Abstract
Background: Consumption of flavonoid-containing foods may be useful for the management of hypertension.
Objective: We investigated whether 100% Concord grape juice lowers blood pressure in patients with prehypertension and stage 1 hypertension.
Design: We conducted a double-blind crossover study to compare the effects of grape juice (7 mL · kg−1 · d−1) and matched placebo beverage on 24-h ambulatory blood pressure, stress-induced changes in blood pressure, and biochemical profile. Participants consumed each beverage for 8 wk with a 4-wk rest period between beverages. They ceased consumption of grapes and other flavonoid-containing beverages throughout the study.
Results: We enrolled 64 otherwise healthy patients taking no antihypertensive medications (31% women, 42% black, age 43 ± 12 y). Baseline mean (±SD) cuff blood pressure was 138 ± 7 (systolic)/82 ± 7 (diastolic) mm Hg. No effects on the primary endpoint of 24-h mean systolic blood pressure, diastolic blood pressure, or stress-induced changes in blood pressure were observed. A secondary endpoint was nocturnal dip in systolic pressure. At baseline, nocturnal pressure was 8.3 ± 7.1% lower at night than during daytime. The mean nocturnal dip increased 1.4 percentage points after grape juice and decreased 2.3 percentage points after placebo (P = 0.005). Fasting blood glucose was 91 ± 10 mg/dL at baseline for the entire cohort. Glucose decreased 2 mg/dL after consumption of grape juice and increased 1 mg/dL after consuming the placebo (P = 0.03).
Conclusions: We observed no effect of grape juice on ambulatory blood pressure in this cohort of relatively healthy individuals with modestly elevated blood pressure. Secondary analyses suggested favorable effects on nocturnal dip and glucose homeostasis that may merit further investigation. This trial was registered at clinicaltrials.gov as NCT00302809.
INTRODUCTION
Hypertension affects >25% of the US adult population. There is considerable interest in dietary and other nonpharmacologic approaches to improve blood pressure control. Epidemiologic studies suggest that higher consumption of fruit and vegetables is associated with lower blood pressure and reduced cardiovascular mortality (1–3). In the well-controlled Dietary Approaches to Stop Hypertension (DASH) study, a low-fat diet rich in fruit and vegetables lowered blood pressure (4). Increased consumption of foods containing flavonoids has been proposed as a possible contributing mechanism for the benefits of the DASH diet.
Grapes are rich in flavonoids, and a body of work suggests that consumption of grapes and grape-containing products might lower blood pressure (5). In hypertensive animals, oral administration of red wine or grape extracts lowers blood pressure (6, 7). Mechanistic studies showed that grape flavonoids have favorable effects on endothelial function and inflammation that might reduce arterial stiffness and lower blood pressure (5). In population studies, wine consumption has been linked to lower blood pressure and reduced cardiovascular disease risk (8–10). Finally, a randomized intervention study showed a reduction in blood pressure after consumption of grape juice for 8 wk in 40 untreated hypertensive Korean men (11, 12).
The present study was designed to evaluate the effects of grape juice on blood pressure in otherwise healthy individuals with modest blood pressure elevation. It is conceivable that this population could benefit from a dietary intervention and avoid drug therapy. In contrast with the previous study by Park et al (11, 12), we sought to use more accurate 24-h ambulatory blood pressure monitoring as the primary endpoint (13) and to study a larger sample of both men and women. In addition to ambulatory blood pressure, we examined stress-induced changes in blood pressure and tested the hypothesis that grape juice would blunt the adverse effects of stress on blood pressure.
SUBJECTS AND METHODS
Study subjects
We identified subjects for screening by advertising for individuals with mild or borderline blood pressure elevation who were taking no antihypertensive medications. All potential subjects provided written informed consent. All procedures were in accordance with the ethical standards of the Boston Medical Center Institutional Review Board.
Screening
We enrolled individuals with stage 1 hypertension (systolic blood pressure of 140–159 mm Hg or diastolic blood pressure of 90–99 mm Hg) and individuals in the upper range of prehypertension (systolic blood pressure of 130–139 mm Hg or diastolic blood pressure of 85–89 mm Hg) (13). After providing consent, potential subjects underwent 2 screening visits separated by 1 wk. At each visit, we measured brachial blood pressure 3 times (3 min between measurements) using an automatic physiologic recorder (Dinamap Pro Series 100; GE Health Care, Piscataway, NJ). Subjects rested in a semirecumbent position for ≥10 min before the first measurement. Subjects were eligible for inclusion in the study if the average of 3 systolic or diastolic pressure measurements was within the specified range at both screening visits. At the first visit, eligible subjects were interviewed to obtain a medical history and underwent measurement of height and weight. We excluded patients with a clinical history of coronary artery disease, stroke, diabetes mellitus, congestive cardiac failure, Raynaud syndrome, body mass index (in kg/m2) >35, or use of antioxidant vitamins or estrogen replacement therapy within 4 wk. Pregnancy was excluded by using a urine pregnancy test at all study visits in premenopausal female subjects.
Dietary interventions and study design
After the first screening visit, potentially eligible subjects met with a nutritionist for dietary instruction. They were asked to stop all consumption of grape juice, wine, grape products, green or black tea, dark juices (eg, cranberry and pomegranate juice), and all dietary supplements for the duration of the study. Participants were instructed to maintain a diet that met the current recommendations for sodium intake (<2400 mg/d) (13). Subjects were educated about the calorie content of the study beverages and were instructed to consume the beverage in place of other juice or sugar-sweetened drinks or to cut 2 portions of carbohydrate-rich foods each day.
After qualifying at the second screening visit, subjects were enrolled into the study, which had a double-blind, placebo-controlled, crossover design. Subjects were randomly assigned by computer to receive 100% Concord grape juice first or placebo beverage first. There was a separate randomization for subjects with prehypertension or stage 1 hypertension to ensure balanced numbers of subjects within each group. The study beverages were supplied by the study sponsor (Welch Foods Inc, Concord, MA) and have been used in previous clinical studies (14–16). The 100% Concord grape juice contained 160 kcal, including 39 g natural sugar (52% fructose and 48% glucose) and 472.8 mg total polyphenols per 8 oz (240 mL). The Concord grape–flavored placebo beverage matched the flavor, color, calorie, and sugar profile of the juice, but did not contain any juice or polyphenolics. The amount of beverage consumed was individualized to 7 mL · kg−1 · d−1. Thus, a 70-kg person would have consumed 490 mL/d containing 965 mg total polyphenols and ≈327 kcal. Each beverage was consumed for 8 wk with a 4-wk rest period between beverages. All subjects received both beverages. Participants were given a marked “sports bottle” and instructed to fill the bottle to the mark each day and consume about half the juice with breakfast and half with dinner.
Grape juice and the placebo beverage were coded, and the investigators and participants were blinded to the identity of the beverage until completion of data analysis. The beverages were maintained in 24-oz (710-mL) glass bottles at 4°C by a commercial storage company (Millbrook Cold Storage Inc, Somerville, MA) and delivered weekly to the participants by a delivery company experienced in clinical trials involving beverages (Inquil Solutions LLC, Melrose, MA). Subjects were asked to return the juice container caps after each beverage consumption period, and the average compliance was 86%. We completed a “per protocol” subgroup analysis for the participants with evidence of ≥75% compliance (n = 52).
Ambulatory blood pressure measurements
Subjects underwent 24-h ambulatory blood pressure monitoring before consuming the first beverage (model 90207; Spacelabs Medical, Issaquah, WA). The monitor was programmed to record blood pressure measurements 4 times per hour during the day and 2 times per hour at night. The mean systolic blood pressure during the entire 24-h monitoring period served as the primary endpoint of the study. Additional endpoints included 24-h mean diastolic blood pressure, heart rate, and pulse pressure. We also calculated the percentage decrease in systolic and diastolic blood pressure measured at night (2200 to 0600) compared with the daytime pressures (nocturnal dip). The ambulatory blood pressure recordings were repeated in an identical fashion before the second beverage consumption period and during the last 24 h of each beverage consumption period.
Other endpoints measured at all 4 study visits
In addition to 24-h ambulatory blood pressure measurements, we collected blood samples, measured “office blood pressure” by using the physiologic recorder, and assessed vascular function before and after each beverage period. Serum total cholesterol, triglycerides, HDL cholesterol, insulin, and glucose concentrations were analyzed on a commercial basis by Quest Diagnostics Inc (Cambridge, MA). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [(fasting insulin μIU/mL) × (fasting glucose mmol/L)]/22.5. C-reactive protein concentrations were measured on a commercial basis at Children's Hospital (Boston, MA) by using a high-sensitivity nephelometric method as previously described (17). Soluble CD40-ligand concentrations were measured at the University of Massachusetts Medical Center by using a commercially available enzyme-linked immunosorbent assay (Bender MedSystems GmbH, Vienna, Austria) as previously described (18).
With regard to vascular testing, we assessed stiffness of the central aorta by determining carotid-femoral pulse wave velocity at each visit by applanation tonometry (Sphygmacor; AtCor Medical USA, Itasca, IL). Vasodilator function was measured in the fingertip by using digital pulse amplitude tonometry (Endo-PAT 2000R; Itamar Medical Ltd, Caesarea, Israel) as previously described (19). The increase in pulse amplitude in the fingertip during reactive hyperemia depends on nitric oxide and is impaired in patients with metabolic risk factors (20).
Stress testing
We investigated the effects of beverage consumption on stress-induced changes in blood pressure by using 2 established psychological tests and the cold pressure test (21). Subjects were asked to perform a video game challenge (Breakout by Atari Inc, Sunnyvale, CA) that involved moving a cursor to keep a ball from “falling” off the bottom of the video screen and a star-tracing task that involved looking in a mirror and tracing a star pattern. These tasks have been shown to be frustrating and require significant mental concentration. Each psychological test was performed for 3 min. There was a 10-min rest period between the tasks, and the order of task performance was randomized (video game first or star-tracing task first). Baseline blood pressure was measured before the beginning of the tasks and was subsequently repeated once every minute during task performance. After completion of the psychological tests and a 10-min rest period, we conducted the cold pressor test. This thermal pain test involved each subject immersing one hand in a basin of ice water for 45 s. Blood pressure was measured after completion of the cold pressor test. Because it was possible that participants would become familiar with the psychological tasks and experience less stress after repeated performance, we administered the tasks on only 2 occasions (after each beverage), rather than on 4 occasions (before and after each beverage).
Statistical analysis
All analyses were performed with SAS 9.1 (SAS Institute Inc, Cary, NC). We compared the clinical characteristics of the placebo-first and grape juice-first groups using the unpaired t test or chi-square test for continuous and categorical variables, respectively. We evaluated the effect of beverage consumption on blood pressures, biochemical markers, and vascular function measures by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard restricted maximum likelihood estimation. C-reactive protein and CD40 ligand were assessed after log-transformation because they lacked a normal distribution. We considered the effect of grape juice to be different from placebo if the treatment (grape juice or placebo) by follow-up (before beverage or after beverage) interaction had a P value <0.05. We adjusted for potential carryover by including in the model the 3-factor interaction: treatment by follow-up by treatment period (first beverage or second beverage). We completed prespecified analyses that included the subgroups of subjects with prehypertension (n = 38), stage 1 hypertension (n = 26), and evidence of good compliance (n = 52). Data are presented as means ± SDs.
The primary endpoint was a mean systolic blood pressure measured with a 24-h ambulatory blood pressure monitor. By using data from the DASH study (provided by Thomas Moore), we calculated that a sample size of 64 subjects would provide >90% power to detect a treatment effect of 5 mm Hg (22).
RESULTS
Study subjects
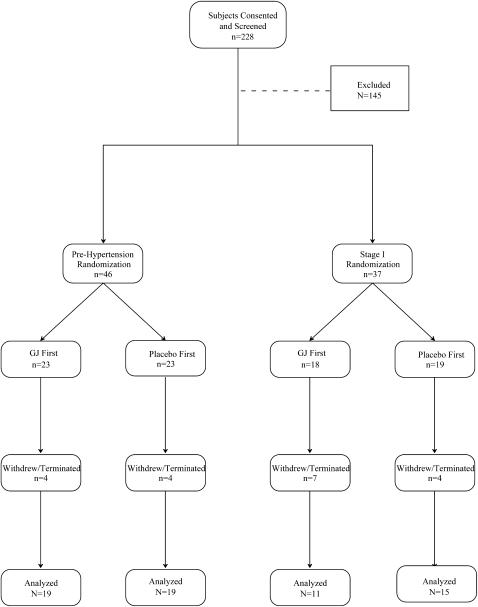
As outlined in Figure 1, 228 individuals underwent screening, and 145 were excluded. The most common reasons for exclusion were failure to meet the blood pressure criteria (n = 95), a body mass index >35 (n = 12), and participant decision after learning the details of study design (n = 25). Participants often qualified on the initial screening visit, but had blood pressure below the entry cutoff on the follow-up screening visit. Of the 83 randomly assigned subjects, 46 had prehypertension and 37 had stage 1 hypertension. Nineteen subjects withdrew or were terminated from the study, mostly by patient preference. One subject withdrew while drinking grape juice after developing diarrhea that may have been related to the study intervention.
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. GJ, grape juice.
The clinical characteristics of the 64 subjects who completed the study are shown in Table 1. The participants had an average age of 43 ± 12 y, were overweight, and were predominantly male. A relatively high proportion of subjects were black (42%). The grape juice–first and placebo-first groups were balanced in regard to clinical characteristics and baseline biochemical profiles. The average cuff blood pressure was 141 ± 7 (systolic)/84 ± 8 (diastolic) mm Hg at the first screening visit and 138 ± 7 (systolic)/82 ± 7 (diastolic) mm Hg at the beginning of the study for the entire group.
TABLE 1.
Baseline characteristics (first screening visit)1
| Characteristic | Grape juice first (n = 30) | Placebo first (n = 34) | P2 |
| Age (y) | 41 ± 133 | 44 ± 11 | 0.44 |
| Male sex [n (%)] | 19 (63) | 25 (74) | 0.38 |
| Black race [n (%)] | 13 (43) | 14 (41) | 0.86 |
| History of hypertension [n (%)] | 10 (33) | 5 (15) | 0.08 |
| Smoking [n (%)] | 11 (37) | 15 (44) | 0.55 |
| Family history of CAD [n (%)] | 3 (10) | 1 (3) | 0.24 |
| Waist (inches)4 | 36 ± 4.8 | 37 ± 5.4 | 0.34 |
| BMI (kg/m2) | 28 ± 3.8 | 28 ± 3.9 | 0.63 |
| Creatinine (mg/dL) | 0.94 ± 0.17 | 0.91 ± 0.17 | 0.45 |
CAD, coronary artery disease.
P for unpaired t test or chi-square test for continuous and categorical variables, respectively.
Mean ± SD (all such values).
One inch = 2.54 cm.
Effects of beverage consumption on biochemical markers and body weight
The effect of beverage consumption on the biochemical markers and body weight is shown in Table 2. No effects on serum lipids, C-reactive protein, soluble CD40 ligand, or body weight were observed. Fasting serum glucose was 2 mg/dL lower after 8 wk of grape juice consumption and was 1 mg/dL higher after 8 wk of placebo consumption (P = 0.03). No differences in the effect of either beverage on fasting insulin concentration or HOMA-IR were observed.
TABLE 2.
Effects of beverage consumption on biochemical profile and weight1
| Variable | Sample size | Before grape juice | After grape juice | Before placebo | After placebo | P2 |
| Total cholesterol (mg/dL) | 63 | 187 ± 343 | 189 ± 32 | 189 ± 37 | 193 ± 37 | 0.41 |
| LDL (mg/dL) | 63 | 114 ± 31 | 114 ± 29 | 114 ± 33 | 117 ± 33 | 0.27 |
| HDL (mg/dL) | 63 | 54 ± 15 | 54 ± 15 | 55 ± 18 | 54 ± 16 | 0.68 |
| Triglyceride (mg/dL) | 63 | 93 ± 53 | 104 ± 70 | 101 ± 78 | 105 ± 66 | 0.54 |
| Glucose (mg/dL) | 64 | 91 ± 10 | 89 ± 11 | 90 ± 11 | 91 ± 13 | 0.03 |
| Insulin (mU/L) | 52 | 6.2 ± 5.0 | 6.0 ± 5.4 | 6.7 ± 6.2 | 7.9 ± 13.0 | 0.46 |
| HOMA-IR | 52 | 1.4 ± 1.2 | 1.4 ± 1.3 | 1.5 ± 1.5 | 2.0 ± 3.8 | 0.31 |
| C-reactive protein (mg/L) | 59 | 1.1 (0.4, 2.5)4 | 0.8 (0.4, 2.5) | 1.2 (0.6, 2.9) | 1.4 (0.6, 4.0) | 0.61 |
| Soluble CD40 ligand (mg/mL) | 58 | 1.0 (0.4, 3.5) | 1.0 (0.4, 3.6) | 0.9 (0.5, 3.2) | 1.0 (0.6, 3.9) | 0.58 |
| Weight (kg) | 64 | 85 ± 15 | 85 ± 15 | 85 ± 16 | 85 ± 16 | 0.82 |
HOMA-IR, homeostasis model assessment of insulin resistance.
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard restricted maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
Mean ± SD (all such values).
Median; first and third quartile cutoffs in parentheses (all such values).
In the subgroup analyses (data not shown), a similar favorable effect of grape juice consumption was observed on serum glucose concentrations in the subgroups with prehypertension (n = 38; P = 0.03) and with good compliance (n = 52; P = 0.03). No effect was observed in the subgroup with stage 1 hypertension.
Effects of beverage consumption on blood pressure
The effects of beverage consumption on ambulatory blood pressure and office blood pressure are shown in Table 3. No significant difference in the effect of grape juice compared with that of placebo on mean systolic blood pressure by 24-h ambulatory monitor was observed. No effects on diastolic blood pressure, heart rate, or office blood pressure were observed. In the subgroup analyses (data not shown), no significant effects of beverage consumption on the primary endpoint of ambulatory systolic blood pressure in the prehypertension subgroup (n = 38; P = 0.47) or in the stage 1 hypertension subgroup (n = 26; P = 0.92) were observed. Furthermore, no effects on ambulatory diastolic pressure or office blood pressure were observed in the participants in the prehypertension or stage 1 hypertension subgroups, and no effects on any blood pressure variable were observed in the subgroup with good compliance.
TABLE 3.
Effect of beverage consumption on ambulatory and office blood pressure
| Variable | Sample size | Before grape juice | After grape juice | Before placebo | After placebo | P1 |
| 24-h Ambulatory blood pressure | ||||||
| Systolic blood pressure (mm Hg) | 64 | 124 ± 112 | 122 ± 10 | 124 ± 12 | 124 ± 10 | 0.67 |
| Diastolic blood pressure (mm Hg) | 64 | 77 ± 8 | 76 ± 7 | 78 ± 9 | 77 ± 8 | 0.90 |
| Pulse pressure (mm Hg) | 64 | 47 ± 7 | 46 ± 7 | 47 ± 8 | 47 ± 7 | 0.35 |
| Heart rate (beats/min) | 64 | 76 ± 10 | 76 ± 12 | 76 ± 10 | 76 ± 12 | 0.53 |
| Nocturnal dip in systolic blood pressure (%) | 63 | 6.8 ± 7.4 | 8.2 ± 7.4 | 9.9 ± 7.1 | 7.6 ± 8.3 | 0.005 |
| Nocturnal dip in diastolic blood pressure (%) | 63 | 9.9 ± 9.8 | 11.4 ± 8.6 | 13.0 ± 8.7 | 11.1 ± 9.7 | 0.03 |
| Office blood pressure (mm Hg) | ||||||
| Systolic blood pressure | 63 | 133 ± 12 | 132 ± 12 | 133 ± 11 | 132 ± 10 | 0.76 |
| Diastolic blood pressure | 64 | 80 ± 10 | 79 ± 10 | 80 ± 8 | 78 ± 8 | 0.32 |
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard restricted maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
Mean ± SD (all such values).
The nocturnal dip in systolic blood pressure increased by 1.4 percentage points after grape juice and decreased by 2.3 percentage points after placebo (P = 0.005), which reflected a favorable effect of grape juice. A significant difference in the effects of the 2 beverages on the nocturnal dip in diastolic blood pressure (P = 0.03) was also observed. In the subgroup analysis (data not shown), the effect of grape juice also differed from the effect of placebo on the nocturnal dip in systolic blood pressure in subjects with prehypertension (n = 38; P = 0.04), stage 1 hypertension (n = 26; P = 0.04), and good compliance (n = 52; P = 0.04).
Effect of beverage consumption on blood pressure reactivity
The effects of beverage consumption on the blood pressure responses to the 2 psychological stress tasks and the cold pressor test are displayed in Table 4. Compared with baseline, each stress test was associated with a significant change in systolic blood pressure, with increases in blood pressure during the star-tracing and cold pressor tests (P < 0.001 for both). Unexpectedly, the video game led to a net decrease in systolic blood pressure (P = 0.02). Beverage consumption had no effect on the blood pressure responses to the stress tasks for the group as a whole.
TABLE 4.
Stress-induced changes in blood pressure (n = 64)1
| Variable | After grape juice | After placebo | P2 |
| Systolic blood pressure (mm Hg) | |||
| Before stress | 132 ± 123 | 132 ± 10 | — |
| Video game | 130 ± 14 | 131 ± 13 | 0.17 |
| Star tracing | 134 ± 14 | 136 ± 15 | 0.51 |
| Cold pressor test | 137 ± 17 | 139 ± 16 | 0.20 |
| Diastolic blood pressure (mm Hg) | |||
| Before stress | 79 ± 10 | 78 ± 8 | — |
| Video game | 80 ± 10 | 78 ± 9 | 0.08 |
| Star tracing | 83 ± 12 | 81 ± 11 | 0.19 |
| Cold pressor test | 81 ± 12 | 81 ± 11 | 0.71 |
Each stress stimulus was associated with a significant change in systolic blood pressure compared with before stress (P < 0.05).
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard restricted maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
Mean ± SD (all such values).
In the subgroup of participants with prehypertension, the video game decreased systolic blood pressure from 130 ± 9 to 126 ± 11 mm Hg after consumption of grape juice and increased systolic blood pressure from 129 ± 9 to 132 ± 13 mm Hg after consumption of placebo. The differential effect of beverage consumption on blood pressure response was statistically significant (n = 38; P = 0.02). In the subgroup of participants with good compliance, the cold pressor increased blood pressure from 133 ± 12 to 137 ± 14 mm Hg after grape juice and from 132 ± 11 to 140 ± 16 after placebo. The differential effect of beverage was also statistically significant (n = 52; P = 0.03). No other significant effects of beverage consumption on the other stress-induced changes in blood pressure were observed in the subgroups (data not shown).
Vascular function
The effects of beverage consumption on vascular function are shown in Table 5. As shown, no effects of grape juice or placebo beverage were observed on carotid-femoral or carotid-radial pulse wave velocity—measures of stiffness of the central aorta and upper extremity conduit arteries, respectively. Also, no effects of the 2 beverages on the hyperemic increase in pulse amplitude in the finger tip, as measured by digital pulse amplitude tonometry, were observed. No significant effects of beverage on vascular function were observed in any of the prespecified subgroups (data not shown).
TABLE 5.
Effects of beverage consumption on vascular function1
| Variable | Sample size | Before grape juice | After grape juice | Before placebo | After placebo | P2 |
| Carotid-femoral PWV (m/s) | 47 | 7.1 ± 1.43 | 7.1 ± 1.4 | 7.3 ± 2.0 | 7.4 ± 1.5 | 0.92 |
| Carotid-radial PWV (m/s) | 62 | 8.1 ± 1.4 | 8.1 ± 1.5 | 8.4 ± 1.8 | 8.3 ± 1.9 | 0.82 |
| ln PAT ratio | 56 | 0.44 ± 0.32 | 0.35 ± 0.40 | 0.42 ± 0.40 | 0.35 ± 0.41 | 0.64 |
PWV, pulse wave velocity; PAT, pulse amplitude tonometry.
P for treatment by follow-up interaction as determined by using a general linear model for correlated data with PROC MIXED, an unstructured covariance matrix, and standard restricted maximum likelihood estimation in SAS (SAS Institute Inc, Cary, NC).
Mean ± SD (all such values).
DISCUSSION
In this randomized, double-blind, placebo-controlled, crossover study, consumption of Concord grape juice for 8 wk had no effect on the primary endpoint, ie, mean systolic blood pressure measured by 24-h ambulatory monitor. The subjects were otherwise healthy, were taking no antihypertensive medications, and had a mean baseline blood pressure in the prehypertension range (138/82 mm Hg). The subjects consumed a relatively large amount of grape juice (nearly 500 mL/d for a 70-kg person), and the study had >90% power to detect a decrease of 5 mm Hg. Thus, it is unlikely that an achievable and clinically meaningful antihypertensive effect of grape juice in this cohort was missed. Grape juice consumption had no effect on many secondary endpoints, including diastolic blood pressure, office blood pressure, stress-induced increases in blood pressure, vascular function, arterial stiffness, or blood markers of inflammation and platelet activity.
Although the study was negative for the primary endpoint, we did observe several potentially beneficial effects of Concord grape juice consumption compared with placebo. First, consumption of grape juice was associated with a modest enhancement of the normal nocturnal dip in blood pressure. Second, it is interesting that there was a modest, but significant, decrease in fasting blood glucose after consumption of grape juice, despite concerns that the additional 327 kcal/d for a 70-kg person might have adverse effects. Finally, grape juice had beneficial effects in several prespecified subgroup analyses, including beneficial effects of grape juice consumption on the stress blood pressure responses in participants with prehypertension and the group with good compliance.
The findings of our study differ from the prior study in Korean men with hypertension. In that study, consumption of a smaller amount of grape juice (5.5 mL · kg−1 · d−1) for 8 wk was associated with a 7.2-mm Hg reduction in systolic blood pressure compared with a 3.5-mm Hg reduction with placebo (11). Grape juice consumption also reduced oxidative damage to lymphocyte DNA and improved plasma radical scavenging capacity (12). These apparently discrepant findings likely reflect differences in the study population. It was observed in the DASH study that individuals with a higher blood pressure had a larger response to the dietary intervention (4), and the participants in the Korean study had higher blood pressure at baseline (≈150/95 mm Hg) than did the participants in the present study (138/82 mm Hg). It also is possible that the different results reflect methodologic differences between studies. The present study may be more reliable because we used 24-h ambulatory monitoring rather than office blood pressure measurements. Furthermore, the present study had a much larger sample size, with 64 participants receiving both beverages in a crossover study, whereas the study by Park et al randomly assigned separate groups of 19 and 21 men to treatment with grape juice and placebo, respectively (11). Nevertheless, it remains possible that grape juice may have a beneficial effect in untreated individuals with more elevated blood pressure.
Many studies have reported antihypertensive effects after treatment with other foods and beverages that are rich in flavonoids. For example, several studies have reported a blood pressure–lowering effect of high-polyphenol dark chocolate in patients with hypertension (23, 24). Pomegranate juice has also been reported to lower blood pressure in hypertensive patients (25). In general, the studies showing beneficial effects of polyphenol-rich foods and beverages were completed in patients with hypertension. On the other hand, a study of patients with prehypertension showed no benefit of dark chocolate (26), a finding consistent with the present study.
An interesting finding of our study was the decrease in fasting blood glucose observed after grape juice consumption. This result is consistent with a prior study that demonstrated a reduction in fasting blood glucose, insulin, and hemoglobin A1C after consumption of grape juice, wine, or dealcoholized wine (27). Favorable effects of chocolate (24) and tea (28, 29) on insulin sensitivity have also been reported. Such findings could indicate increased insulin sensitivity or insulin release. The mechanisms accounting for improved insulin sensitivity or release after consumption of grape-derived polyphenols and other dietary sources of these bioactive compounds remain incompletely understood. However, experimental studies suggest that these compounds may improve insulin signaling via the PI3 kinase-Akt pathway and activate sirtuin-1 and AMP kinase (30, 31).
Another effect of grape juice consumption in the present study was the increase in the nocturnal dip in blood pressure. Large-scale studies have shown that a greater degree of nocturnal dip is associated with lower mortality after adjustment for other risk factors (32). Failure to dip >10% is associated with worse cardiovascular outcome and is more common in black individuals (33). It is interesting that there was a large proportion of black individuals in the present study (42%) and that, on average, the study cohort was in the “nondipper” range (mean nocturnal decrease: 8.3%). The dip in blood pressure at night has been attributed to the inhibition of the sympathetic nervous system activity, and studies suggest that failure to dip reflects excessive sympathetic activity (34). Further study will be needed to confirm our finding that grape juice consumption increased nocturnal dipping and to elucidate the mechanism of the effect.
In predefined subgroup analyses, we observed favorable effects of grape juice consumption on changes in blood pressure induced by psychological stress and the cold pressor test. In the CARDIA (Coronary Artery Risk Development in Young Adults) study, blood pressure response induced by the video game challenge, star-tracing task, or the cold pressor test predicted incident hypertension in 4202 normotensive subjects (21). In that study, stress testing was performed between 1987 and 1988, and the video game challenge produced an increase in blood pressure of 9–10 mm Hg. In our study, the video game challenge had a neutral to blood pressure–lowering effect, possibly reflecting greater familiarity of our cohort with video games. In the subgroup with confirmed compliance, it was interesting that the blood pressure response to the cold pressor test was reduced by 50% after grape juice consumption; however, because they represent only subgroup analyses, these findings should be interpreted with caution. Confirmation is required before firm conclusions can be drawn about a protective effect of grape juice. These results and our observations about nocturnal dipping are consistent with the premise that grape juice might have modest favorable effects on sympathetic nervous system–induced changes in blood pressure.
Prior studies in experimental models and in sicker populations have shown beneficial effects of grape juice consumption on other aspects of vascular function and inflammation. As we recently reviewed, grape products improve endothelial function, inhibit platelet aggregation, and reduce inflammation (5, 14, 15). Possibly because of the modest blood pressure elevation and generally healthy study cohort, we observed no benefits of grape juice consumption on arterial stiffness assessed as pulse wave velocity (35) or on endothelial function in the fingertip as measured by peripheral arterial tonometry (20). We also observed no effect on C-reactive protein or soluble CD40 ligand—markers of inflammation and platelet activation.
Our study had several limitations. As mentioned, the cohort had only a modestly elevated blood pressure. Although much larger than the prior grape juice study, the sample size was modest compared with other studies showing favorable effects of dietary interventions on blood pressure. We had a high rate of screen failures, which reflected the difficulty in identifying individuals with elevated blood pressure who were not taking antihypertensive medications. Furthermore, we had a 23% withdrawal rate, largely reflecting the subjects' unwillingness to continue consuming a large volume of beverage on a daily basis. We did not record alcohol consumption, which could have influenced blood pressure. Finally, our study involved withholding grape products and other foods containing polyphenols. This aspect of the study design might explain the apparent worsening of some endpoints during consumption of the placebo beverage and reflect a lack of steady state in regard to flavonoid status. The study limitations were balanced by the placebo-controlled crossover study design and the use of 24-h ambulatory blood pressure monitoring to accurately measure changes in blood pressure.
In conclusion, we observed no effect of Concord grape juice consumption on ambulatory mean blood pressure in a group of otherwise healthy individuals taking no antihypertensive medications, with prehypertension, and with stage 1 hypertension. We also observed no effect on stress-induced increases in blood pressure, vascular function, or markers of inflammation. Interestingly, we observed modest, but potentially favorable, effects of grape juice on fasting blood glucose and the nocturnal dip in blood pressure. Further studies are needed to confirm these latter findings and identify the responsible mechanisms.
Acknowledgments
We gratefully acknowledge Thomas Moore for his assistance with study design and the statistical power calculations.
The authors' responsibilities were as follows—JAV: conceived the study hypothesis and secured funding; JAV, MH, M-AD, MT, WBC, FBV, and JFK: designed the study and implemented the study procedures; MMD, MH, BHK, M-AD, AL, MT, WBC, FBV, TLC, and AAF: collected data; and MMD, NMH, and JAV: conducted the analyses and drafted the manuscript. All authors critically reviewed and contributed to the intellectual content of the manuscript. Employees of Welch's Food Inc reviewed and agreed to fund the study as designed by the investigators. They had no input on the conduct of the study or the data analysis and interpretation. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Moline J, Bukharovich IF, Wolff MS, Phillips R. Dietary flavonoids and hypertension: is there a link? Med Hypotheses 2000;55:306–9 [DOI] [PubMed] [Google Scholar]
- 2.Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev 2005;63:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Rissanen TH, Voutilainen S, Virtanen JK, et al. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J Nutr 2003;133:199–204 [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 5.Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr 2009;139:1788S–93S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension 2001;38:159–65 [DOI] [PubMed] [Google Scholar]
- 7.Soares DR, Costa Viana FS, Souza MA, et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J Pharm Pharmacol 2002;54:1515–20 [DOI] [PubMed] [Google Scholar]
- 8.Nevill AM, Holder RL, Fentem PH, et al. Modelling the associations of BMI physical activity and diet with arterial blood pressure: some results from the Allied Dunbar National Fitness Survey. Ann Hum Biol 1997;24:229–47 [DOI] [PubMed] [Google Scholar]
- 9.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, de Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002;105:2836–44 [DOI] [PubMed] [Google Scholar]
- 10.Carollo C, Presti RL, Caimi G. Wine, diet, and arterial hypertension. Angiology 2007;58:92–6 [DOI] [PubMed] [Google Scholar]
- 11.Park YK, Kim JS, Kang MH. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double-blind, placebo controlled intervention trial. Biofactors 2004;22:145–7 [DOI] [PubMed] [Google Scholar]
- 12.Park YK, Lee SH, Park E, Kim JS, Kang MH. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Ann N Y Acad Sci 2009;1171:385–90 [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52 [DOI] [PubMed] [Google Scholar]
- 14.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999;100:1050–5 [DOI] [PubMed] [Google Scholar]
- 15.Freedman JE, Parker C, III, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001;103:2792–8 [DOI] [PubMed] [Google Scholar]
- 16.Albers AR, Varghese S, Vitseva O, Vita JA, Freedman JE. The antiinflammatory effects of purple grape juice consumption in subjects with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2004;24:e179–80 [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9 [DOI] [PubMed] [Google Scholar]
- 18.Keaney JF, Jr, Lipinska I, Larson MG, et al. Clinical correlates, heritability, and genetic linkage of circulating CD40 ligand in the Framingham Offspring Study. Am Heart J 2008;156:1003–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117:2467–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009;19:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews KA, Katholi CR, McCreath H, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 2004;110:74–8 [DOI] [PubMed] [Google Scholar]
- 22.Vollmer WM, Appel LJ, Svetkey LP, et al. Comparing office-based and ambulatory blood pressure monitoring in clinical trials. J Hum Hypertens 2005;19:77–82 [DOI] [PubMed] [Google Scholar]
- 23.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA 2003;290:1029–30 [DOI] [PubMed] [Google Scholar]
- 24.Grassi D, Desideri G, Necozione S, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 2008;138:1671–6 [DOI] [PubMed] [Google Scholar]
- 25.Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001;158:195–8 [DOI] [PubMed] [Google Scholar]
- 26.Ried K, Frank OR, Stocks NP. Dark chocolate or tomato extract for prehypertension: a randomised controlled trial. BMC Complement Altern Med 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banini AE, Boyd LC, Allen JC, Allen HG, Sauls DL. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006;22:1137–45 [DOI] [PubMed] [Google Scholar]
- 28.Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr 2009;28:355–61 [DOI] [PubMed] [Google Scholar]
- 29.Stote KS, Baer DJ. Tea consumption may improve biomarkers of insulin sensitivity and risk factors for diabetes. J Nutr 2008;138:1584S–8S [DOI] [PubMed] [Google Scholar]
- 30.Zang M, Xu S, Maitland-Toolan KA, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006;55:2180–91 [DOI] [PubMed] [Google Scholar]
- 31.Zunino S. Type 2 diabetes and glycemic response to grapes or grape products. J Nutr 2009;139:1794S–800S [DOI] [PubMed] [Google Scholar]
- 32.Ben Dov IZ, Kark JD, Ben Ishay D, Mekler J, Ben Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007;49:1235–41 [DOI] [PubMed] [Google Scholar]
- 33.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006;354:2368–74 [DOI] [PubMed] [Google Scholar]
- 34.Sayk F, Becker C, Teckentrup C, et al. To dip or not to dip: on the physiology of blood pressure decrease during nocturnal sleep in healthy humans. Hypertension 2007;49:1070–6 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular Risk. Artery Res 2009;3:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]