Abstract
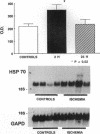
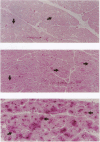
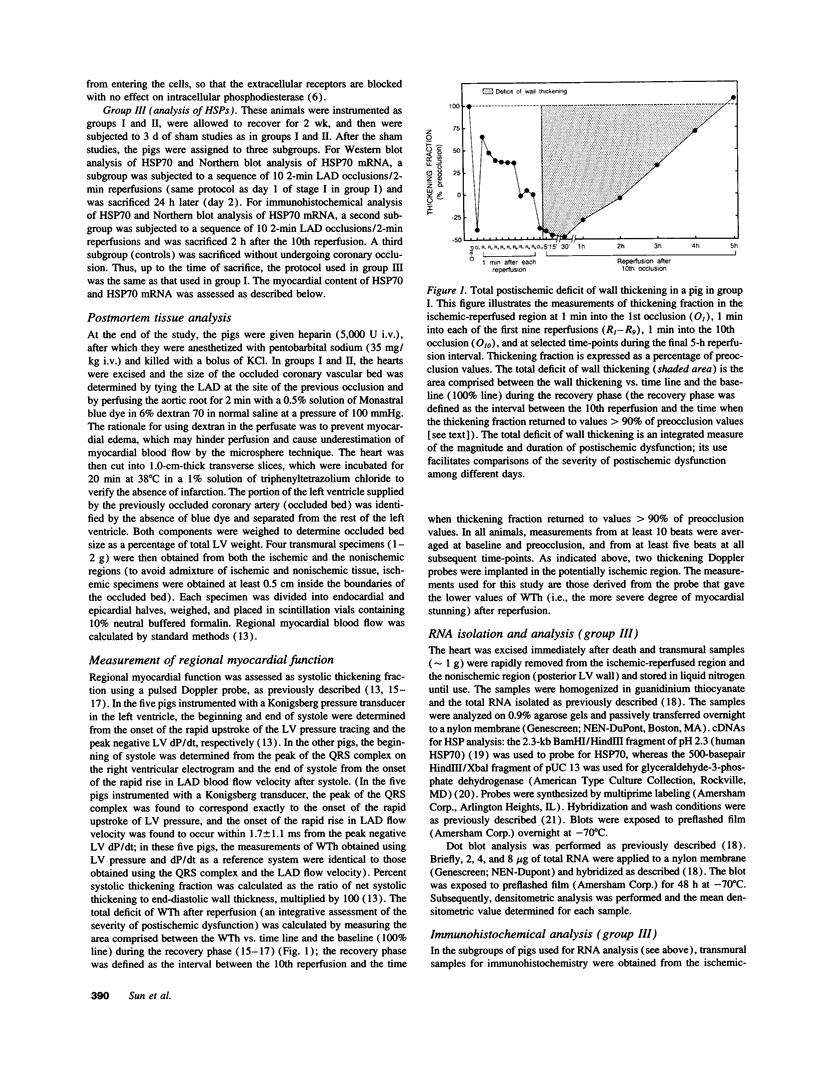
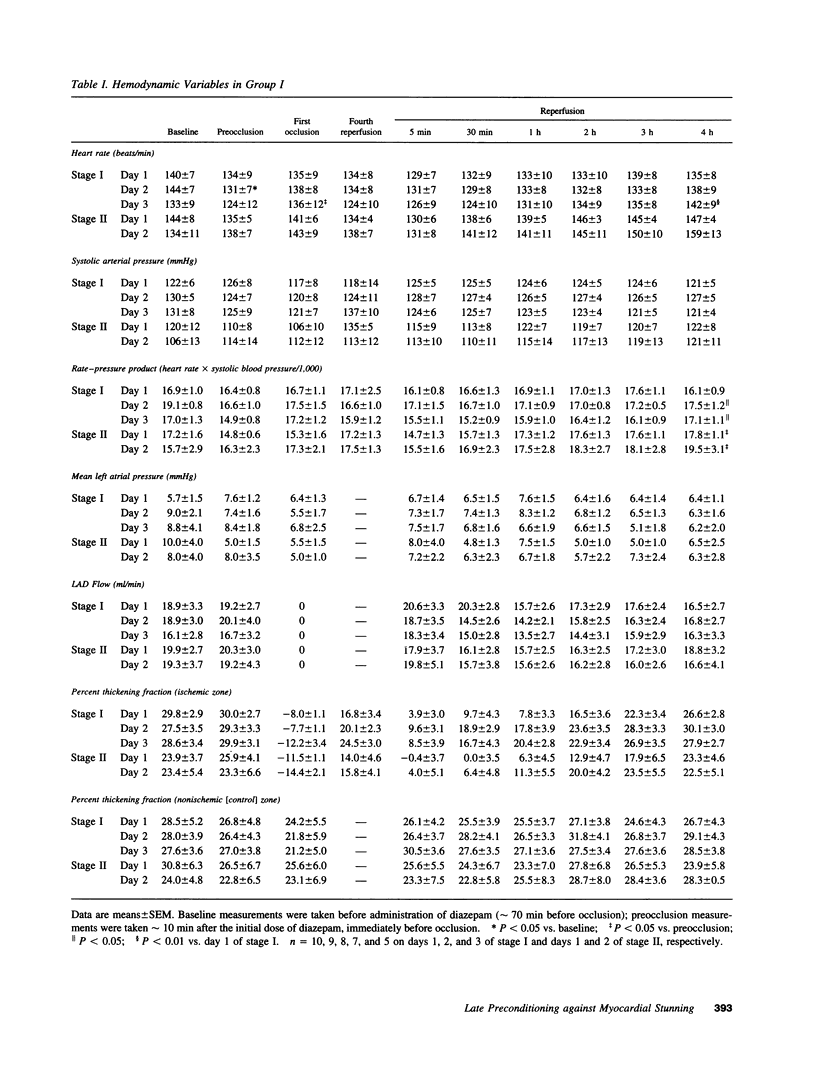
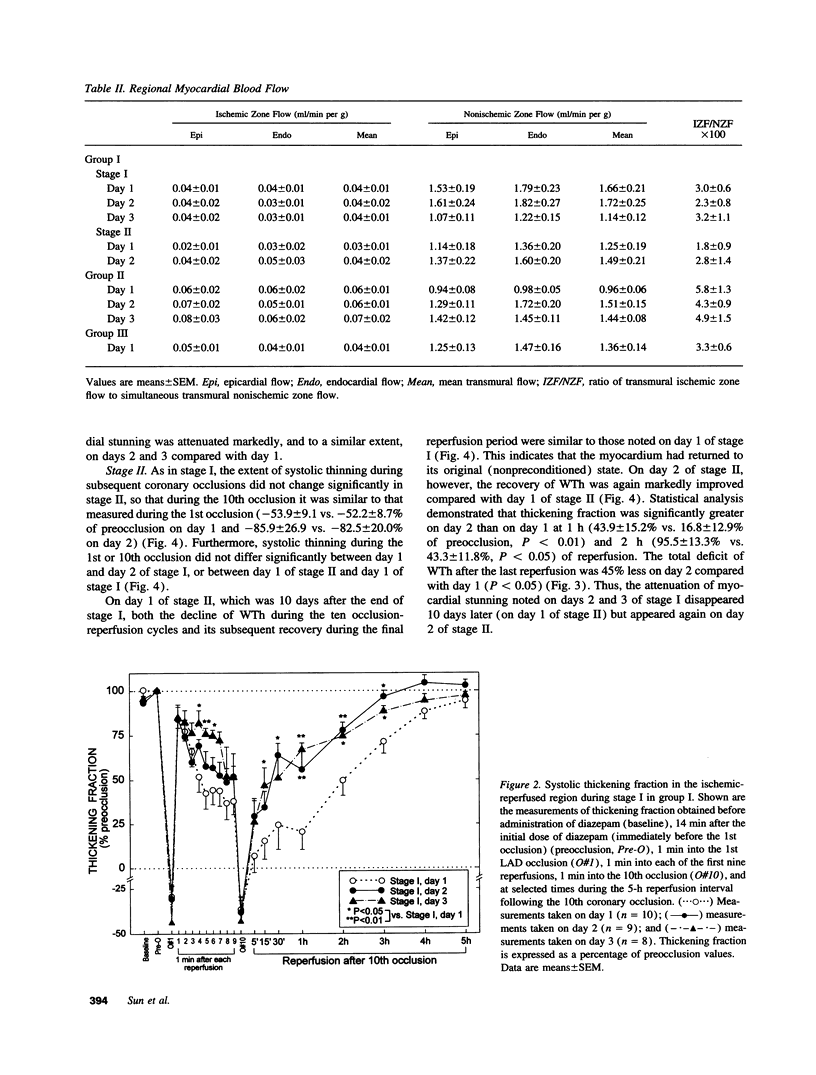
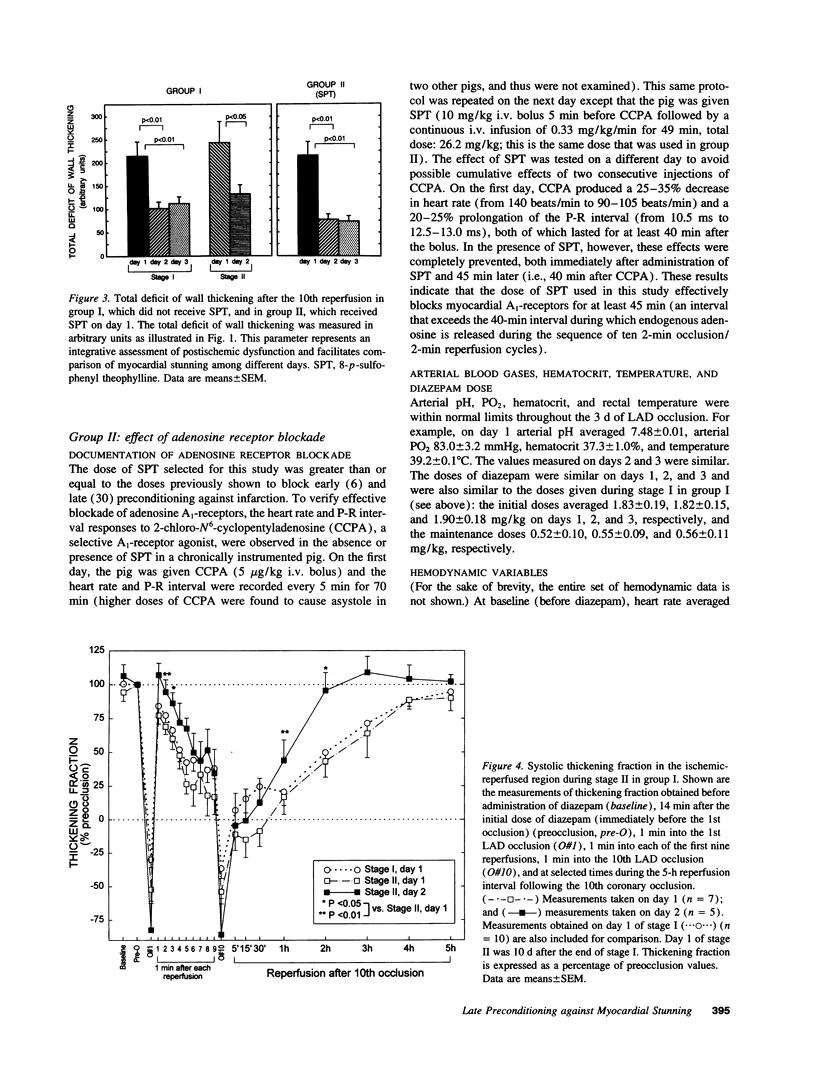
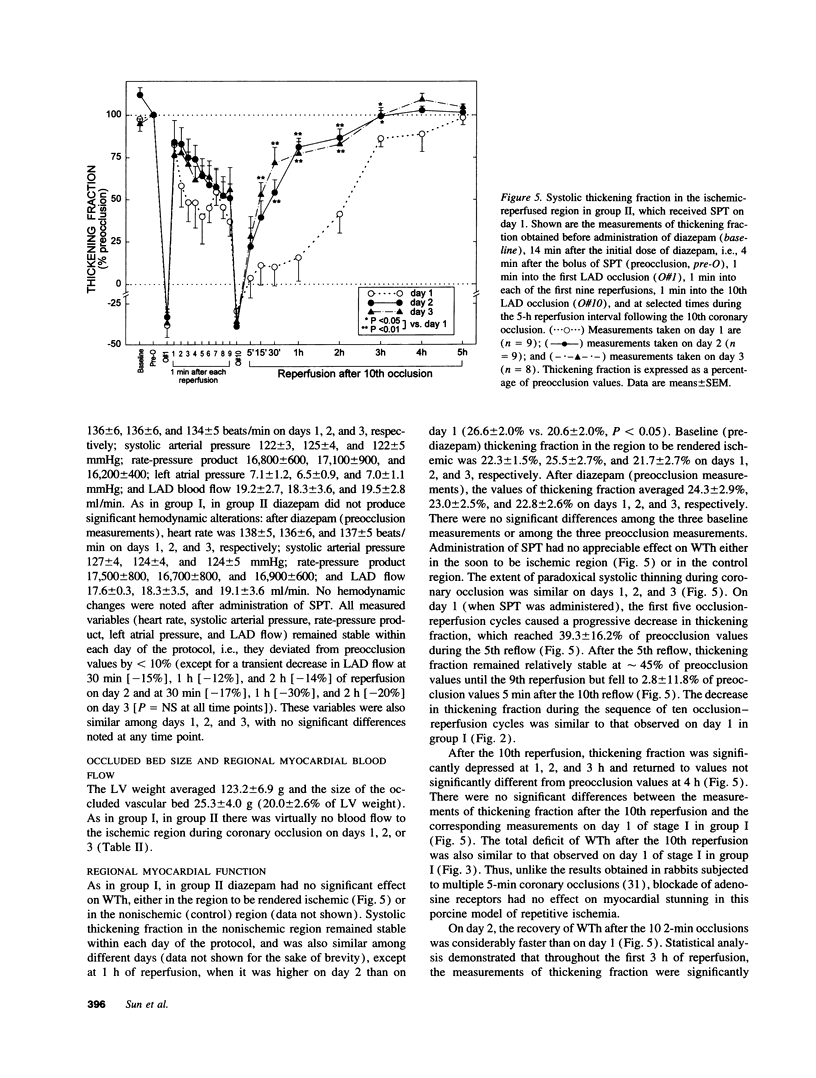
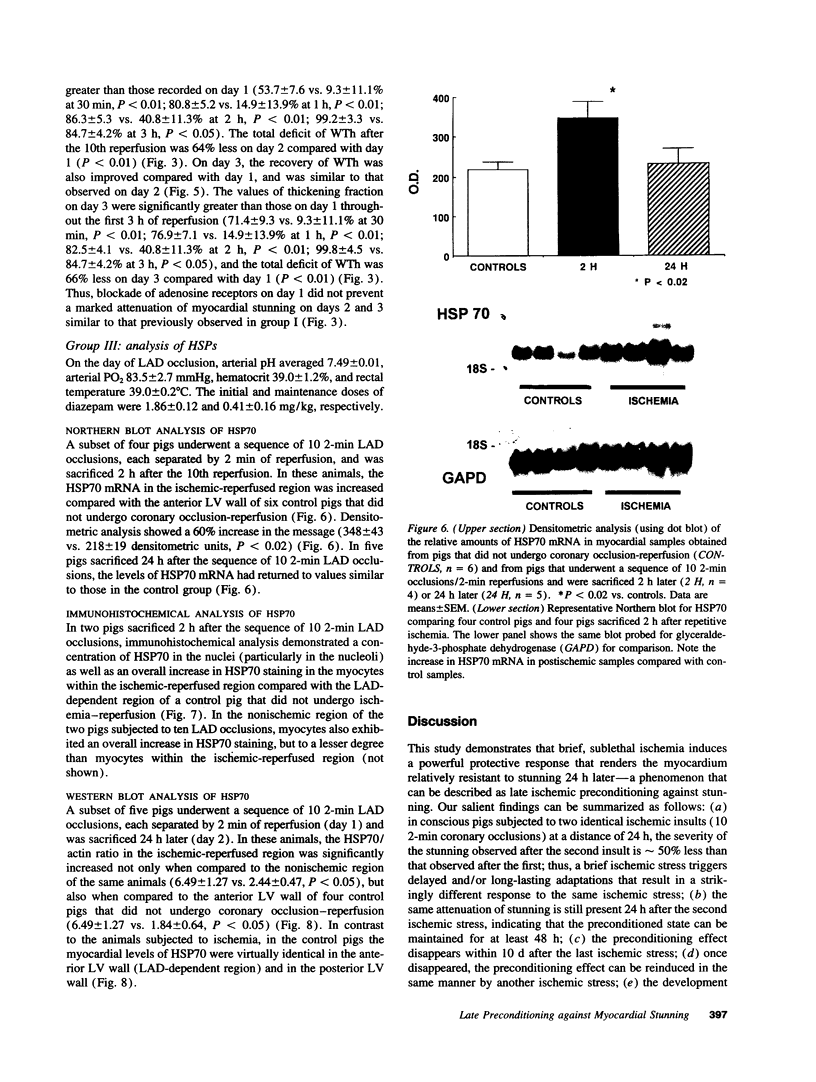
Conscious pigs underwent a sequence of 10 2-min coronary occlusions, each separated by 2 min of reperfusion, for three consecutive days (days 1, 2, and 3 of stage I). The recovery of systolic wall thickening (WTh) after the 10th reperfusion was markedly improved on days 2 and 3 compared with day 1, indicating that the myocardium had become preconditioned against "stunning." 10 d after stage I, pigs underwent again a sequence of 10 2-min coronary occlusions for two consecutive days (days 1 and 2 of stage II). On day 1 of stage II, the recovery of WTh after the 10th reperfusion was similar to that noted on day 1 of stage I; on day 2 of stage II, however, the recovery of WTh was again markedly improved compared with day 1. Blockade of adenosine receptors with 8-p-sulfophenyl theophylline failed to prevent the development of preconditioning against stunning. Northern blot analysis demonstrated an increase in heat stress protein (HSP) 70 mRNA 2 h after the preconditioning ischemia; at this same time point, immunohistochemical analysis revealed a concentration of HSP70 in the nucleus and an overall increase in staining for HSP70. 24 h after the preconditioning ischemia, Western dot blot analysis demonstrated an increase in HSP70. This study indicates the existence of a new, previously unrecognized cardioprotective phenomenon. The results demonstrate that a brief ischemic stress induces a powerful, long-lasting (at least 48 h) adaptive response that renders the myocardium relatively resistant to stunning 24 h later (late preconditioning against stunning). This adaptive response disappears within 10 d after the last ischemic stress but can be reinduced by another ischemic stress. Unlike early and late preconditioning against infarction, late preconditioning against stunning is not blocked by adenosine receptor antagonists, and therefore appears to involve a mechanism different from that of other forms of preconditioning currently known. The increase in myocardial HSP70 is compatible with, but does not prove, a role of HSPs in the pathogenesis of this phenomenon.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke C. W., Kaplinsky E., Michelson E. L., Naito M., Dreifus L. S. Reperfusion ventricular tachyarrhythmias: correlation with antecedent coronary artery occlusion tachyarrhythmias and duration of myocardial ischemia. Am Heart J. 1981 Apr;101(4):449–456. doi: 10.1016/0002-8703(81)90135-6. [DOI] [PubMed] [Google Scholar]
- Bolli R. Mechanism of myocardial "stunning". Circulation. 1990 Sep;82(3):723–738. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- Bolli R. Myocardial 'stunning' in man. Circulation. 1992 Dec;86(6):1671–1691. doi: 10.1161/01.cir.86.6.1671. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Thornby J. I., O'Neill P. G., Roberts R. Time course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol. 1988 Jan;254(1 Pt 2):H102–H114. doi: 10.1152/ajpheart.1988.254.1.H102. [DOI] [PubMed] [Google Scholar]
- Bunch F. T., Thornton J., Cohen M. V., Downey J. M. Adenosine is an endogenous protectant against stunning during repetitive ischemic episodes in the heart. Am Heart J. 1992 Dec;124(6):1440–1446. doi: 10.1016/0002-8703(92)90055-z. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., White F. C., Roth D. M., Bloor C. M. Heparin accelerates coronary collateral development in a porcine model of coronary artery occlusion. Circulation. 1993 Jul;88(1):198–207. doi: 10.1161/01.cir.88.1.198. [DOI] [PubMed] [Google Scholar]
- Cohen M. V., Liu G. S., Downey J. M. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation. 1991 Jul;84(1):341–349. doi: 10.1161/01.cir.84.1.341. [DOI] [PubMed] [Google Scholar]
- Crottogini A. J., Guth B. D., Barra J. G., Willshaw P., Lascano E. C., Pichel R. H. Interventricular coronary steal induced by stenosis of left anterior descending coronary artery in exercising pigs. Circulation. 1991 Apr;83(4):1361–1370. doi: 10.1161/01.cir.83.4.1361. [DOI] [PubMed] [Google Scholar]
- Crottogini A. J., Willshaw P., Barra J. G., Lascano E. C., Pichel R. H. Differential effects of left anterior descending coronary occlusion on left and right ventricular anterior wall thickening in the conscious pig. Cardiovasc Res. 1992 Mar;26(3):221–225. doi: 10.1093/cvr/26.3.221. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Karmazyn M., Kloc M., Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988 Sep;63(3):543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Tanguay R. M., Kingma J. G., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993 Mar;87(3):963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Shamim M. T., Butts-Lamb P., Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985 Apr;28(4):487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Donnelly T. J., Sievers R. E., Vissern F. L., Welch W. J., Wolfe C. L. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992 Feb;85(2):769–778. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- Downey J. M., Cohen M. V., Ytrehus K., Liu Y. Cellular mechanisms in ischemic preconditioning: the role of adenosine and protein kinase C. Ann N Y Acad Sci. 1994 Jun 17;723:82–98. [PubMed] [Google Scholar]
- Duncker D. J., Heiligers J. P., Saxena P. R., Verdouw P. D. Nisoldipine and perfusion of post-stenotic myocardium in conscious pigs with different degrees of concentric stenosis. Br J Pharmacol. 1988 May;94(1):219–227. doi: 10.1111/j.1476-5381.1988.tb11518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Cohen M., Ambrose J. A., Badimon J. J., Chesebro J. Insights into the pathogenesis of acute ischemic syndromes. Circulation. 1988 Jun;77(6):1213–1220. doi: 10.1161/01.cir.77.6.1213. [DOI] [PubMed] [Google Scholar]
- Grum C. M., Gallagher K. P., Kirsh M. M., Shlafer M. Absence of detectable xanthine oxidase in human myocardium. J Mol Cell Cardiol. 1989 Mar;21(3):263–267. doi: 10.1016/0022-2828(89)90741-4. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Murry C. E., Reimer K. A. Preconditioning myocardium with ischemia. Cardiovasc Drugs Ther. 1991 Oct;5(5):933–938. doi: 10.1007/BF00053555. [DOI] [PubMed] [Google Scholar]
- Karmazyn M., Mailer K., Currie R. W. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990 Aug;259(2 Pt 2):H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Connelly C. M., Romo G. M., Mamuya W., Apstein C. S., Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992 Apr;89(4):1060–1068. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A. A., Eberli F. R., Brecher P., Romo G. M., Owen A., Apstein C. S. A single myocardial stretch or decreased systolic fiber shortening stimulates the expression of heat shock protein 70 in the isolated, erythrocyte-perfused rabbit heart. J Clin Invest. 1991 Dec;88(6):2018–2025. doi: 10.1172/JCI115529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja R. C., Hess M. L. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992 Jul;26(7):641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- Kuzuya T., Hoshida S., Yamashita N., Fuji H., Oe H., Hori M., Kamada T., Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993 Jun;72(6):1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Li X. Y., McCay P. B., Zughaib M., Jeroudi M. O., Triana J. F., Bolli R. Demonstration of free radical generation in the "stunned" myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993 Aug;92(2):1025–1041. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. S., Thornton J., Van Winkle D. M., Stanley A. W., Olsson R. A., Downey J. M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991 Jul;84(1):350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Marber M. S., Latchman D. S., Walker J. M., Yellon D. M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993 Sep;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Miyamae M., Fujiwara H., Kida M., Yokota R., Tanaka M., Katsuragawa M., Hasegawa K., Ohura M., Koga K., Yabuuchi Y. Preconditioning improves energy metabolism during reperfusion but does not attenuate myocardial stunning in porcine hearts. Circulation. 1993 Jul;88(1):223–234. doi: 10.1161/01.cir.88.1.223. [DOI] [PubMed] [Google Scholar]
- Mohri M., Tomoike H., Noma M., Inoue T., Hisano K., Nakamura M. Duration of ischemia is vital for collateral development: repeated brief coronary artery occlusions in conscious dogs. Circ Res. 1989 Feb;64(2):287–296. doi: 10.1161/01.res.64.2.287. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986 Nov;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Muxfeldt M., Schaper W. The activity of xanthine oxidase in heart of pigs, guinea pigs, rabbits, rats, and humans. Basic Res Cardiol. 1987 Sep-Oct;82(5):486–492. doi: 10.1007/BF01907096. [DOI] [PubMed] [Google Scholar]
- Ovize M., Przyklenk K., Hale S. L., Kloner R. A. Preconditioning does not attenuate myocardial stunning. Circulation. 1992 Jun;85(6):2247–2254. doi: 10.1161/01.cir.85.6.2247. [DOI] [PubMed] [Google Scholar]
- Pinto J. M., Kirby D. A., Johnson D. A., Lown B. Diazepam administered prior to coronary artery occlusion increases latency to ventricular fibrillation. Life Sci. 1991;49(8):587–594. doi: 10.1016/0024-3205(91)90257-c. [DOI] [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Roth D. M., Maruoka Y., Rogers J., White F. C., Longhurst J. C., Bloor C. M. Development of coronary collateral circulation in left circumflex Ameroid-occluded swine myocardium. Am J Physiol. 1987 Nov;253(5 Pt 2):H1279–H1288. doi: 10.1152/ajpheart.1987.253.5.H1279. [DOI] [PubMed] [Google Scholar]
- Roth D. M., Maruoka Y., Rogers J., White F. C., Longhurst J. C., Bloor C. M. Development of coronary collateral circulation in left circumflex Ameroid-occluded swine myocardium. Am J Physiol. 1987 Nov;253(5 Pt 2):H1279–H1288. doi: 10.1152/ajpheart.1987.253.5.H1279. [DOI] [PubMed] [Google Scholar]
- Roth D. M., White F. C., Nichols M. L., Dobbs S. L., Longhurst J. C., Bloor C. M. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation. 1990 Nov;82(5):1778–1789. doi: 10.1161/01.cir.82.5.1778. [DOI] [PubMed] [Google Scholar]
- Savage R. M., Guth B., White F. C., Hagan A. D., Bloor C. M. Correlation of regional myocardial blood flow and function with myocardial infarct size during acute myocardial ischemia in the conscious pig. Circulation. 1981 Oct;64(4):699–707. doi: 10.1161/01.cir.64.4.699. [DOI] [PubMed] [Google Scholar]
- Schaper W., Görge G., Winkler B., Schaper J. The collateral circulation of the heart. Prog Cardiovasc Dis. 1988 Jul-Aug;31(1):57–77. doi: 10.1016/0033-0620(88)90011-4. [DOI] [PubMed] [Google Scholar]
- Schott R. J., Rohmann S., Braun E. R., Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990 Apr;66(4):1133–1142. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- Sekili S., McCay P. B., Li X. Y., Zughaib M., Sun J. Z., Tang L., Thornby J. I., Bolli R. Direct evidence that the hydroxyl radical plays a pathogenetic role in myocardial "stunning" in the conscious dog and demonstration that stunning can be markedly attenuated without subsequent adverse effects. Circ Res. 1993 Oct;73(4):705–723. doi: 10.1161/01.res.73.4.705. [DOI] [PubMed] [Google Scholar]
- Shiki K., Hearse D. J. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am J Physiol. 1987 Dec;253(6 Pt 2):H1470–H1476. doi: 10.1152/ajpheart.1987.253.6.H1470. [DOI] [PubMed] [Google Scholar]
- Symons J. D., Firoozmand E., Longhurst J. C. Repeated dipyridamole administration enhances collateral-dependent flow and regional function during exercise. A role for adenosine. Circ Res. 1993 Sep;73(3):503–513. doi: 10.1161/01.res.73.3.503. [DOI] [PubMed] [Google Scholar]
- Symons J. D., Longhurst J. C., Stebbins C. L. Response of collateral-dependent myocardium to vasopressin release during prolonged intense exercise. Am J Physiol. 1993 May;264(5 Pt 2):H1644–H1652. doi: 10.1152/ajpheart.1993.264.5.H1644. [DOI] [PubMed] [Google Scholar]
- Symons J. D., Pitsillides K. F., Longhurst J. C. Chronic reduction of myocardial ischemia does not attenuate coronary collateral development in miniswine. Circulation. 1992 Aug;86(2):660–671. doi: 10.1161/01.cir.86.2.660. [DOI] [PubMed] [Google Scholar]
- Thornton J., Striplin S., Liu G. S., Swafford A., Stanley A. W., Van Winkle D. M., Downey J. M. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990 Dec;259(6 Pt 2):H1822–H1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- Triana J. F., Li X. Y., Jamaluddin U., Thornby J. I., Bolli R. Postischemic myocardial "stunning". Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res. 1991 Sep;69(3):731–747. doi: 10.1161/01.res.69.3.731. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Welch W. J. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- White F. C., Roth D. M., Bloor C. M. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci. 1986 Aug;36(4):351–356. [PubMed] [Google Scholar]
- Yellon D. M., Iliodromitis E., Latchman D. S., Van Winkle D. M., Downey J. M., Williams F. M., Williams T. J. Whole body heat stress fails to limit infarct size in the reperfused rabbit heart. Cardiovasc Res. 1992 Apr;26(4):342–346. doi: 10.1093/cvr/26.4.342. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Latchman D. S. Stress proteins and myocardial protection. J Mol Cell Cardiol. 1992 Feb;24(2):113–124. doi: 10.1016/0022-2828(92)93148-d. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Pasini E., Cargnoni A., Marber M. S., Latchman D. S., Ferrari R. The protective role of heat stress in the ischaemic and reperfused rabbit myocardium. J Mol Cell Cardiol. 1992 Aug;24(8):895–907. doi: 10.1016/0022-2828(92)91102-b. [DOI] [PubMed] [Google Scholar]