in diverse tissues over time, including the salivary glands, hemolymph and midgut (Figures 1B, C, D and E). Immunoblots also demonstrated an increased amount of mosGCTL-1 in WNV-infected mosquitoes (Figure 1F).
Figure 1. Effect of WNV infection on mosGCTL-1 expression.
(A) Viral distribution in A. aegypti. WNV (1 × 103 M.I.D50) was inoculated into the mosquito thorax. (B-E) mosGCTL-1 mRNA was induced by viral infection in whole A. aegypti (B), salivary glands (C), hemolymph (D), and midgut (E). Total RNA was isolated from various tissues or whole mosquitoes at 5 time points following viral infection.
(A-E) The viral load and mosGCTL-1 mRNA levels were determined by Taqman® RT-QPCR, and normalized using A. aegypti actin (AAEL011197). WNV (1×103 M.I.D50) was microinjected into each mosquito. Data are shown as the mean ± standard error (SEM). The experiments were repeated 3 times.
(F) Immunoblot to detect mosGCTL-1 in WNV-infected mosquitoes. Three WNV infected or control mosquitoes were pooled and homogenized. The supernatant was then isolated, separated by SDS-PAGE, and probed with rabbit mosGCTL-1 antisera. UI, uninfected mosquitoes. I, WNV infected mosquitoes. dpi, days post infection. 50 μg of protein from mosquito lysates was loaded into each lane. Also see Figure S1 and Table S1.
mosGCTL-1 facilitates WNV infection in A. aegypti
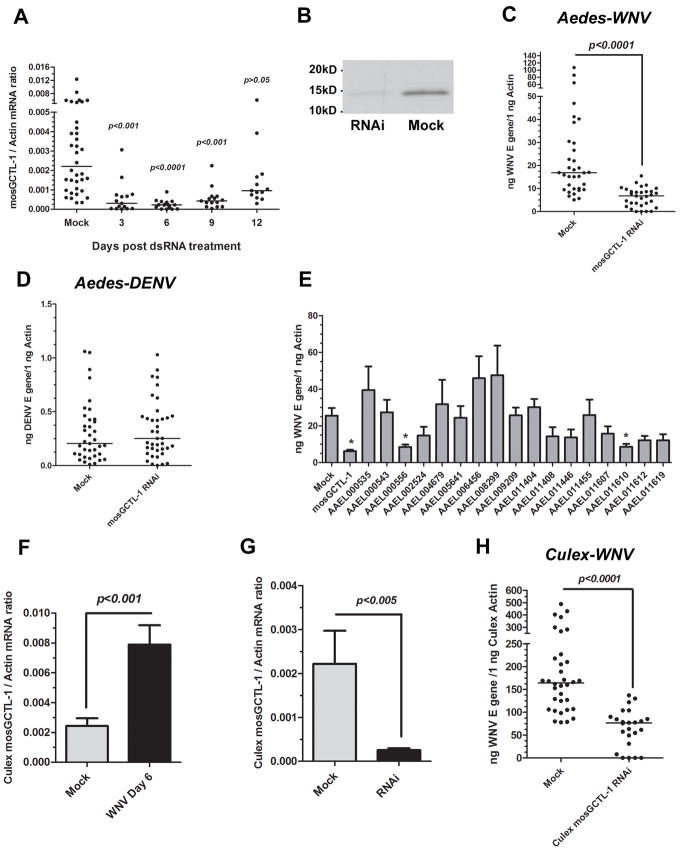
dsRNA-mediated gene silencing studies then assessed the role of mosGCTL-1 in WNV infection of mosquitoes. Total RNA from mock-injected and mosGCTL-1 dsRNA-injected mosquitoes was analyzed by RT-QPCR. Compared to the mock group, the target gene was silenced 6- to 10-fold from day 3 through 9 (Figure 2A and B). WNV was therefore inoculated into mosquitoes on day 3 following dsRNA-treatment, and the viral load was quantified on day 9. There was a 3-fold reduction in the WNV burden in mosGCTL-1 silenced A. aegypti (Figure 2C, p<0.0001), compared to controls. In contrast, the burden of a related flavivirus, dengue virus, was unaffected by mosGCTL-1 silencing (Figure 2D), suggesting that mosGCTL-1 has a specific role in WNV infection of mosquitoes.
Figure 2. The function of mosGCTL-1 in WNV infection.
(A-B) mosGCTL-1 silencing. The mock group was treated with the same amount of GFP dsRNA. mosGCTL-1- or GFP-dsRNA-treated mosquitoes were used to isolate total RNA at several time points post dsRNA injection. mRNA levels were determined by SYBR Green® RT-QPCR (A). mosGCTL-1-dsRNA-treated or mock-treated mosquitoes were collected at 6 days post gene silencing. The supernatant separated by SDS-PAGE and probed with rabbit mosGCTL-1 antisera (B).
(C-D) Silencing mosGCTL-1 impairs WNV (C), but not dengue virus (D), infection. The viral burden was examined at day 6 post-infection. 10 M.I.D50 WNV or dengue virus was used to challenge mosquitoes. The viral load was determined by Taqman® RT-QPCR, and normalized using A. aegypti actin. The result shown was representative of 4 independent experiments.
(E) The role of the mosGCTL paralogues in WNV infection. The sample number was no less than 12 mosquitoes in each group. The viral burden was determined by Taqman® RT-QPCR, and normalized with A. aegypti actin. *, p<0.05. The results were pooled from 2 independent experiments.
(F) WNV induces mosGCTL-1 homologue expression in C. quinquefasciatus. WNV-infected and mock mosquitoes were collected at 6 days post infection. Culex mosGCTL-1 mRNA was determined by SYBR Green® RT-QPCR, and normalized using C. quinquefasciatus actin (CPIJ012570). The experiment was repeated 3 times with similar results.
(G) Efficiency of Culex mosGCTL-1 RNA interference. The mock group was treated with the same amount of GFP dsRNA. mRNA levels were determined by SYBR Green® RT-QPCR.
(H) Silencing Culex mosGCTL-1 decreases the WNV burden in C. quinquefasciatus. The viral burden was determined by Taqman® RT-QPCR. The results were combined from 3 independent experiments.
(A, C-H) Statistical analysis was done with the Mann-Whitney test in all experiments. Each dot represents the mRNA levels in an individual mosquito. The horizontal line depicts the medians.
(E-G) Data are shown as the mean ± standard error (SEM).
Also see Figure S2 and Table S2.
As mosGCTL-1 is part of a multigene family with 25 members, and shares 22-48% identity with many of these genes, we examined the role of the mosGCTL paralogues in WNV and DENV infection. Twenty-one of these 25 genes were expressed in adult female A. aegypti (Table S1 and S2). Since AAEL014382 and AAEL014390 share greater than 95% identity with AAEL011607 and AAEL011619, respectively, AAEL011607 and AAEL011619 were selected for functional assays. We silenced each of the expressed mosGCTL genes and then infected the mosquitoes with virus. In the WNV challenge, two additional mosGCTL subtypes (AAEL000556 and AAEL011610) showed the similar phenotype to mosGCTL-1 (Figure 2E), and in DENV infection, silencing of 5 mosGCTL-related genes reduced the DENV burden (Figure S2).
Culex mosquitoes are a common vector for WNV in nature. We therefore extended the studies to Culex quinquefasciatus, a example of this species that is abundant in the southeast U.S.. We identified a mosGCTL-1 homologue (CPIJ010995, Culex mosGCTL-1) in C. quinquefasciatus, which has a higher degree of similarity (64%) with mosGCTL-1 than other homologues. We then determined whether Culex mosGCTL-1 had a similar role in WNV infection. Culex mosGCTL-1 was up regulated by WNV infection (Figure 2F). Silencing Culex mosGCTL-1 in C. quinquefasciatus reduced the WNV burden compared to the control group (Figure 2G and 2H, p<0.0001), suggesting that Culex mosGCTL-1 plays a role in facilitating WNV infection in Culex mosquitoes.
mosGCTL-1 interacts with WNV
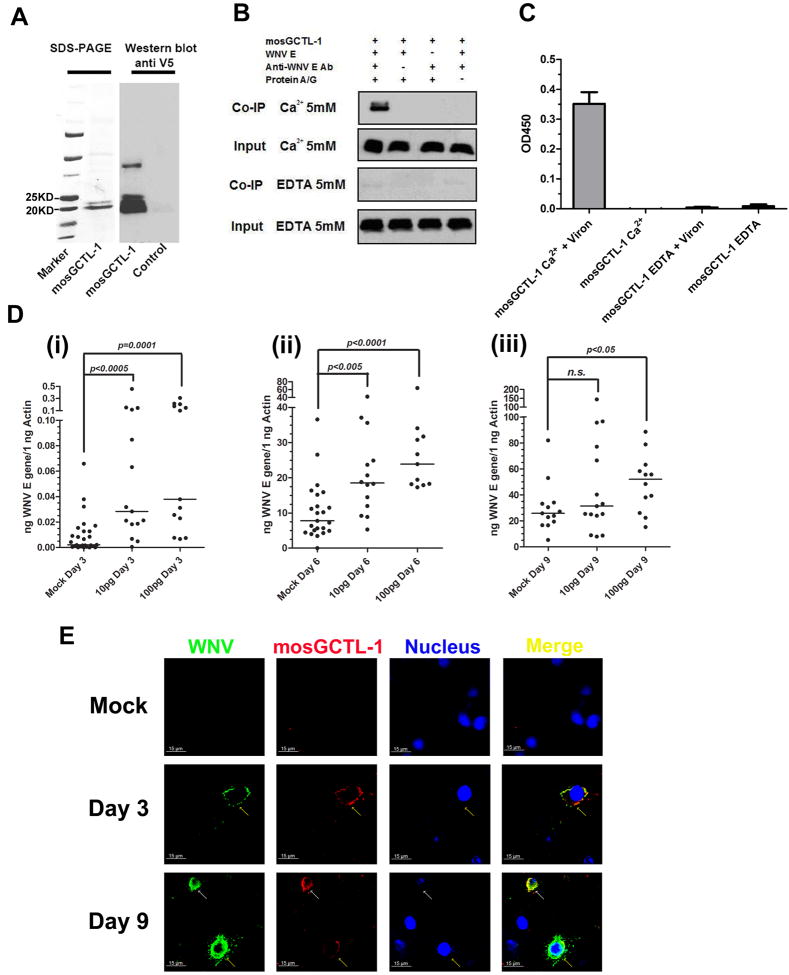
Our results show that silencing mosGCTL-1 in A. aegypti and C. quinquefasciatus reduces WNV infection. The mechanism by which mosGCTL-1 facilitates viral infection in mosquitoes was , therefore, investigated. We first generated A. aegypti mosGCTL-1 protein in a Drosophila expression system (Figure 3A), and investigated whether mosGCTL-1 associates with WNV envelope (E) protein. Immuno-precipitation experiments revealed that these 2 proteins strongly interacted in a calcium-dependent manner (Figure 3B). We then determined, by ELISA, that mosGCTL-1 bound to WNV virions. mosGCTL-1 efficiently captured virus in the presence of calcium, and this interaction was inhibited by EDTA (Figure 3C). Since this association suggests a role during infection, we premixed mosGCTL-1 and WNV and then co-injected the combination into A. aegypti and determined the viral burden. mosGCTL-1 protein significantly enhanced the viral load at days 3 (Figure 3D, i, p=0.0001) and 6 (Figure 3D, ii, p<0.0001) after viral challenge.
Figure 3. mosGCTL-1 interacts with WNV.
(A) Recombinant mosGCTL-1, produced in Drosophila cells, and purified using a Ni-His column (Left panel). Glycosylated mosGCTL-1 was detected with a V5-HRP mAb (Right panel). The control was the supernatant of mock-infected S2 cells.
(B) mosGCTL-1 interacted with WNV E protein in a co-immunoprecipitation assay. The protein complex was pulled down with a flavivirus E mAb and probed with anti-V5-HRP mAb. The experiments were repeated 3 times.
(C) mosGCTL-1 captured West Nile virions by ELISA. The interaction was determined using a flavivirus E mAb. Data are expressed as the mean ± standard error from 3 independent experiments.
(D) Co-microinjecting the purified mosGCTL-1 with WNV enhanced virus infection in A. aegypti. The various amount of purified mosGCTL-1 were pre-mixed with WNV (10 M.I.D50 per mosquito) for 1 hour at 4°C. The mixture was microinjected into mosquito and compared to the control group inoculated with the same amount WNV. The mosquitoes were collected at 3 days (i), 6 days (ii) and 9 days (iii) post WNV inoculation. Total RNA was isolated to determine the viral burden by Taqman® RT-QPCR, normalized using A. aegypti actin. Each dot represents the mRNA level in one mosquito. The horizontal line represents the medians. n.s., nonsignificance (p > 0.05). The Mann-Whitney test was used for analysis. Three independent experiments yielded similar data.
(E) The association between mosGCTL-1 and WNV in A.aegypti hemolymph. Hemolymph was collected from WNV-infected or mock-infected mosquitoes for immunofluorescence staining. WNV E protein was stained with anti-mouse IgG Alexa-488 (Green), and mosGCTL-1 was identified using anti-rabbit IgG Alexa-546 (Red). Nuclei were stained blue with To-Pro-3 iodide. The white arrow represents the induced expression of mosGCTL-1 in WNV-infected hemocytes. The yellow arrows show the infected hemocytes. Images were examined using a Zeiss LSM 510 meta confocal 63×objective lens.
Also see Figure S3.
To further characterize the association between mosGCTL-1 and WNV infection in vivo, immunofluorescence was used to examine the hemolymph and salivary glands of WNV-infected A. aegypti, at different time points. Co-localization of mosGCTL-1 and WNV was clearly observed in both tissues at various intervals after WNV infection (Figure 3E and Figure S3). In the hemolymph, some hemocytes were highly infected by WNV, and mosGCTL-1 was induced in these infected hemocytes (Figure 3E). We counted the number of mosGCTL-1-positive and WNV-infected hemocytes by microscopy. All these infected cells stained positive for mosGCTL-1. The virus spread rapidly to the salivary glands after inoculation (Figure S3), consistent with the Q-PCR data (Figure 1A). mosGCTL-1 was identified on the basement membrane of salivary glands in uninfected mosquitoes, and throughout this tissue following infection, suggesting that mosGCTL-1 was induced by WNV infection of the salivary glands (Figure S3).
mosPTP-1 captures mosGCTL-1 onto the cell surface
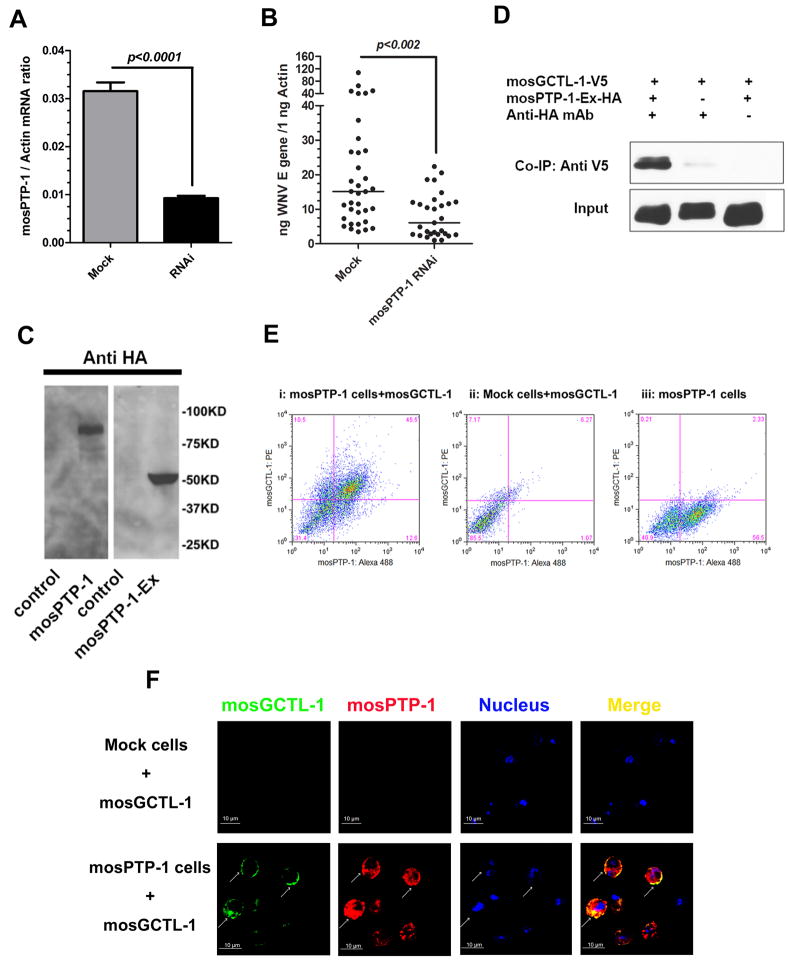
Human mannose-binding lectin (MBL) (Zelensky and Gready, 2005) and mosGCTL-1 are both secreted. Human MBL is thought to interact with several surface receptors to exert pleotrophic effects (Baldwin and Ostergaard, 2001; Arnold et al., 2006). We therefore postulated that secreted mosGCTL-1 captures WNV and presents it to a ligand on the cell surface, thereby facilitating viral entry. To assess this, we identified 11 A. aegypti homologues of human proteins that putatively interact with human MBL (Table S3) from the Human Protein Reference Database (http://www.hprd.org) and examined their roles in WNV infection of A. aegypti (Figure S4A). One mosquito CD45 homolog (AAEL013105, mosPTP-1) exhibited a similar phenotype to mosGCTL-1. The viral burden was significantly decreased in the mosPTP-1 dsRNA-treated mosquitoes (p<0.002) (Figures 4A and 4B). To further determine whether mosPTP-1 interacts with mosGCTL-1, we cloned and expressed the extracellular region of mosPTP-1 (mosPTP-1-Ex) in a Drosophila cell line (Figure 4C). mosGCTL-1 strongly interacted with mosPTP-1-Ex in a co-immunoprecipitation assay (Figure 4D). We then expressed the mosPTP-1 gene, including the transmembrane region (91bp-2202bp), in S2 cells (Figure 4C) and examined the ability of mosPTP-1 to bind mosGCTL-1 on the cell surface. A mock DNA vector transfected stable S2 cell line was used as control. More than 40% of the mosPTP-1-expressing cells bound mosGCTL-1 and showed double-positive staining in FACS (Figure 4E, i), compared with controls (Figure 4E, ii, iii). Confocal microscopy demonstrated that cells expressing high levels of mosPTP-1 in the cytoplasm, also had substantial mosGCTL-1 on the cell surface. (Figure 4F). These results suggest that mosGCTL-1 binds mosPTP-1.
Figure 4. mosPTP-1 captures mosGCTL-1 onto the cell surface.
(A) RNAi efficiency for the mosPTP-1 gene at 6 days post dsRNA treatment. The amount of mosPTP-1 mRNA was determined by SYBR Green® RT-QPCR, and normalized using A. aegypti actin. Data are represented as the mean ± standard error.
(B) Silencing mosPTP-1 decreased WNV infection. The viral burden was measured at day 6. 10 M.I.D50 of WNV was injected into each mosquito. The viral load was determined by Taqman® RT-QPCR, and normalized using A. aegypti actin. Statistical analysis was done with the Mann-Whitney test. The horizontal line depicts the medians. The result shown is a combination of 3 independent experiments.
(C) Expression of recombinant mosPTP-1 and mosPTP-1-Ex in S2 cells. mosPTP-1 and mosPTP-1-Ex genes were isolated from cDNA library of A. aegypti, and expressed as recombinant proteins with an HA tag at the N-terminus. The left panel is mosPTP-1 and the right panel is mosPTP-1-EX, probed by anti-HA tag mAb in western-blot. The control was the products from S2 cells transfected with mock DNA vector.
(D) mosGCTL-1 interacts with mosPTP-1-Ex peptide by co-immunoprecipitation. The protein complex was pulled down with a rabbit HA antibody, and probed with a V5-HRP mAb. The experiments were repeated 3 times.
(E) mosPTP-1 captured mosGCTL-1 to the cell surface by flow cytometry. A stable cell line was generated to express mosPTP-1 in S2 cells. The purified mosGCTL-1 was inoculated with mosPTP-1 expressing cells at 4°C. An empty DNA vector transfected stable S2 cell line was used as the control cells. The interaction between mosPTP-1 and mosGCTL-1 was investigated by FACS. mosPTP-1 was stained by Alexa-488; mosGCTL-1 was stained by Phycoerythrin (PE). Three independent experiments yielded similar results, and one representative study is shown in this figure.
(F) Confocal microscopy to examine for mosPTP-1 and mosGCTL-1. mosGCTL-1 was stained with Alexa-488 (Green) and mosPTP-1 was identified with Alexa-546 (Red). Nuclei were stained by To-Pro-3 iodide (blue). The images were collected using a Zeiss LSM 510 meta confocal microscopy 63×objective lens. The arrows represent the overlap between mosPTP-1 and mosGCTL-1. Also see Figure S4 and Table S3.
To test whether mosPTP-1 has a conserved role in Culex spp., we identified the mosPTP-1 homologue from the Culex quinquefasciatus genome (CPIJ014098, Culex mosPTP-1). Silencing this gene in Culex also influenced the viral burden (Figures S4B and S4C), similar to mosPTP-1 in A.aegypti (Figure 4B), suggesting that mosPTP-1 in both these mosquito species have a similar role in WNV infection.
The mosGCTL-1/mosPTP-1 pathway has a dominant role in WNV infection
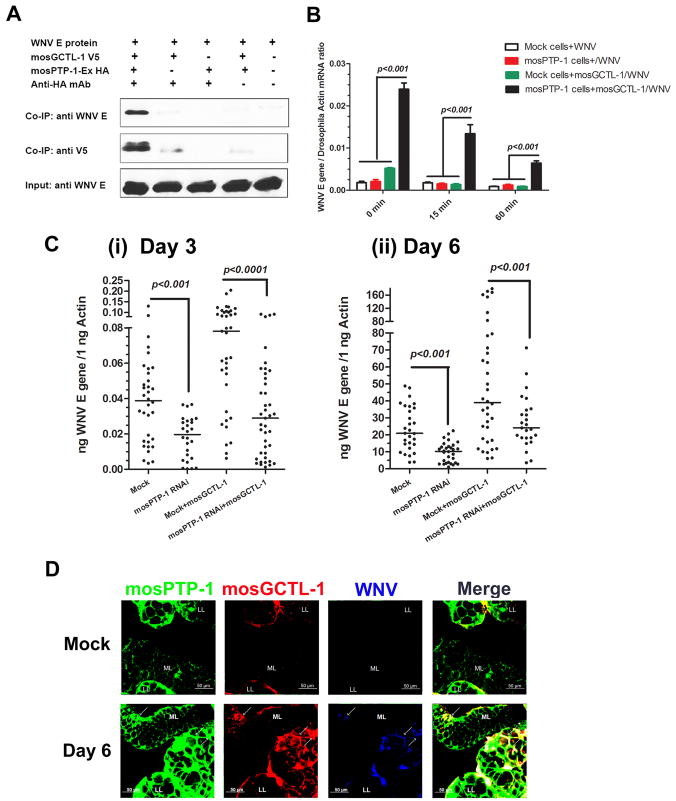
mosGCTL-1 and mosPTP-1 each facilitate WNV infection of A. aegypti in vivo and in vitro. We therefore assessed whether mosGCTL-1 and mosPTP-1 cooperate to enable viral infection. WNV E protein, mosGCTL-1 and mosPTP-1-Ex form a complex, where mosGCTL-1 is the key factor linking the other 2 proteins (Figure 5A). WNV E protein did not interact directly with mosPTP-1-Ex (Figure 5A). We then examined whether mosPTP-1 expressing cells could recruit more WNV in the presence of mosGCTL-1. mosGCTL-1 and WNV were added to cells and incubated at 4°C for membrane attachment. After washing, the cells were transferred to room temperature and collected at different time points to determine the viral burden (Krishnan et al., 2007). mosPTP-1 expressing cells incorporated up to 5-10 fold more WNV in the presence of mosGCTL-1, compared to control groups (Figure 5B). We then examined the role of the mosGCTL-1/mosPTP-1 pathway for WNV infection in A. aegypti. We knocked down the mosPTP-1 gene using dsRNA, and then inoculated the mosGCTL-1/WNV mixture at 3 days post RNAi-silencing. Silencing mosPTP-1 interfered with the ability of mosGCTL-1 to facilitate WNV infection (shown in Figure 3D) at 3 and 6 days post-challenge (Figure 5C). The viral burden of the mosPTP-1 RNAi/mosGCTL-1/WNV group was 2-3 fold lower than that of the mock/mosGCTL-1/WNV group (p<0.001), and decreased to a level similar to the group that did not receive mosGCTL-1, suggesting that mosGCTL-1 and mosPTP-1 cooperate in the same pathway to enhance WNV infection. To further determine the relationship between mosGCTL-1 and mosPTP-1 in infection, we silenced both of these genes using dsRNA. These genes were successfully knocked down in mosquitoes in the co-silenced group (Figures S5A and S5B). The decrease in the viral burden was similar in the co-silenced and individually silenced groups (Figure S5C), suggesting that mosPTP-1 is the dominant downstream receptor for mosGCTL-1 in the process of WNV infection of A. aegypti.
Figure 5. The mosGCTL-1/mosPTP-1 pathway in WNV infection.
(A) mosGCTL-1 associated with WNV E protein bound to mosPTP-1-Ex. The protein complex was pulled down with an HA antibody and probed with V5-HRP and flavivirus E protein antibody. The experiment was repeated 3 times.
(B) mosPTP-1-expressing cells recruit WN virions in the presence of mosGCTL-1. mosGCTL-1 and WNV were added to cells and incubated at 4°C for 1 hr, for membrane attachment. Cells were gently washed 3 times using cold PBS, and then moved to room temperature. At different time points, 0, 15 and 60 min, cells were collected for total RNA isolation. Control cells were the empty DNA vector transfected stable S2 cell line. Viral attachment was determined by SYBR Green® RT-QPCR, and normalized with Drosophila actin 5C (CG4027). Statistical analysis was done with ANOVA. Data are represented as the mean ± standard error. The results are representative of 3 independent experiments.
(C) Silencing mosPTP-1 impairs the function of mosGCTL-1. The mosPTP-1 gene was knocked down using dsRNA treatment. Then the mosGCTL-1/WNV mixture was inoculated at 3 days post RNAi-silencing. The virus burden was measured at 3 (i) and 6 (ii) days after the introduction of virus by Taqman® RT-QPCR. 10 M.I.D50 WNV and 100 pg mosGCTL-1 were inoculated into each mosquito. Each dot represents the mRNA level in an individual mosquito. Statistical analysis was done using the Mann-Whitney test. The horizontal line depicts the medians. The result shown is the combination of 5 independent experiments.
(D) Immunostaining of mosGCTL-1, mosPTP-1 and WNV in A. aegypti salivary glands. mosPTP-1 was stained with anti-mouse IgG Alexa-488 (Green); mosGCTL-1 was identified by anti-rabbit IgG Alexa-546 (Red); WNV E protein was probed with horse anti-E protein IgG and detected by anti-horse IgG Alexa-633 (Blue). The arrows show the sites of overlap between mosGCTL-1, mosPTP-1 and WNV at 6 days post infection. LL, Lateral Lobe; ML, Median Lobe in female A.aegypti salivary glands. Images were examined using a Zeiss LSM 510 meta confocal 25×objective lens. Also see Figure S5.
To better understand the association between mosGCTL-1, mosPTP-1 and WNV in vivo, we examined the distribution of mosPTP-1 in A. aegypti. mosPTP-1 was highly expressed in various mosquito tissues, but not induced by WNV infection (Figure S4D). The salivary glands and hemolymph were sites of abundant mosPTP-1 expression, while expression in midgut was comparatively lower (Figure S4E, F, G). We next generated mosPTP-1 antibody in mice, which recognized native mosPTP-1 protein (Figure S5E) and the mosPTP-1-Ex expressed by S2 cells (Figure S5F). We then determined the relationship between mosGCTL-1, mosPTP-1 and WNV in salivary glands by immunofluorescence. mosPTP-1 was copiously expressed on the cell surface of salivary glands (Figure 5D), thereby providing additional data to complement and extend the initial QPCR expression data (Figure S4E). Several regions in the salivary glands with substantial mosPTP-1 also demonstrated staining for mosGCTL-1 and WNV (Figure 5D).
mosGCTL-1 antisera interferes with WNV infection of mosquitoes
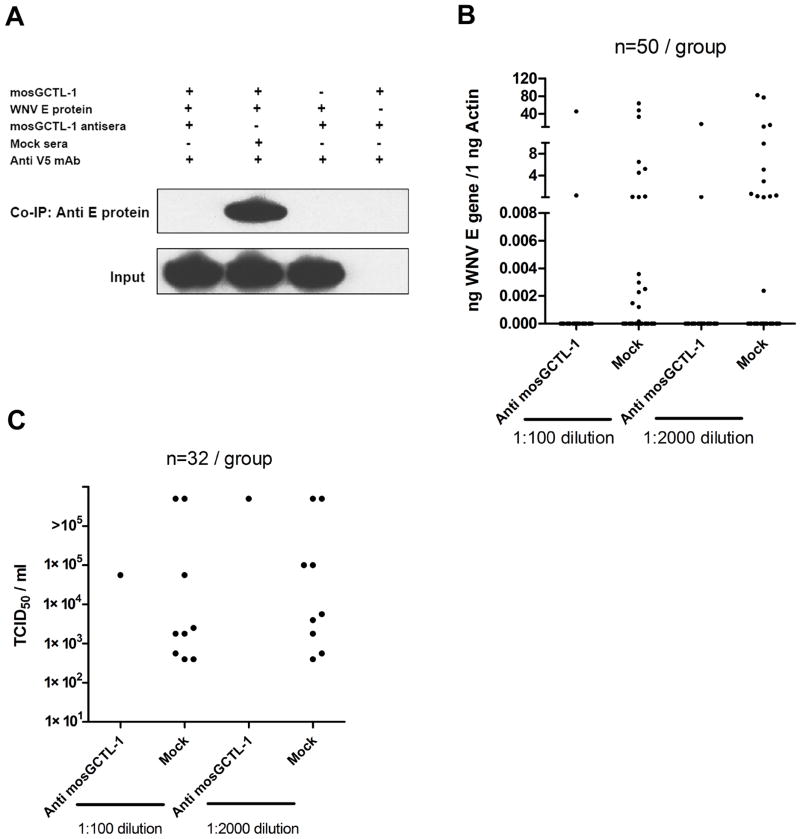
Disrupting the transfer of WNV from the vertebrate to arthropod host could theoretically diminish viral dissemination in nature. We therefore investigated whether mosGCTL-1 antisera reduces WNV infection in mosquitoes during the blood meal. We generated mosGCTL-1 antisera in rabbits, and showed that mosGCTL-1 antisera interfered with mosGCTL-1 binding to WNV E protein in vitro (Figure 6A). Then, we examined whether mosGCTL-1 antisera influenced the ability of WNV to infect mosquitoes during a blood meal. We mixed mosGCTL-1 antisera and WNV with fresh whole blood and performed membrane blood-feeding using a Hemotek®. Seven days later, mosquitoes were sacrificed to determine the infectivity rate. mosGCTL-1 antisera efficiently blocked WNV infection of A. aegypti. The number of infected mosquitoes was reduced in mosGCTL-1 antisera treated groups, compared to mock group, by QPCR (Figures 6B) or a 50% tissue culture infective doses (TCID50) assay (Figure 6C). Hence, a humoral response against mosGCTL-1 in a vertebrate host may alter WNV infection of mosquitoes during the feeding process. This hypothetically affords a strategy to develop a transmission blocking vaccine to control WNV dissemination in nature.
Figure 6. mosGCTL-1 antiserum interferes with WNV infection of mosquitoes.
(A) mosGCTL-1 antisera blocked the interaction between mosGCTL-1 and WNV E protein. mosGCTL-1 antisera or mock sera was diluted 1000-fold. The protein complex was pulled down with a V5 mAb and WNV E protein was detected using an E protein antibody.
(B-C) mosGCTL-1 antisera interrupted WNV infection during the blood meal. The antisera or control sera were diluted 100- or 2000-fold with fresh whole blood containing 5×106 pfu/ml WNV. Membrane blood-feeding was then performed using a Hemotek®. Seven days later, mosquitoes were sacrificed to determine the infectivity rate by Taqman® RT-QPCR (B) and TCID50 (C). Each group included 50 mosquitoes in the QPCR assay, and 32 mosquitoes in TCID50 assay. One dot corresponds to a mosquito. n, the number of mosquitoes in each group. The result is representative of 3 independent experiments.
DISCUSSION
As mosquitoes are prominent vectors for flaviviruses, specific interactions between the virus and arthropod likely enhance pathogen survival. In the mammalian host, C-type lectins such as DC-SIGN and the mannose receptor augment viral entry into specific DCs and macrophages (Tassaneetrithep et al., 2003; Davis et al., 2006; Miller et al., 2008). Our results show that a secreted mosquito C-type lectin, mosGCTL-1, binds to WNV in a calcium-dependent manner, and enhances viral infection. A mosquito homologue of human CD45 (mosPTP-1) recruits mosGCTL-1 to facilitate viral attachment to cells. Based on our findings, we envision a model whereby WNV that is inoculated into mosquitoes binds to secreted mosGCTL-1 in the hemolymph, thereby forming a complex in the extracellular milieu which has the ability to interact with the membrane protein, mosPTP-1, to facilitate cellular entry. The virus rapidly replicates in the mosquito thorax. This induces additional mosGCTL-1 expression, which accelerates formation of the mosGCTL-1/WNV complex -- enabling WNV to invade different mosquito tissues, and enhancing viral spread throughout the mosquito body. This mechanism, which involves WNV associating with mosGCTL-1 and then being captured by mosPTP-1 onto the cell surface in mosquitoes, suggests that an extracellular soluble protein is an important receptor for flavivirus in arthropods.
mosGCTL-1 shares homology with human MBL. In mammals, MBL is a pattern recognition molecule that recognizes carbohydrate moieties on invading microbes (Neth et al., 2002). As examples, MBL interacts with HIV envelope protein (gp120) (Saifuddin et al., 2000) and HBV surface antigen (HBsAg) (Chong et al., 2005), and has a role in the opsonization of HIV (Ezekowitz et al., 1989). In these processes, MBL associates with serine proteases, MASPs, and activates the complement system (Neth et al., 2002). Homologues of the proteins that associate with mammalian MBL have not been found in A. aegypti (Table S3), suggesting that the A. aegypti mosGCTL-1 may have different physiological functions than mammalian MBL. Invertebrates lack antibody- and interferon-based immune responses (Cheng et al., 2009). Since lectin expression is significantly up regulated by microbial infection, these molecules are presumed to participate in non-self recognition and pathogen resistance (Wilson et al., 1999; Tanji et al., 2006). Indeed, recent studies have shown that a complement-like system exists in the hemolymph of Anopheles gambiae and mediates parasite killing (Blandin et al., 2004; Povelones, et al. 2009). It is possible that mosGCTL-1 and other subtypes in this family, similar to their mammalian homologues, may normally recognize most pathogens and be involved in the arthropod complement-like system. Nevertheless, our studies showed that the expression of mosGCTL-1 is induced by WNV infection. The induced mosGCTL-1 that then binds to virus, amplifies WNV infection. Overall, these suggest a critical role for mosGCTL-1 in WNV infection of mosquitoes.
In the mammalian host, the association between MBL and the CD45 external domain primarily occurs in immature T cells, and affect the development of thymocytes (Baldwin and Ostergaard, 2001). Mammalian CD45 is expressed on the hemopoietic-originated nucleated cells (Thomas, 1989); however, the mosquito CD45 homologue, mosPTP-1, does not appear to be restricted to particular cells. As a transmembrane protein, mosPTP-1 was abundantly detected in the salivary glands and hemolymph of mosquitoes. The pattern of mosPTP-1 expression correlated with the distribution of WNV in A. aegypti. In our model, after binding to mosGCTL-1, WNV binds to membrane bound mosPTP-1. This implies that mosGCTL-1 and mosPTP-1 are recruited as receptors to facilitate cellular invasion by WNV.
Mosquito control is a common strategy to influence WNV numbers in nature (van der Meulen et al., 2005; Dauphin and Zientara, 2007). The increase in viral spread and fatalities over the last decade (Reisen and Brault, 2007; Lindsey et al., 2009) (http://www.cdc.gov/ncidod/dvbid/westnile/index.htm) suggests that additional strategies could assist in combating WNV. For arthropod-borne microbes, vector ligands that interact with pathogens are potential targets for interfering with the successful acquisition of the microbe from the vertebrate host. As an example, blocking the tick gut receptor for the Lyme disease agent limits the colonization of ticks by Borrelia. burgdorferi (Pal et al., 2004). Our studies show that blocking mosGCTL-1 within A. aegypti reduced the vector competence for WNV and interrupted the infective cycle of WNV. These results indicate that it is theoretically possible to develop a transmission-blocking vaccine to interfere with the migration of WNV from vertebrates to mosquito, thereby restricting viral dissemination in the environment.
In summary, we identified a lectin-based pathway to facilitate flaviviral entry, in which mosGCTL-1 and mosPTP-1 are cascade receptors in WNV infection of A. aegypti and C. quinquefasciatus. Characterization of mosquito ligands for WNV enhances our understanding of flavivirus-arthropod interactions, and may aid in the development of strategies to target selected points in the flaviviral life cycle and interfere with these pathogens in nature.
EXPERIMENTAL PROCEDURES
Mosquitoes and viruses
A. aegypti and C. quinquefasciatus mosquitoes were maintained in a sugar solution at 27°C and 80% humidity according to standard rearing procedures (Keene et al., 2004; Xi et al., 2008). WNV strain 2471 and DENV-2 (DENV New Guinea C strain) were passaged in mosquito C6/36 cells (Hanna et al., 2005; Lin et al., 2007; Krishnan et al., 2008). The titer of WNV for cell culture was determined by a plaque formation assay as described previously (Bai et al., 2007). The viruses for in vivo experiments were titrated in mosquitoes through thoracic microinjection. The dose of viruses was determined by 10-fold serial dilutions (i.e., 10-4, 10-5, 10-6, and 10-7) in PBS. The mosquitoes (12 in each group) were inoculated in the thorax by microinjection with 300 nl of diluted virus. On day 6, the mosquitoes were sacrificed, total RNA was isolated, and the viral load determined by RT-QPCR (Figure S1). The 50% mosquito infective dose (M.I.D50) was estimated by the Reed-Muench method (Pizzi, 1950).
Gene silencing and viral challenge in A. aegypti
dsRNA synthesis was performed as described previously (Brackney et al., 2008). The primers are shown in the Table S4. For silencing the target genes, adult female mosquitoes were kept on ice for 15 min, and then transferred to a cold tray to receive a systemic injection of dsRNA into the hemocoele. 2 μg of dsRNA/300 nl in PBS was microinjected into the thorax of each mosquito. Following a 3 days recovery period, the mosquitoes were microinjected with either WNV 10 M.I.D50/ 300nl for functional studies or 1000 M.I.D50/ 300nl for expression profile assays.
Purification of mosGCTL-1 using Drosophila expression system
mosGCTL-1 was amplified by RT-PCR from adult female A. aegypti. The primers are shown in Table S4. The PCR product was subcloned into the pMT/BiP/V5-His A vector (Invitrogen, Carlsbad, CA) and transfected into Drosophila S2 cells in combination with the hygromycin selection vector pCo-Hygro for stable transfection. The cells were selected using 300 μg/ml Hygromycin-B (Invitrogen) for 4 weeks. The resistant cells were grown in spinner flasks, switched to Express Five® serum-free medium (GIBCO, Invitrogen) for 3 days, and induced with copper sulfate at a final concentration of 500 μM for 4 days. The culture medium was cleared by centrifugation at 1000 × g for 5 min and collected for protein purification with the Talon metal affinity resin (Clontech, Mountain View, CA). The protein was eluted with 150 mM imidazole, extensively dialyzed against PBS (pH 7.8), and concentrated by centrifugal filtration through a 5 kDa filter (Millipore Corp., Bedford, MA). The protein purity was checked by SDS-PAGE and immunoblots performed with a anti-V5-HRP mouse mAb (Invitrogen).
Isolation and image of mosquito tissues
Salivary glands were dissected as the previously described (Coleman et al., 2007). Tissues were isolated, placed on sialylated slides (PGC Scientific, Gaithersburg, MD), washed in PBS, and fixed in 4% PFA at 37°C for 1 hr. For hemolymph isolation, the mosquitoes were anesthetized on ice tray, and then the proboscis was removed by forceps. Hemolymph was expelled in a droplet using the tip of the proboscis upon pressure to the thorax. Only clear droplets were collected to avoid contamination by the fat body (Han et al., 1999). Allow the liquid to be dried on sialylated slides, and fixed in 4% PFA. Samples were blocked in PBS with 1% BSA and 0.1% Triton X-100 at room temperature for 2 hrs before antibody incubations. After staining by primary and secondary antibodies, slides were imaged using the Multi-Track mode of Zeiss LSM 510 meta confocal microscope.
Membrane blood feeding
Fresh whole blood was obtained from C57BL/6J mice, placed in tubes with anticoagulant, and centrifuged at 3000 rpm for 10 min to separate the serum and cells. The serum was collected and heat inactivated at 57°C for 1 hr. The blood cells were washed in PBS 3 times to remove the anticoagulant. The cells were resuspended with serum. 5×106 pfu/ml WNV and antisera (or pre-immune sera) were added to the treated blood. About 1 ml of the virus/sera/blood mixture was used on each Hemotek® feeder. The feeder was placed on the mosquito container and the mosquitoes were allowed to feed for 1 hr. The container was transferred to 4°C for 20 min to anesthetize the mosquitoes. Carefully selected fed mosquitoes were placed into new containers and reared for 7 days. The mosquitoes were sacrificed; the whole mosquitoes were homogenized in RLT buffer (RNeasy Mini kit, QIAGEN) to measure the viral load by RT-QPCR, or grinded in PBS for virologic assays.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI 50031 and AI 070343. We thank Drs. Yue Zhang, Lei Liu, Lili Zhang and Sukanya Narasimhan for help with the biochemical studies, Ms. Debbie Beck for technical assistance, and Dr. Michel Ledizet for providing the purified WNV E protein and the flaviviral E protein antibodies. G. C. is a James Hudson Brown-Alexander Brown Coxe Postdoctoral Fellow at the Yale School of Medicine. P. W. is supported by a Career Development Award from Northeast Biodefense Center (U54-AI057158-Lipkin). E. F. is an investigator with the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTIONS G. C. and E. F. designed the experiments and wrote the manuscript; G. C. performed the majority of the experiments and analyzed data; J. C. assisted the mosquito techniques; J. F. A. provided the A. aegypti and C. quinquefasciatus mosquitoes, and helped with the mosquito feeding; J. D. and F. Q. assisted in FACS and confocal microscopy; P. W., M.N.K. and J. D. provided suggestions for the project. All authors reviewed, critiqued and provided comments to the text.
SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Experimental Procedures, five figures, and four tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett. 2006;106:103–110. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bai FW, et al. Antiviral peptides targeting the West Nile virus envelope protein. J Virol. 2007;81:2047–2055. doi: 10.1128/JVI.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TA, Ostergaard HL. Developmentally regulated changes in glucosidase II association with, and carbohydrate content of, the protein tyrosine phosphatase CD45. J Immunol. 2001;167:3829–3835. doi: 10.4049/jimmunol.167.7.3829. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Brackney DE, Foy BD, Olson KE. The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J Trop Med Hyg. 2008;79:267–274. [PMC free article] [PubMed] [Google Scholar]
- Byth KF, Conroy LA, Howlett S, Smith AJ, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhao X, Li Z, Liu X, Yan W, Zhang X, Zhong Y, Zheng Z. Identification of a putative invertebrate helical cytokine similar to the CNTF/LIF family by PSI-BLAST-based approach. J Interf Cytok Res. 2009;29:461–468. doi: 10.1089/jir.2008.0078. [DOI] [PubMed] [Google Scholar]
- Chong WP, To YF, Ip WK, Yuen MF, Poon TP, Wong WH, Lai CL, Lau YL. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037–1045. doi: 10.1002/hep.20891. [DOI] [PubMed] [Google Scholar]
- Coleman J, Juhn J, James AAA. Dissection of Midgut and Salivary Glands from Ae. aegypti Mosquitoes. JoVE 5. 2007 doi: 10.3791/228. http://www.jove.com/index/details.stp?id=228. [DOI] [PMC free article] [PubMed]
- Dauphin G, Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007;25:5563–5576. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Davis CW, Nguyen HY, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz RA, Kuhlman M, Groopman JE, Byrn RA. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Gould EA, Solomon T. Pathogenic flaviviruses. Lancet. 2008;371:500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Dengue virus-mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- Han YS, Chun J, Schwartz A, Nelson S, Paskewitz SM. Induction of mosquito hemolymph proteins in response to immune challenge and wounding. Dev Comp Immunol. 1999;23:553–562. doi: 10.1016/s0145-305x(99)00047-6. [DOI] [PubMed] [Google Scholar]
- Hanna SL, Pierson TC, Sanchez MD, Ahmed AA, Murtadha MM, Doms RW. N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J Virol. 2005;79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek Z, Halouzka J. West Nile fever: a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of Dengue and West Nile Viruses. J Virol. 2007;81:4881–4885. doi: 10.1128/JVI.02210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan MN, et al. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis AA, Stokic DS, Polk JL, Dostrow V, Winkelmann M. A poliomyelitis-like syndrome from West Nile virus infection. N Engl J Med. 2002;347:1279–1280. doi: 10.1056/NEJM2002c021587. [DOI] [PubMed] [Google Scholar]
- Lin CC, Yang CF, Tu CH, Huang CG, Shih YT, Chuang CK, Chen WJ. A novel tetraspanin C189 upregulated in C6/36 mosquito cells following dengue 2 virus infection. Virus Res. 2007;124:176–183. doi: 10.1016/j.virusres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Lindsey NP, Hayes EB, Staples JE, Fischer M. West Nile virus disease in children, United States, 1999-2007. Pediatrics. 2009;123:e1084–1089. doi: 10.1542/peds.2008-3278. [DOI] [PubMed] [Google Scholar]
- Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Heinz FX. Flaviviruses. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Nene V, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- Nir Y, Goldwasser R, Lasowski Y, Margalit J. Isolation of West Nile virus strains from mosquitoes in Israel. Am J Epidemiol. 1968;87:496–501. doi: 10.1093/oxfordjournals.aje.a120839. [DOI] [PubMed] [Google Scholar]
- Pal U, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Pizzi M. Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum Biol. 1950;22:151–190. [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W, Brault AC. West Nile virus in North America: perspectives on epidemiology and intervention. Pest Manag Sci. 2007;63:641–646. doi: 10.1002/ps.1325. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reise Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- Saifuddin M, Hart ML, Gewurz H, Zhang Y, Spear GT. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. J Gen Virol. 2000;81:949–955. doi: 10.1099/0022-1317-81-4-949. [DOI] [PubMed] [Google Scholar]
- Sessions OM, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1450. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilleux EJ, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- Tanji T, Kobayashi AO, Natori S. Participation of a galactose-specific C-type lectin in Drosophila immunity. Biochem J. 2006;396:127–138. doi: 10.1042/BJ20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneetrithep B, et al. DC-SIGN (CD209) mediates dengue virus infection of human Dendritic Cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am J Trop Med Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- van der Meulen KM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637–657. doi: 10.1007/s00705-004-0463-z. [DOI] [PubMed] [Google Scholar]
- Vanlandingham DL, et al. Short report: relative susceptibilities of south Texas mosquitoes to infection with West Nile virus. Am J Trop Med Hyg. 2007;77:925–928. [PubMed] [Google Scholar]
- Wilson R, Chen CW, Ratcliffe NA. Innate immunity in insects: the role of multiple, endogenous serum lectins in the recognition of foreign invaders in the Cockroach, Blaberus discoidalis. J Immunol. 1999;162:1590–1596. [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.