Abstract
Aberrant activation of Akt is a common finding in adult malignant gliomas, resulting in most cases from mutations or deletions involving PTEN, which allows constitutive Akt phosphorylation. In contrast, we have previously reported that pediatric malignant gliomas, which are morphologically similar to lesions arising in adults, have a substantially lower incidence of genomic alterations of PTEN. The objective of this study was to determine whether Akt activation was also an uncommon finding in childhood malignant gliomas and whether this feature was associated with survival. To address this issue, we examined the frequency of Akt activation, determined by overexpression of the activated phosphorylated form of Akt (Se473) on immunohistochemical analysis, in a series of 53 childhood malignant gliomas obtained from newly diagnosed patients treated on the Children’s Oncology Group ACNS0126 and 0423 studies. The relationship between Akt activation and p53 over-expression, MIB1 labeling, and tumor histology was evaluated. The association between Akt activation and survival was also assessed. Overexpression of activated Akt was observed in 42 of 53 tumors, far in excess of the frequency of PTEN mutations we have previously observed. There was no association between Akt activation and either histology, p53 overexpression, or MIB1 proliferation indices. Although tumors that lacked Akt overexpression had a trend toward more favorable event-free survival and overall survival (p = 0.06), this association reflected that non-overexpressing tumors were significantly more likely to have undergone extensive tumor removal, which was independently associated with outcome. Activation of Akt is a common finding in pediatric malignant gliomas, although it remains uncertain whether this is an independent adverse prognostic factor. In view of the frequency of Akt activation, the evaluation of molecularly targeted therapies that inhibit this pathway warrants consideration for these tumors.
Keywords: Anaplastic glioma, Childhood, Glioblastoma, Akt, Prognostic factors, Treatment resistance
Introduction
Brain tumors are collectively the leading cause of childhood cancer-related mortality. High-grade gliomas have a particularly poor prognosis, with a long-term survival rate of less than 20%, despite conventional surgical, radio-therapeutic, and chemotherapeutic interventions [1]. Although these tumors are comparable morphologically to malignant gliomas that arise in adults, childhood gliomas have a number of distinctive molecular characteristics, which suggests the involvement of somewhat different pathways of tumorigenesis. For example, childhood primary malignant gliomas rarely exhibit EGFR amplification, which is a common feature of adult primary malignant gliomas, but often demonstrate overexpression of the EGFR protein [2–9], presumably mediated by distinct mechanisms. Conversely, pediatric lesions commonly exhibit p53 mutations, similar to adult glioblastomas that progress secondarily from lower grade lesions [10–16]. In addition, previous studies from our group [2] and others [3, 5, 6] have shown that childhood malignant gliomas infrequently exhibit PTEN mutations, another of the hallmarks of adult primary glioblastomas [17], which leads to constitutive activation of Akt (protein kinase B), although these lesions may exhibit activation of Akt by mechanisms other than genomic PTEN alterations [8, 18].
Given the recent development of molecularly targeted agents that inhibit the aberrantly activated signaling pathways of various tumors, the elucidation of the molecular alterations involved in tumorigenesis is relevant not only from a biological perspective, but also from a therapeutic standpoint. Akt is a serine/threonine kinase that is of particular interest in this regard, owing to its central role as a regulator of cell growth, migration, and survival [19, 20], and the availability of several novel agents that inhibit Akt [21], its upstream activator, phosphatidylinositol 3-kinase (PI3 K) [22–28], or components of its signaling network [29–32]. We therefore sought to define the frequency of Akt activation in a centrally reviewed cohort of childhood malignant gliomas, and to determine whether this factor was associated with p53 expression status and MIB1 proliferation index, and whether Akt activation status had an impact on overall survival following conventional multimodality therapy.
Methods
Tumor samples
Tumor samples were obtained from children enrolled on the Children’s Oncology Group ACNS0126 and 0423 studies, both of which involved post-surgical administration of alkylator-based therapy and irradiation. Each study required institutional IRB approval for protocol enrollment. Children on ACNS0126 received temozolomide daily with radiation and on a 5-day per 28-day cycle post-irradiation; and those on ACNS0423 received daily temozolomide during irradiation followed by post-irradiation temozolomide and lomustine. Eligibility for both studies required an institutional histopathology diagnosis of high-grade glioma (i.e., glioblastoma (GBM), anaplastic astrocytoma (AA), or other eligible malignant glioma, such as gliosarcoma), and each incorporated central pathology review to refine classification.
Tissue accrual for the current study was coordinated by the Pediatric Branch of the Cooperative Human Tissue Network (CHTN). Specimens were de-identified by the CHTN to mask clinical and outcome data from investigators.
Immunohistochemistry
Paraffin-embedded specimens were used for all analyses. Slides were reviewed by a neuropathologist (RLH) to confirm that tumor tissue of sufficient quantity was available for the planned studies. Tumor-containing sections were baked at 60°C for 30 min, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Endogenous peroxidase activity was quenched by incubation in 0.3% hydrogen peroxide solution. Antigen retrieval [33] was performed by heating the slides in 10 mmol citrate buffer (pH 6.0) for 20 min. Nonspecific antibody binding was blocked by incubation in Protein Blocking Reagent (Thermo Corp, Pittsburgh, PA) for 20 min. Sections were then incubated with primary antibodies against p-Akt (Se473) (Cell Signaling Technology, Inc., Danvers, MA; 1:100), Ki-67 (Immunotech, Westbrook, ME; 1:100), and p53 (DO-7, Dako Corporation, Carpinteria, CA; 1:300), in Common Antibody Diluent (BioGenex, San Ramon, CA) at room temperature for 2 h. The p53 antibody recognizes a denaturation-stable determinant of wild-type and mutant p53 [34]. Negative control sections were treated with diluent and mouse IgG (5 μg/ml, Dako Corporation, Carpinteria, CA) alone. Slides were then rinsed twice with PBS and antibody binding was localized using a Universal Labeled Streptavidin–Biotin 2 System (LSAB 2—HRP, Dako). Slides were incubated for 30 min in Biotinylated Link reagent at room temperature, followed by a 10 min PBS wash. Slides were then incubated in Streptavidin-HRP solution for 30 min at room temperature. Antibody binding was visualized using 3,3′-diaminobenzidine [35]. The slides were counterstained with Mayer’s hematoxylin, dehydrated through graded concentrations of ethanol, cleared in xylene, mounted and examined using a light microscope. Positive controls were included with each batch of sections to confirm the consistency of the analysis. Where sufficient slides were available, staining for PTEN was also performed, using an primary antibody against wild-type PTEN (Clone 138G6, Cell Signaling. 1:100).
Immunohistochemical analysis
Immunoreactivity of tumor specimens for pAKT was assessed semiquantitatively by a neuropathologist (RLH), and in accordance with other reports, tumors were considered positive for pAkt expression if dense staining at an intensity equal to or greater than that of the positive control was seen in at least 20% of cells [36, 37]. Reliability of categorization was subsequently confirmed by blinded independent review of the specimens by a second neuropathologist (GHM). Percent MIB-1 labeling was quantitated as previously reported by our group by counting stained and unstained cells in 5–10 high-power fields (approximately 2,000 cells) that incorporated the most anaplastic regions of the specimen [38]. P53 labeling was assessed semiquantitatively as previously reported [7]. Tumors with more than 10% positive cells, either focally or diffusely within the lesion, were categorized as exhibiting overexpression in relation to normal brain. PTEN staining was considered deficient if absent in at least 25% of the tumor-containing section and present in adjacent endothelial cells or normal tissue elements.
PTEN LOH Assay
Because previous studies from our group [2] and others [5, 6] have shown the mutations of PTEN are extremely uncommon in pediatric malignant gliomas, but that loss of heterozygosity (LOH) involving the region of the PTEN locus on chromosome 10q is a substantially more frequent event [2], we examined the relationship between Akt immunoreactivity and 10q LOH. Formalin-fixed paraffin-embedded (FFPE) tissue specimens were used for all analyses. Tumor targets were manually microdissected from 4-μm unstained histologic sections under the guidance of a hematoxylin and eosin-stained slide using an Olympus SZ61 stereo microscope (Olympus, Hamburg, Germany). DNA was isolated from each target with the DNeasy Blood and Tissue kit on the automated QIAcube (Qiagen, Valencia, CA) instrument according to the manufacturer’s instructions. The quantity of isolated DNA was assessed using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Detection of loss of heterozygosity involving chromosome 10q was performed using a series of microsatellite markers (D10S520, D10S1 171, D10S1173). PCR amplification was performed with primers labeled either with TET, NED or HEX fluorophores at the 5′ end and carried out on a 9700 GeneAmp thermocycler (Applied Biosystems, Foster City, CA) using standard PCR profiles (denaturation at 94°C for 10 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 1 min). Post-PCR amplification products were detected using capillary gel electrophoresis on an ABI 3730 platform (Applied Biosystems). The relative fluorescence values (peak heights) were analyzed using GeneScan 3.7 software (Applied Biosystems), with a 1.5-fold or greater difference in peak height ratios indicative of LOH.
Central pathology review
Both ACNS0126 and 0423 used a prospective central review panel to restrict the eligible cohort to those cases with high-grade gliomas and refine histological classification according to consistent guidelines [39]. The immunohistochemical analysis was performed independently of this review, so the review diagnosis of each specimen was not known during the assessment.
Statistical analysis
For outcome analysis, tumors were classified according to the presence or absence of Akt activation. The endpoints for analysis were overall survival (OS) and event-free survival (EFS). Overall survival was defined as the time from study entry to death from any cause. Event-free survival was defined as the time from study enrollment to the first occurrence of disease progression, relapse, second malignancy, or death from any cause. Nonparametric EFS and OS curves were computed using the product-limit estimates, with standard error via the Greenwood formula [40]. Comparisons of outcome were based on one-sided log-rank test. Comparisons of the distribution of Akt activation status between different patient subgroups defined by age, gender, histology, extent of resection, p53 expression, and MIB1 labeling were based on two-sided Fisher exact test [41].
Results
Frequency of Akt activation
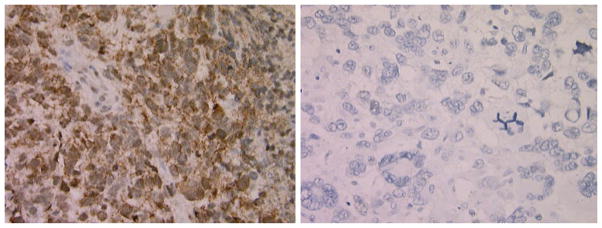
Fifty-three patients had specimens that were available for analysis of Akt activation and both p53 and MIB1 labeling, with 48 patients from ACNS0423 and 5 from ACNS0126. There were 106 and 90 eligible patients enrolled on ACNS0423 and ACNS0126, respectively. As a group, the 53 patients who had specimens had a more favorable outcome than the cohort without specimens. Of the 53 evaluable tumors, 11 showed little or no pAkt immunoreactivity, whereas 42 (79%) showed overexpression, with dense staining in more than 20 percent of cells (Fig. 1). The typical pattern of pAkt staining included both cytoplasmic and nuclear immunoreactivity. This is consistent with most other reports for malignant cells regarding the subcellular localization of pAkt staining [36, 37, 42, 43].
Fig. 1.
Pediatric malignant gliomas exhibiting positive (left) and negative (right) staining for pAkt
The distribution of pAkt immunoreactivity as a function of age, gender, histology, extent of resection, p53 expression status, and MIB1 labeling is shown in Table 1. Among the 42 tumors with Akt activation, 22 (52.4%) also showed p53 overexpression. Among the 11 tumors lacking Akt activation, p53 overexpression was seen in 6 (54.5%). Average MIB labeling in the Akt activated subset was 31.8 ± 3.2 versus 24.7 ± 3.8 in those without pAkt expression (p = 0.28). The frequency of particularly high MIB1 indices (>36%), which has been previously associated with an adverse prognosis, did not differ significantly between the two groups (2 of 11 vs. 15 of 42, p = 0.30).
Table 1.
Association Between pAkt overexpression and histology, p53 overexpression and MIB1 labeling
| pAkt overexpression N (%) | No pAkt overexpression N (%) | p value | |
|---|---|---|---|
| Study | 0.005 | ||
| ACNS0423 | 41 (97.6) | 7 (63.6) | |
| ACNS0126 | 1 (2.4) | 4 (36.4) | |
| Age | 0.26 | ||
| <10 years | 10 (23.8) | 5 (45.5) | |
| ≥10 years | 32 (76.2) | 6 (54.5) | |
| Gender | 1.0 | ||
| Male | 25 (59.5) | 6 (54.5) | |
| Female | 17 (40.5) | 5 (45.5) | |
| Histology | 0.74 | ||
| Grade III | 20 (47.6) | 4 (36.4) | |
| Grade IV | 22 (52.4) | 7 (63.6) | |
| P53 Overexpression | 1.0 | ||
| Present | 22 (52.4) | 6 (54.5) | |
| Absent | 20 (47.6) | 5 (45.5) | |
| MIB Labeling | 0.59 | ||
| Very High (>36) | 15 (35.7) | 2 (18.2) | |
| Intermediate (18–36) | 14 (33.3) | 5 (45.5) | |
| Low (<18) | 13 (31.0) | 4 (36.4) | |
| Extent of resection | 0.02 | ||
| <90% resection | 24 (60) | 2 (18.2) | |
| ≥90% resection | 16 (40) | 9 (81.8) |
We also examined whether Akt activation might be more common in grade IV tumors compared to grade III lesions, given that PTEN mutations in adult malignant gliomas are seen predominantly in GBMs. Contrary to this assumption, Akt activation was seen at relatively comparable frequencies in the two histological subgroups, being present in 20 of 24 grade III gliomas (83.3%) and 22 of 29 grade IV lesions (68.9%) (p = 0.74). However, a significantly higher percentage of patients with no pAkt overexpression had ≥90% extent of resection (81.8%) compared to 40% in the pAkt overexpressing group (p = 0.02).
Akt activation and outcome
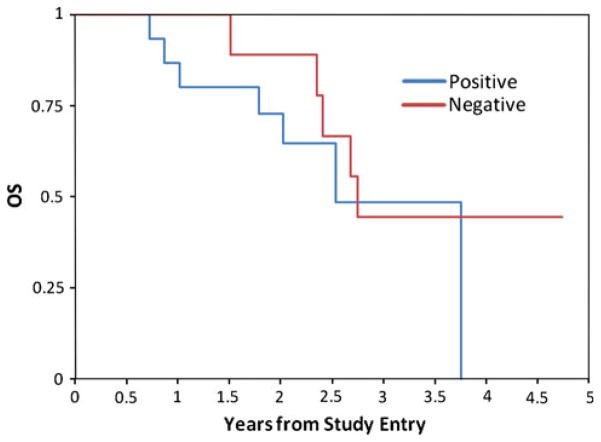
To assess the prognostic significance of Akt activation in pediatric malignant gliomas, overall survival and event-free survival were compared between the subgroup of tumors showing overexpression of pAkt and those lacking this feature. One-year EFS was 59 ± 8% for patients with Akt overexpression and and 91 ± 8% for those with no overexpression (p = 0.16, logrank test) (Fig. 2). One-year OS was 78 ± 7% for patients with Akt overexpression versus 100% for those with no overexpression (p = 0.06, logrank test) (Fig. 3). The median follow up for non-failures was 2.1 years. Based on the figures presented, it appeared that patients who had tumors with Akt overexpression seemed to fail earlier than those lacking this feature, although more mature follow up data is needed for the comparison of long-term outcome. Given the known association of extent of resection with outcome, we also examined the association between pAkt expression and outcome, after stratifying for extent of resection. After this stratification, some of the early difference in outcome as a function of pAkt status disappeared, suggesting that this difference may in part reflect differences in outcome due to the impact of resection extent. Figures 4 and 5 show the EFS and OS in relation to Akt expression level in patients who had ≥90% extent of resection. Corresponding figures in patients with <90% resection are not shown due to small patient numbers.
Fig. 2.
Event-free survival in pediatric malignant gliomas exhibiting positive and negative staining for pAkt
Fig. 3.

Overall survival in pediatric malignant gliomas exhibiting positive and negative staining for pAkt
Fig. 4.
Event-free survival in patients with 90% extent of resection exhibiting positive and negative staining for pAkt
Fig. 5.
Overall survival in patients with 90% extent of resection exhibiting positive and negative staining for pAkt
Reliability of pAkt categorization
We assessed the reliability of Akt categorization in these specimens by having the immunohistochemical results reviewed independently by a second neuropathologist in a blinded fashion. Concordance among the two reviewers in categorization of Akt immunoreactivity was extremely high, with complete agreement in 51 of 53 cases (96.2%). The two discrepancies related to cases judged to have overexpression by the initial reviewer in which the second reviewer felt the 20% threshold for pAkt immunoreactivity was not reached throughout the tumor specimen.
Association of pAkt overexpression with PTEN immunoreactivity and LOH
Given the known impact of PTEN deletion or inactivation on Akt activation status, we examined whether pAkt overexpression was associated with either LOH of chromosome 10q in the region of the PTEN locus or loss of expression of PTEN. Because this was not one of the initial objectives of the study, availability of tissue sections for these analyses was a limiting factor in many instances. LOH studies were performed in 30 of the samples, 26 of which had pAkt overexpression and four of which did not. Seven of the 26 pAkt-overexpressing tumors had chromosome 10q LOH, although in two of these cases, only one of three loci examined showed this alteration. In contrast, 19 had no evidence of LOH. Two of four tumors without Akt overexpression had 10q LOH. Thus, no consistent association between pAkt immunoreactivity and 10q LOH was apparent (two-tail Fisher’s exact test p value: 0.56). Likewise, in the 15 cases amenable to PTEN immunohistochemistry, 9 of 13 with pAkt overexpression showed loss of PTEN immunoreactivity, whereas 1 of 2 without pAkt ovexpression showed loss of PTEN (two-tail Fisher’s exact test p value: 1.0).
Discussion
Activation of the PI3K/Akt pathway is a common feature of malignant gliomas that arise in adults [16]. Mechanistically, this occurs most commonly as a consequence of mutations of PTEN, a tumor suppressor gene that normally regulates downstream inhibition of Akt phosphorylation. Loss of this suppressive signal leads to constitutive Akt activation. Activated Akt phosphorylates several proteins involved in cell survival and cell growth signaling, such as Bad, forkhead transcription factor, glycogen synthase kinase, and the mammalian target of rapamycin (mTOR) [19, 20], which promotes tumor cell proliferation, survival and invasion. Although previous studies from our laboratory [2] and others [3, 5, 6] have shown that PTEN mutations are rare in pediatric malignant gliomas, it has been demonstrated that other factors can contribute to dysregulation of the Akt pathway. These include activating mutations of the PIK3CA and PIK3R1 genes [16, 44, 45], methylation-mediated inactivation of PTEN [46] and constitutive activation of upstream signaling elements, such as growth factor receptors [16]. Because Akt is a focus of interest for molecularly targeted therapies in gliomas, we questioned whether Akt activation was a frequent finding in pediatric malignant gliomas.
The results of this study demonstrate that high levels of Akt phosphorylation are commonly observed in pediatric malignant gliomas. It is important to note that phosphorylation of the Ser473 site represents only one surrogate for Akt activation, in addition to phosphorylation of the Thr308 site and activation of downstream signaling elements, such as S6 kinase and S6, among others [36, 37, 42, 47–50]. The Ser473 site has been most commonly profiled in previous studies as a marker of Akt activation status, owing to the reasonably robust immunoreactivity of this antigen in formalin-fixed, paraffin-embedded tissue, which provided a rationale for its use as a marker for Akt activation in the current study.
The high frequency of Akt phosphorylation in this centrally reviewed cohort of tumors is consistent with results from smaller institutional cohorts. For example, Faury et al. [18] noted phosphorylation of Akt in 10 of 18 pediatric malignant gliomas analyzed by western blotting, and 10 of 17 institutional samples examined by immunohistochemistry. They also observed this feature in 13 of 21 samples from an independent data set [18] and noted an adverse association between Akt activation and outcome. Similarly, Thorarinsdottir et al. [8] noted Akt phosphorylation in more than 80% of independent tissue samples derived from 22 pediatric malignant gliomas. High expression of pAkt was associated with high levels of EGFR expression in these tumors.
The association between a more adverse prognosis in tumors with Akt phosphorylation than those lacking this feature fits with observations in a variety of tumor types [36, 37, 42, 43, 47–50]. In part, this may reflect that Akt activation represents a common event in the evolution toward treatment-resistant biological behavior. Because Akt activation is involved in mediating apoptosis resistance, this change may render tumor cells more resistant to the cytotoxic effects of irradiation and conventional chemotherapy. Our observation fits with the findings of Faury et al. [18] that those patients whose tumors had a “Ras active” phenotype, which generally encompassed Akt activation, had a trend toward worse overall survival than those with tumors lacking this feature.
Because previous studies of primary adult malignant gliomas have indicated that PTEN alterations are seen most commonly in grade IV gliomas, and infrequently in grade III lesions [51], we examined whether the frequency of Akt activation was associated with tumor histology or other biological factors, such as p53 overexpression and high MIB1 proliferation indices, which have been noted to be more common in grade IV than grade III lesions. Although the latter trend was apparent in this cohort as well, our analysis showed no clear association between tumor grade and the frequency of Akt activation, which was detected in the majority of both grade III and grade IV malignant gliomas. This finding is consistent with the observation of Wiencke et al. [46] in adult secondary malignant gliomas, which exhibit Akt activation at similarly high frequencies in both grade III and grade IV lesions. Secondary adult malignant gliomas resemble pediatric malignant gliomas in terms of their frequency of p53 mutations and infrequency of PTEN mutations and EGFR amplification, although exhibit key biological differences in terms of their progression from lower grade (i.e. grade II) histologies, which is an uncommon pattern in childhood lesions. It has been suggested that the predominant mechanism of Akt activation in these tumors is PTEN inactivation by promoter methylation, rather than mutation [46], and in this setting, it represents an earlier step in malignant progression. Such a mechanism remains to be established in the pediatric glioma context, although it has been shown that a significant percentage of childhood malignant gliomas have loss of PTEN immunoreactivity [8, 9], despite their low incidence of PTEN mutations. In agreement with these previous results, we also observed that among 15 tumors amenable to PTEN immunohistochemistry, 9 of 13 (69%) with pAkt overexpression exhibited loss of PTEN immunoreactivity, although the molecular basis for this observation remains conjectural. LOH of chromosome 10q was noted in 27% of evaluable tumors with pAkt overexpression, which suggests that other mechanisms, such as PTEN methylation, may play a contributory role in at least a subset of these tumors.
An unexpected observation of this study was that the subset of tumors that lacked pAkt overexpression were more likely to have undergone extensive (≥90%) tumor resections than those with pAkt overexpression. This may reflect distinctive features in the growth properties of these tumors or their location of origin. However, there was no apparent difference in the frequency of pAkt overexpression in tumor subsets defined by superficial (e.g. cerebral or cerebellar cortical) versus deep (e.g., basal ganglion, diencephalic, or brainstem) localization, so the impact of location as a potential correlative factor remains conjectural. Another potentially important observation was that the frequency of positive and negative pAkt immunoreactivity varied significantly between the ACNS0126 and ACNS0423 study samples. This may reflect true differences between these cohorts in the biology of the evaluable tumors or differences in staining of the pAkt antigen as a function of specimen age, given that the ACNS0126 samples were obtained several years prior to the ACNS0423 samples.
Notwithstanding the uncertainty regarding independent prognostic relevance of pAkt overexpression, the finding of high levels of Akt activation in a sizeable percentage of pediatric malignant gliomas has important implications from a therapeutic perspective. With the availability of molecularly targeted agents that directly inhibit Akt phosphorylation [21] or that interfere with signaling elements upstream or downstream from Akt, such as PI3 K and mTOR [22–32], it may be possible to counteract the growth and survival promoting effects of Akt, and such tumors may be particularly sensitive to these inhibitors [52, 53]. These agents are already being examined in a number of solid tumors, including adult malignant gliomas [26, 29–31], and the results of this study provide a rationale to further explore the potential applicability of this strategy in pediatric malignant gliomas.
Acknowledgments
This work was supported in part by NIH grants NS37704 (IFP), and CA98543 to the Children’s Oncology Group. The authors wish to acknowledge Judith Burnham for technical assistance.
Contributor Information
Ian F. Pollack, Email: ian.pollack@chp.edu, Department of Neurosurgery, Children’s Hospital of Pittsburgh, University of Pittsburgh School of Medicine, 4401 Penn Avenue, Pittsburgh, PA 15224, USA
Ronald L. Hamilton, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, USA
Geoffrey H. Murdoch, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, USA
Peter C. Burger, Department of Pathology, Johns Hopkins University, Baltimore, MD, USA
Daniel J. Brat, Department of Pathology, Emory University, Atlanta, USA
Marc K. Rosenblum, Department of Pathology, Memorial Sloan-Kettering Cancer Center, Newyork, USA
Marina N. Nikiforova, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, USA
Emiko J. Holmes, The Children’s Oncology Group, Arcadia, CA, USA
Tianni Zhou, The Children’s Oncology Group, Arcadia, CA, USA.
Kenneth J. Cohen, Department of Oncology, Johns Hopkins University, Baltimore, MD, USA
Regina I. Jakacki, Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, USA
References
- 1.Pollack IF. Current concepts: brain tumors in children. N Engl J Med. 1994;331:1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 2.Pollack IF, Hamilton RL, James CD, Finkelstein SD, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg Pediatr. 2006;105:3431–3437. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 3.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor (EGFR) expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 5.Cheng Y, Ng H-K, Zhang S-F, Ding M, Pang JC-S, Zheng J, Poon W-S. Genetic alterations in pediatric high-grade astrocytomas. Hum Pathol. 1999;30:1284–1290. doi: 10.1016/s0046-8177(99)90057-6. [DOI] [PubMed] [Google Scholar]
- 6.Raffel C, Frederick L, O’Fallon JR, et al. Analysis of oncogene and tumor suppressor gene alterations in pediatric malignant astrocytomas reveals reduced survival for patients with PTEN mutations. Clin Cancer Res. 1999;5:4085–4090. [PubMed] [Google Scholar]
- 7.Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R. Expression of p53 and prognosis in malignant gliomas in children. N Engl J Med. 2002;346:420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 8.Thorarinsdottir HK, Santi M, McCarter R, Rushing EJ, Cornelison R, Jales A, MacDonald TJ. Protein expression of platelet-derived growth factor receptor correlates with malignant histology and PTEN with survival in childhood gliomas. Clin Cancer Res. 2008;14:3386–3394. doi: 10.1158/1078-0432.CCR-07-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang M-L, Ma J, Ho M, et al. Tyrosine kinase expression in pediatric high grade astrocytoma. J Neurooncol. 2008;87:247–253. doi: 10.1007/s11060-007-9513-1. [DOI] [PubMed] [Google Scholar]
- 10.Sure U, Ruedi D, Tachibana O, et al. Determination of p53 mutations, EGFR overexpression, and loss of p16 expression in pediatric glioblastomas. J Neuropathol Exp Neurol. 1997;56:782–789. [PubMed] [Google Scholar]
- 11.Pollack IF, Finkelstein SD, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Finlay J, Sposto R. Age and TP53 mutation frequency in childhood malignant gliomas. Results in a multi-institutional cohort. Cancer Res. 2001;61:7404–7407. [PubMed] [Google Scholar]
- 12.Collins VP. Progression as exemplified by human astrocytic tumors. Semin Cancer Biol. 1999;9:267–276. doi: 10.1006/scbi.1999.0132. [DOI] [PubMed] [Google Scholar]
- 13.Ichimura K, Bolin MB, Goike HM, Schmidt EE, Moshref A, Collins VP. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000;60:417–424. [PubMed] [Google Scholar]
- 14.von Deimling A, von Ammon K, Schoenfeld D, Wiestler OD, Seizinger BR, Louis DN. Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol. 1993;3:19–26. doi: 10.1111/j.1750-3639.1993.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe K, Tachibana O, Sato K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–224. doi: 10.1111/j.1750-3639.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 16.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 18.Faury D, Nantel A, Dunn SE, Guiot M-C, Haque T, Hauser P, Garami M, Bognar L, Hanzély Z, Liberski PP, Lopez-Aguilar E, Valera ET, Tone LG, Carret A-S, Del Maestro RF, Gleave M, Montes J-L, Pietsch T, Albrecht S, Jabado N. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 19.Cross D, Alessi D, Cohen P, Andjelkovich M, Hemmings B. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 20.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes N, Heerding DA, Duckett DR, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 22.Prevo R, Deutsch E, Sampson O, et al. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res. 2008;68:5915–5923. doi: 10.1158/0008-5472.CAN-08-0757. [DOI] [PubMed] [Google Scholar]
- 23.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with anti-tumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 24.Fan Q-W, Cheng CK, Nicolaides TP, et al. A dual phosphoinositide-3-kinase α/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN–mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3 K signaling and inhibits growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 26.Thaker NG, Pollack IF. Molecularly targeted therapies for malignant glioma: rationale for combinatorial strategies. Expert Rev Neurother. 2009;9:1815–1836. doi: 10.1586/ern.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T-J, Koul D, LaFortune T, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 30.Doherty L, Gigas DC, Kesari S, et al. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology. 2006;67:156–158. doi: 10.1212/01.wnl.0000223844.77636.29. [DOI] [PubMed] [Google Scholar]
- 31.Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- 32.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 33.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 34.Vojtesek B, Bartek J, Midgley CA, Lane DP. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992;151:237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 35.Hsu S, Raine L, Fanger H, et al. Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 36.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 37.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated Akt in pancreas cancer. Molec Cell Pathol. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack IF, Hamilton RL, Burnham J, Holmes EJ, Finkelstein SD, Sposto R, Yates AJ, Boyett JM, Finlay JL. Impact of proliferation index on outcome in childhood malignant gliomas: results in a multi-institutional cohort. Neurosurgery. 2002;50:1238–1244. doi: 10.1097/00006123-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Pollack IF, Boyett JM, Yates AJ, Burger PC, Gilles FH, Davis RL, Finlay JL. The influence of central review on outcome associations in childhood malignant gliomas: Results from the CCG-945 experience. Neuro Oncol. 2003;5:197–207. doi: 10.1215/S1152-8517-03-00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalbfleisch JD, Prentice RI. The statistical analysis of failure time data. Wiley; New York: 1980. pp. 163–180. [Google Scholar]
- 41.Dixon WJ, Massey FJ. Introduction to statistical analysis. 3. McGraw-Hill; New York: 1969. [Google Scholar]
- 42.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, Rosenthal DI, Bakanauskas VJ, Cerniglia GJ, Bernhard EJ, Weber RS, Muschel RJ. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- 44.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 45.Gallia GL, Rand V, Siu I-M, Eberhart CG, James CD, Marie SKN, Oba-Shinjo SM, Carlotti CG, Caballero OL, Simpson AJG, Brock MV, Massion PP, Carson BS, Jr, Riggins GJ. PI3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4:709–713. doi: 10.1158/1541-7786.MCR-06-0172. [DOI] [PubMed] [Google Scholar]
- 46.Wiencke JK, Zheng S, Jelluma N, Tihan T, Vandenberg S, Tamgüney T, Baumber R, Parsons R, Lamborn KR, Berger MS, Wrensch MR, Haas-Kogan DA, Stokoe D. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol. 2007;9:271–279. doi: 10.1215/15228517-2007-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chadha KS, Khoury T, Yu J, et al. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13:933–939. doi: 10.1245/ASO.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Petricoin EF, III, Espina V, Araujo RP, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 49.Opel D, Poremba C, Simon T, Debatin K-M, Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- 50.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 51.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O’Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB. PTEN mutation, EGFR amplication, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 52.Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 53.Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, Mills GB, Hung MC, Meric-Bernstam F. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]