Abstract
We previously demonstrated that adding pyruvate to Perfadex® increased graft metabolism during 24 hours storage and improved reperfusion lung function. This increased metabolism was associated with progressively lower storage solution pH over the preservation interval. We hypothesized that more effective pH regulation would result in further improvements in lung survival after hypothermic storage. Rat lungs were stored for 24 hours in Perfadex, Perfadex with N-2-hydroxyethylpiperazine-propanesulfonic acid buffer (HEPES), pyruvate-modified Perfadex, and pyruvate-modified Perfadex with HEPES. pH change in the storage solution was measured. Structural lung injury was evaluated on hematoxylin-eosin stained tissue sections. Cell death was quantified by measuring necrotic cells by trypan blue exclusion and apoptotic cells by TUNEL assay. Lungs stored in Perfadex® demonstrated the greatest degree of cell death. Lungs from the Pyruvate group exhibited decreased cell death despite greater acidosis. The addition of HEPES reduced cell death and preservation solution acidosis in both Perfadex and pyruvate-modified Perfadex (p<.05). Almost all cell death resulted from necrosis. Adding pyruvate to the preservation solution increases acid formation during storage but reduces cell death. HEPES ameliorates this acidosis and reduces allograft cell destruction. Increasing the preservation solution buffering capacity may be a simple strategy to improve lung preservation for transplantation.
Introduction
Organs preserved for transplantation are stored under deep hypothermia in solutions designed to reduce metabolism, conserve energy stores, and maintain the cellular integrity of the organ. Despite these measures, some degree of substrate metabolism continues even under these extreme conditions. Progressive acidosis is a consequence of these low levels of metabolic activity, particularly as the preservation interval is extended. Modern preservation solutions therefore contain various buffers to maintain a physiologic pH and limit graft acidosis [1]. Most solid organs are stored depleted of oxygen and generate acidic species as a consequence of anaerobic metabolism, especially lactate produced via glycolysis. However, lungs stored for transplantation are inflated with oxygen and also undergo oxidative metabolism during the storage interval [2]. This ongoing aerobic metabolism produces carbon dioxide in addition to organic acids further increasing the proton burden that must be buffered to ensure graft viability.
We previously demonstrated that superior lung preservation may be achieved if metabolic support of the graft is optimized by adding pyruvate to low-potassium dextran (LPD) preservation solution [3]. However, an unwanted consequence of this increased metabolism may be the development of worsening cellular acidosis during storage and strategies designed to improve the buffering capacity of the solution could result in superior graft preservation. The purpose of the current study was to evaluate the effects of increased buffering capacity within the storage solution on pH and cellular survival under conditions of low and increased lung metabolism.
Methods
Male Sprague-Dawley rats weighing 370–480 grams were given access to food and water ad libitum and used in an institutionally approved animal research protocol. All animals were cared for in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources (National Academy of Sciences) and published by the National Institutes of Health (NIH publication number 86-23, revised 1996).
Organ Harvest and Preservation
Rats were anesthetized by intraperitoneal injection of 4 % chloral hydrate (1ml/100g) and ventilated via tracheostomy with a Columbus Instruments (Columbus, OH) ventilator at 50 breaths per minute, 10 ml/kg tidal volume, 5cm H2O positive end-expiratory pressure, and 1.00 FiO2. After heparin administration (1000 units/kg) and median sternotomy, lungs were passively flushed with 60ml/kg of cold (4°C) storage solution and rapidly cooled with saline slush. Lungs were placed in 100ml preservation solution, and stored semi-inflated at functional residual capacity for 24 hours at 10°C.
Experimental Groups
Five groups were evaluated to measure the effects of increased metabolism and pH buffer on cellular survival. Storage solution pH in all groups was 7.4 ± .2. The Perfadex Group consisted of standard Perfadex in which the pH was adjusted with sodium bicarbonate. In the Perfadex/HEPES Group, Perfadex was supplemented with 30 mmoles/liter of HEPES to provide increased buffer capacity and pH was adjusted to 7.4 with potassium hydroxide. The Pyruvate Group consisted of pyruvate-enriched (32 mmoles/liter) LPD solution designed to increase allograft metabolism as previously described [3]. The pH in this solution was adjusted with sodium bicarbonate as in the Perfadex Group. In the Pyruvate/HEPES Group, rat lungs were preserved in the same solution as the Pyruvate Group except that 30 mmoles/liter of HEPES was added. See Table 1. A Control Group of lungs which was flushed with Perfadex but not preserved was also evaluated. The pH in the preservation solution after 24 hours of storage was measured with a calibrated pH probe (Beckman-Coulter Inc., Fullerton, CA) and the pH difference between initial and final (24 hour) measurements was calculated.
Table 1.
Preservation Solution Composition
| Component | Perfadex Group | Perfadex/HEPES Group |
Pyruvate Group | Pyruvate/HEPES Group |
|---|---|---|---|---|
| Na | 138 | 138 | 133 | 133 |
| Cl | 142 | 142 | 105 | 105 |
| K | 6.0 | 6.0 | 6.0 | 6.0 |
| Mg | 0.8 | 0.8 | 0.8 | 0.8 |
| SO4 | 0.8 | 0.8 | 0.8 | 0.8 |
| PO4 | 0.8 | 0.8 | 0.8 | 0.8 |
| Glucose | 5 | 5 | 5 | 5 |
| Pyruvate | - | - | 32 | 32 |
| Buffer | HCO3 to 7.4 | 30mM HEPES | HCO3 to 7.4 | 30mM HEPES |
| Dextran 40 | 5% | 5% | 5% | 5% |
Concentrations are in millimoles per liter unless otherwise noted
Quantification of Cell Death and Histology
Cellular viability in stored allografts after 24 hours of preservation was assessed by trypan blue exclusion (necrotic cell death) or terminal deoxynucleotidyl transferase biotin-dUTP nick-end-labeling (TUNEL) assay (apoptotic cell death). At the end of the storage interval, lungs were flushed with 20 mL 0.5millimoles/liter trypan blue via a pulmonary artery cannula. After 5 minutes, lungs were washed with normal saline through the pulmonary artery until a clear effluent was obtained. Finally, lungs were flushed with 20 mL 4% paraformaldehyde and fixed for 24 hours. Representative 4µm tissue sections were then obtained and paraffin-embedded for later analysis. TUNEL assays on tissue sections were performed using the Promega DEAD End TUNEL kit (Madison, WI) according to the manufacturer’s instructions. Rat thymus and unstained tissue sections were utilized as positive and negative controls, respectively. Light and fluorescence microscopy were performed. Cell death was quantified by the fraction of trypan blue and TUNEL positive cells per high power field (HPF, 400X) as described by Fischer and colleagues [4]. Only cells that demonstrated nuclear characteristics of apoptosis were considered TUNEL positive [5]. Nine high power fields per lung were counted – three each in the upper, middle and lower lung zones. The observer was blinded to the experimental group in all cases. Cells that stained trypan blue positive only were considered to have undergone cell death by necrosis. TUNEL positive and cells that were positive for both trypan blue and TUNEL were classified as apoptotic cell death. The total apoptotic fraction equaled: TUNEL positive nuclei / total number of cells. The total necrotic fraction was trypan blue positive only cells / total number of cells. Hematoxylin – eosin (HE) stained tissue sections from lungs were evaluated for tissue architectural abnormalities or evidence of cellular injury by a pathologist (SM) who was blinded to the experimental group.
Statistics
Results are reported as mean and standard error of the mean (SEM). Commercially available software (SigmaStat®, STSS Inc., Chicago, IL) was used for statistical analysis. Data were compared by one-way analysis of variance. Differences between groups were evaluated with the Fisher’s LSD post-hoc test and a p value less than 0.05 was considered significant.
Results
After 24 hours storage, significant differences in preservation solution pH were noted. See Table 2. The lowest pH was encountered in lungs stored with pyruvate-modified LPD, buffered with sodium bicarbonate (Δ pH = 0.53 ± 0.03). This change on pH was significantly greater than in lungs stored in Perfadex buffered with sodium bicarbonate (Δ pH = 0.42 ± .03) [p<.05]. Both these groups had greater pH changes than lungs stored in either of the HEPES buffered solutions (Perfadex/HEPES Group Δ pH = 0.12 ± .01, Pyruvate/HEPES Group Δ pH = 0.15 ± .02) [p<.05].
Table 2.
pH Change after 24 Hour Storage
| Group | Delta pH |
|---|---|
| Perfadex | −0.42±0.03† |
| Perfadex/HEPES | −0.12±0.01 |
| Pyruvate | −0.53±0.03* |
| Pyruvate/HEPES | −0.15±0.02 |
p<.05 versus all other groups
p<.05 versus all HEPES containing groups
Significant differences in cell death were noted between groups. See Table 3 for cell count data. As expected, cell death was minimal in the Control group. The dominant mechanism of cell death in all groups was by necrosis, not apoptosis. Among stored groups, improved pH regulation (by adding HEPES) and increased metabolism (by adding pyruvate) appeared to correlate with cellular survival. Lungs stored in Perfadex with bicarbonate buffer alone had significantly greater cell death than all other groups. Improved buffer capacity (Perfadex/HEPES Group) or the addition of pyruvate (Pyruvate Group) improved lung preservation to a similar degree compared to Perfadex alone (p<.05). The least cell death among stored lungs was encountered when allografts were stored in pyruvate and HEPES supplemented preservation solution. This difference was not significant compared to Perfadex/HEPES when expressed as counts per high power field (p=.09) (See Table 3) but reached statistical significance when counts were indexed to the total cell count per HPF (Pyruvate/HEPES p<.05 versus all other stored groups). See Figure 1.
Table 3.
Apoptotic, Necrotic, and Total Cell Counts per High Power Field
| Group | Apoptotic Cells/ HPF |
Necrotic Cells/ HPF |
Total Dead Cells/ HPF |
Total Cells/ HPF |
|---|---|---|---|---|
| Control | .4±.1 | 7.7±4 | 8.1±4 | 541±46 |
| Perfadex | 3.5±3 | 130±14* | 133±15* | 554±35 |
| Perfadex/HEPES | .8±.1 | 70±6 | 71±6 | 506±29 |
| Pyruvate | .6±.3 | 77±11 | 78±11 | 538±21 |
| Pyruvate/HEPES | 3.5±3 | 44±6† | 47±7† | 619±35 |
Data are mean ± SEM
p>.05 versus all other groups
p<.05 versus Pyruvate
Figure 1. Fraction Necrotic, Apoptotic and Total Cell Death.

* - p<.05 versus all other groups
† - p<.05 versus Perfadex
Cell death was significantly lower in the Pyruvate/HEPES group compared to all other experimental groups. Cell death was reduced in Pyruvate and Perfadex/HEPES groups compared to Perfadex alone.
No structural histologic evidence of lung injury was noted in any of the experimental groups. HE stained tissue section evaluation for trypan blue cells correlated with the fluorescence microscopy results. See Figure 2. Again, few dead cells were noted in the Pyruvate/HEPES group. A similar, moderate number of dead cells were present in the Pyruvate and Perfadex/HEPES group, whereas numerous trypan blue positive cells were noted in the Perfadex group.
Figure 2. Histology.
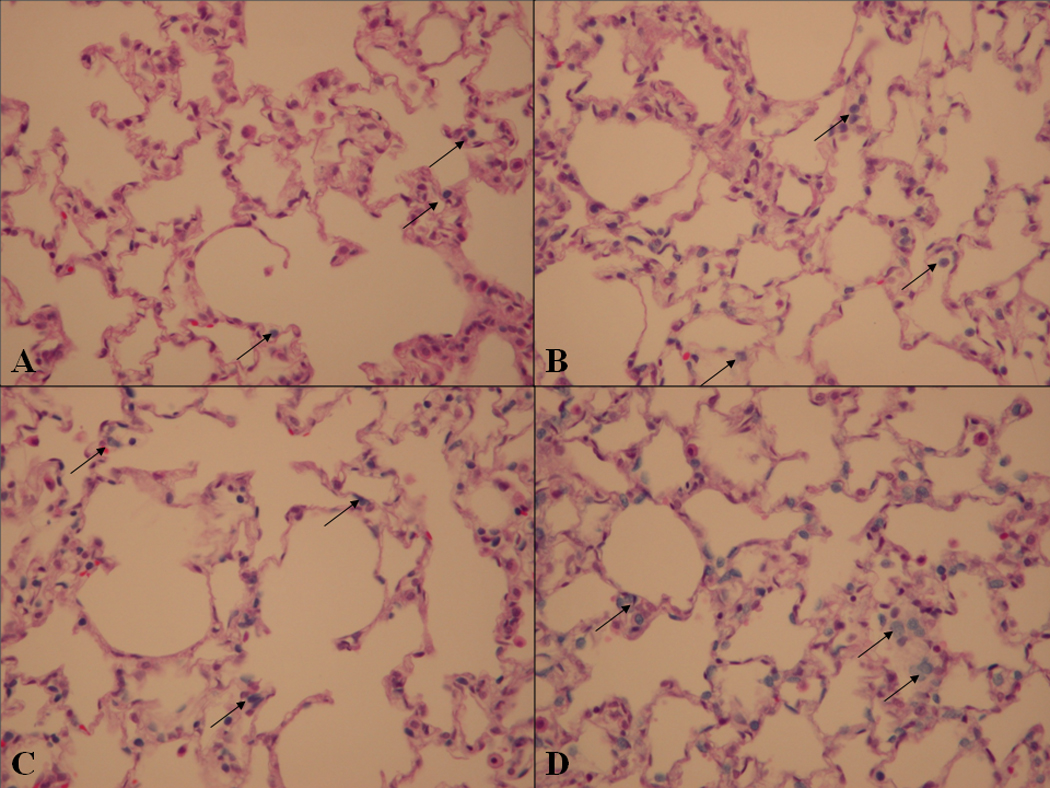
Panel A Pyruvate/HEPES, Panel B Perfadex/HEPES, Panel C Pyruvate, Panel D Perfadex. Arrows point to examples of non-viable cells.
Cell death as measured by trypan blue exclusion is minimal in Panel A. A moderate number of nonviable cells are noted in both Panels B and C. Extensive cell death is present in Panel D.
Discussion
Improved survival after lung transplantation has been reported after organ storage in the LPD preservation solution Perfadex [6,7]. The intrinsic buffering capacity of this solution is quite low compared to other organ preservation solutions such has University of Wisconsin solution or Celsior [8]. The buffer contained in Perfadex consists of small amounts of phosphate buffers resulting in a moderately acidotic pH (5.5 – 6.0). In clinical practice pH adjustments are then made with tromethamine (THAM), sodium bicarbonate or other clinically acceptable alkaline buffers to achieve a physiologic pH prior to flushing the donor organ. Sodium bicarbonate was chosen as the control buffer to correlate findings in this study with our prior experiments. Bicarbonate was superior to THAM in the previous experiments with better pH regulation and a statistically non-significant improvement in reperfusion function (unpublished data). We selected HEPES as an additive in the current study because of its physiologic pKa and superior buffering capacity compared to these other compounds [9].
Lung preservation appears to be influenced by the storage solution pH. Both Hiramatsu [10] and Shiraishi [11] reported improved reperfusion lung function when grafts where stored at physiologic or slightly alkalotic pH. Acidosis is an inevitable consequence after prolonged storage of solid organs. Lung preservation is limited to a relatively brief ischemic period during which standard pH protective strategies appear adequate. Our data suggest that as the storage interval is extended, the buffering capacity of Perfadex is overwhelmed and significant changes in pH occur unless the buffer composition of the solution is modified. Other investigators have modified buffers in preservation solutions with subsequent improvements in organ preservation. By adding histidine and other amino acids to University of Wisconsin Solution, for example, Churchill et al were able to demonstrate improved pH regulation and cellular energy state in a model of liver transplantation [12].
The current lung preservation experiment demonstrated that both regulating pH by adding HEPES and supporting cellular metabolism by adding pyruvate appear to improve pneumocyte survival after prolonged storage. Pyruvate is the preferred oxidative substrate of the lung [13] but also has the potential to act as a free radical scavenger. In prior studies, we noted beneficial effects of pyruvate on reperfusion lung function. In those experiments, it was unclear whether these effects occurred during preservation or if the protective effects of pyruvate were realized during reperfusion as others have speculated [14]. The current study suggests that supporting lung metabolism during the preservation interval with pyruvate substantially reduces allograft cell loss even prior to reperfusion.
Cell death during lung preservation has been previously evaluated using comparable techniques to those applied in the current study, both in animal models and human studies [15,16]. The degree of cell death after 24 hour storage in the Perfadex group correlates well with data reported by Fischer et al. who utilized a similar model and similar methods of analysis. As in our study, Fischer noted that most cell death in the rat after long-term ischemia was by necrosis [4], and standard histologic evaluation of lungs even after extended storage demonstrated minimal histologic abnormalities [17], as in our experience.
Shorter storage intervals have been associated with higher fractions of apoptotic cell death [4]. We hypothesized that as organ preservation is improved, a shift from necrotic to apoptotic cell death might be noted. However, despite substantial decreases in cell death with improved pH control, the fraction and total number of apoptotic cells did not increase. It is possible that as preservation is extended, the capability of pneumocytes to undergo regulated cell death may be altered, leaving necrotic cell death as the dominant mechanism of cell loss [4].
The current study has several limitations. First, measurements of the preservation solution pH may not reflect the intracellular acid-base status of the stored organ. It is possible that intracellular pH might be maintained by exporting protons into the extracellular milieu thus resulting in the observed lower preservation solution pH. Secondly, although we focused on cell death during storage, important metabolic and cellular events occur during reperfusion that may lead to amelioration or progression of cellular acidosis and cell death. Correlation between pH, cell death and histology during storage, and reperfusion function within the same lungs is not possible and any reperfusion data using different animals with four different experimental groups would have to be interpreted cautiously. We therefore chose the cell death assay to directly evaluate the effect of pH and pyruvate on preservation of donor lungs. However, our prior experiments have suggested that some of the preservation solution modifications used in the current study (addition of pyruvate) do translate into improved reperfusion lung function when compared to Perfadex alone [3], and alterations that lead to reduced cell death during storage appear to correlate with superior lung performance after reperfusion [4]. We are planning on further reperfusion experiments once additional modifications to the preservation technique specifically targeting the reperfusion period have been made.
In summary, the current experiments suggest that cellular survival in stored lungs is dependent on maintaining a physiologic pH. Improving the pH buffering capacity in the preservation solution substantially influences allograft cell death during hypothermic storage, even in the presence of substrates that increase metabolism. Increasing the buffering capacity of lung preservation solutions may represent a simple strategy to improve results of lung transplantation.
Acknowledgments
This research was supported by grants from the National Institutes of Health (2 P41 RR02584), the American Lung Association, and the Sarah M. and Charles E. Seay Distinguished Chair in Thoracic Surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sara Milchgrub, Email: sara.milchgrub@utsouthwestern.edu.
Michael E. Jessen, Email: michael.jessen@utsouthwestern.edu.
Dan M. Meyer, Email: dan.meyer@utsouthwestern.edu.
References
- 1.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplant. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Peltz M, Hamilton TT, He TT, Adams GA, Chao RY, Jessen ME, Meyer DM. Lung preservation solution substrate composition affects rat lung oxidative metabolism during hypothermic storage. J Resp Physiol Neurobiol. 2005;148:275–283. doi: 10.1016/j.resp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Peltz M, He TT, Adams GA, Robert Y, Chao MD, Jessen ME, Meyer DM. Pyruvate-modified Perfadex® improves lung function after long-term hypothermic storage. J Heart Lung Transplant. 2005;24:896–903. doi: 10.1016/j.healun.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JFM, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162:1932–1939. doi: 10.1164/ajrccm.162.5.9910064. [DOI] [PubMed] [Google Scholar]
- 5.Jerome KR, Vallan C, Jaggi R. The TUNEL assay in the diagnosis of graft-versus-host disease: caveats for interpretation. Pathology. 2000;32:186–190. [PubMed] [Google Scholar]
- 6.Bertolotti A, Gómez C, Lascano E, Negroni J, Cuniberti L, Yannarelli G, Laguens R, Shiraishi J, Favaloro R. Effect of Preservation Solution on Graft Viability in Single-Lung Transplantation From Heart-Beating Donors in Pigs. Transplant Proc. 2007;39:355–357. doi: 10.1016/j.transproceed.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Strüber M, Wilhelmi M, Harringer W, Niedermeyer J, Anssar M, Künsebeck A, Schmitto JD. Flush perfusion with low potassium dextran solution improves early graft function in clinical lung transplantation. Eur J Cardiothorac Surg. 2001;19:190–194. doi: 10.1016/s1010-7940(00)00631-x. [DOI] [PubMed] [Google Scholar]
- 8.Paddilla AM, Padilla JD. Lung preservation - current practices. Arch Bronchoneumol. 2004;40:86–93. doi: 10.1016/s1579-2129(06)60200-0. [DOI] [PubMed] [Google Scholar]
- 9.Baicu SC, Taylor MJ. Acid-base buffering in organ preservation solutions as a function of temperature: new parameters for comparing buffer capacity and efficiency. Cryobiology. 2002;45:33–48. doi: 10.1016/s0011-2240(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu Y, Muraoka R, Chiba Y, Sasaki M. Influence of pH of preservation solution on lung viability. Ann Thorac Surg. 1994;58:1083–1086. doi: 10.1016/0003-4975(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 11.Shiraishi T, Igisu H, Shirakusa T. Effects of pH and temperature on lung preservation: a study with an isolated rat lung reperfusion model. Ann Thorac Surg. 1994;57:639–643. doi: 10.1016/0003-4975(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 12.Churchill TA, Kneteman N. Investigation of a primary requirement of organ preservation solutions. Supplemental buffering agents improve hepatic energy production during cold storage. Transplantation. 1998;65(4):551–559. doi: 10.1097/00007890-199802270-00017. [DOI] [PubMed] [Google Scholar]
- 13.Peltz M, He TT, Adams GA, Chao RY, Meyer DM, Jessen ME. Characterizing lung metabolism with carbon-13 MRS in a small animal model: evidence of gluconeognesis during hypothermic storage. Transplantation. 2005;80:417–420. doi: 10.1097/01.tp.0000169129.45433.b6. [DOI] [PubMed] [Google Scholar]
- 14.Cicalese L. Pyruvate in organ transplantation. JPEN. 2001;25:216–218. doi: 10.1177/0148607101025004216. [DOI] [PubMed] [Google Scholar]
- 15.Fischer S, Cassivi SD, Xavier AM, Cardella JA, Cutz E, Edwards V, Liu M, Keshavjee S. Cell death in human lung transplantation: apoptosis induction in human lungs during ischemia and after transplantation. Ann Surg. 2000;231:424–431. doi: 10.1097/00000658-200003000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon KY, Cho CH, Kim SP, Park CK, Jheon SH. Apoptosis Induced by Preservation and Reperfusion in Canine Lung Transplantation. Transplant Proc. 2003;35:134–137. doi: 10.1016/s0041-1345(02)03813-7. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Hopkinson D, Liu M, MacLean AA, Edwards V, Cutz E, Keshavjee S. Raffinose Improves 24-Hour Lung Preservation in Low Potassium Dextran Glucose Solution: A Histologic and Ultrastructural Analysis. Ann Thorac Surg. 2001;71:1140–1145. doi: 10.1016/s0003-4975(01)02426-2. [DOI] [PubMed] [Google Scholar]


